Abstract
Renal cell carcinoma (RCC) accounts for the 3% of all solid tumors. Despite continuous improvement in the therapy regimen, less has been achieved in terms of enabling an earlier diagnosis: the neoplasia usually reveals its presence at an advanced stage, obviously affecting prognosis. The most frequent sites of secondary disease are shown to be lungs (50–60%), bone (30–40%), liver (30–40%) and brain (5%); while the head and neck district seems to account for less than 1% of patients with primary kidney lesion. We report here the case of a 70-year old man who presented with acute renal failure due to abdominal recurrence of RCC 18 years post nephrectomy. After a few months of follow up without any systemic therapy due to the renal impairment, the patient presented a vascularized tongue lesion that was demonstrated to be a secondary localization of the RCC. This lesion has, therefore, been treated with microsphere embolization to stop the frequent bleeding and to lessen the unbearable concomitant symptoms it caused, such as dysphagia and pain. A tongue lesion that appears in a RCC patient should always be considered suspect and a multidisciplinary study should be conducted both to assess whether it is a metastasis or a primary new lesion and to understand which method should be selected, if necessary, to treat it (surgery, radiation or embolization). Lingual metastasis should be examined accurately not only because they seem to implicate a poor prognosis, but also because they carry a burden of symptoms that not only threatens patients' lives but also has a strong impact on their quality of life.
Key words: kidney cancer, lingual metastasis.
Introduction
Renal cell carcinoma (RCC) accounts for 3% of all solid tumors and currently causes about 3500 deaths/year in the UK.1 In the US, the incidence rate has increased slightly, but this has been a progressive increase among ethnic groups of different age ranges, with a more rapid growth for smaller tumors at an earlier stage.2 The natural history of RCC suggests that about one-third of patients exhibit secondary lesions at presentation, and about another third develop metastasis during subsequent follow up.3 Radical nephrectomy, the only possible curative treatment option, provides about 96% 5-year survival for stage I patients; the rate dramatically drops to 23% for patients with advanced disease (stage IV). Among the factors that can affect prognosis, a major role is played by the presence of occult metastasis at diagnosis, especially for patients with positive lymph nodes or spread to the renal vein.4 Metastasis are more frequent in lungs (50–60%), bone (30–40%), liver (30–40%) and brain (5%); the head and neck district is the only site of metastasis in as few as 1% of patients with a primary lesion of the kidney.5 Lingual localizations are rarer: a comprehensive review of the relative literature from 1914 to the present day includes only 21 cases of renal tumors with lingual metastasis.6 As a rule, primary lesions metastasizing to the tongue are uncommon, with an incidence as low as 0.2% in a series of 6881 autopsies performed on cancer patients.3
Case Report
G.G. was a 70-year old male patient when, in February 2008, he was referred to our center for emergency hospitalization for acute renal failure. His medical history showed a right nephrectomy in 1992 for a cancer staged as pT2, Nx, M0, histologically diagnosed as a moderately differentiated (Furhman's grade G2) clear cell renal carcinoma. During hospitalization, abdominal computed tomography (CT) showed secondary lesions to the adrenals and some abdominal lymph nodes (which suggested hydroureteronephrosis), while chest CT scan showed secondary lung involvement. Nevertheless the patient was not given any oncological treatment because of his kidney impairment, which was only slightly improving after the positioning of a left ureteral stent. Instead, the patient was just followed with instrumental and clinical examinations after discharge.
In July 2008, the patient reported the appearance of a lesion growing in the oral cavity from the posterior half of the left hemitongue. This was richly vascularized, bright red in color, maximum 3 cm in diameter, ulcerated on part of its outermost surface (Figure 1).
Figure 1.
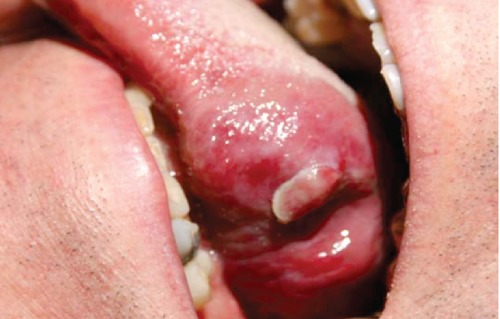
First clinical presentation of the tongue lesion.
The patient, therefore, underwent a CT scan of the head and neck. This showed an oval lesion localized at the free margin of the left hemi-tongue with high contrast enhancement; the lesion does not involve oral floor structures (Figure 2) and there was no involvement of lat-ero-cervical lymph nodes or bones. An oval secondary lesion, approximately a maximum 25 mm in axial diameter, was also seen at the right retrojugular region, medially. This showed infiltrative features and involved the lower portion of the right part of the odontoid process of the second cervical vertebra, and a large part of the body and stem of the vertebra below.
Figure 2.
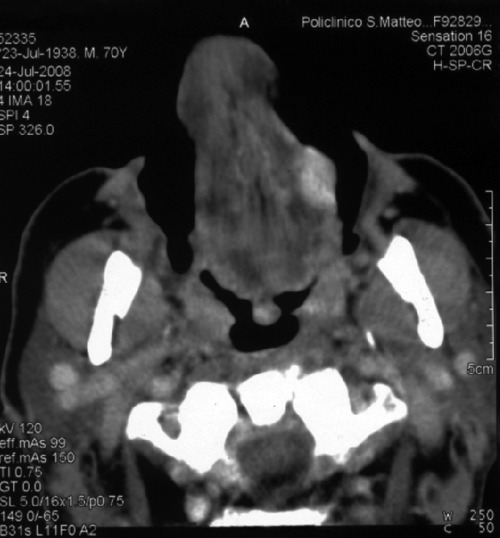
Computed tomography scan appearance of the tongue lesion.
The patient has therefore been referred to our Department and a biopsy of the lingual lesion has been performed; the lesion resulted compatible with a localization of clear cell renal carcinoma (Figure 3). A subsequent neurosurgical visit excluded any surgical option for the management of the cervical vertebra and just suggested the use of a collar with chin support.
Figure 3.
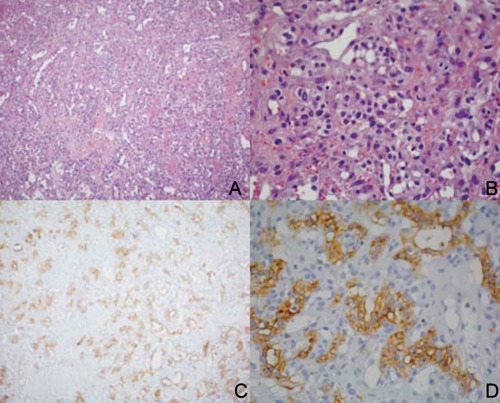
Histological finding obtained by the biopsy of the lingual lesion. A, B) hematoxylin and eosin appearance of the specimen at different magnification; C, D) same levels of the lesion with a positive focal pattern for CD10 expression (immunohistochemistry).
The lesion enlarged drastically and progressively over the next weeks (Figure 4) with repeated episodes of local bleeding, with dysphagia and odynophagia; tracheostomy with gastroenterostomy was therefore performed in August 2008.
Figure 4.
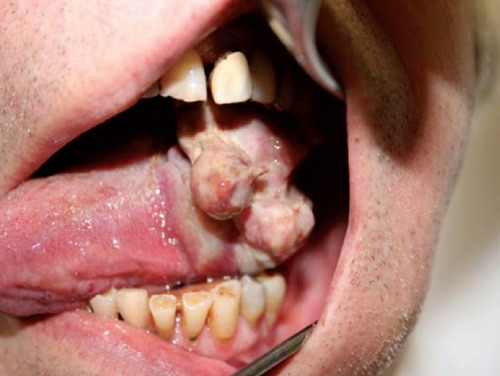
Last appearance of the tongue lesion before embolization.
Surgical ablation of the lingual mass was judged to be unfeasible as it would have been too radical, requiring the resection of the anterior two-thirds of the tongue.
Therefore, the patient was submitted to embolization of the left lingual artery through trans-femoral catheterization and infusion of embolization microspheres (Embosphere™) until complete blockade of regional flow was achieved. Embosphere™ microspheres are small, flexible, hydrophilic, biocompatible spheres made of acrylic polymer and porcine-derived gelatin. The microspheres are packaged in 0.9% saline and are provided in a sterile and non-pyrogenic state; they come in six size ranges to allow physicians to choose the appropriate caliber for the vessels to be embolized.
The procedure, performed at the end of August, aimed to reduce both bleeding episodes and uncontrolled pain. It was considered successful but systemic progression quickly occurred, with the onset of acute respiratory failure from lung progression, ultimately leading to the patient's death in September 2008.
Discussion
Just like primary cancers of the tongue, lingual metastasis from other solid tumors, though uncommon, may present as ulcerating lesions or as polypoid, vegetating lesions. Therefore, the finding of a suspicious lesion requires a thorough, possibly multidisciplinary, diagnostic work-up to distinguish a metastasis from a primary lesion, since treatment and prognosis differ greatly.7,8
Boles and Cerny and Nahum and Bailey have all discussed the pathogenesis of this metastatic site:9,10 the rich venous anastomosis connecting the pre-vertebral, vertebral and epidural districts provides the tumor with a convenient route of spread by means of circulating emboli of different sizes that do not meet much resistance. The increase in intra-abdominal or intra-thoracic pressure causes a retrograde venous flow that can allow tumor cells to bypass capillary filtration at the pulmonary circle and to colonize tissues in the head and neck region. Indeed, there have been reports of paradoxical involvement of this district in the absence of lung or liver localizations. For instance, thyroid is one of the most frequent sites of metastasis from RCC among those that are considered uncommon, while the tongue is a much less common site. However, when the tongue is involved, it is most often at the base, because of its rich vascularization.11
The incidence of lingual metastasis from RCC is almost unknown, as only 21 cases have been reported in the literature (Table1), excluding our own.3,6,12–24 From these scanty reports spread over nearly a century, we can still extract some information; mean patient age is 60 years and 85% of cases also present metastasis to other districts, the most common site being the lung, which was involved in 16 of 22 cases, including our own. As for treatment, 11 patients underwent surgical ablation, 3 radiotherapy, 1 chemotherapy, 1 immunotherapy, and 4 no treatment at all.
Table 1. Literature reports of lingual metastasis from renal cell carcinoma.
| Author, year | Age/Gender | Site/Size | Other metastasis | Therapy | Survival (months) |
|---|---|---|---|---|---|
| Coenen, 19143,12 | 62/F | Not known | Not known | None | 3 |
| McNattin, 19313,12 | 58/M | Not known | Lung, heart, skin | None | 1 |
| Schrag, 19453,12 | 34/M | Not known | Lung | Excision | 5 |
| DelCarmen, 197013 | 77/M | Not known | None | Excision | Not known |
| Satomi, 19743,12 | 41/F | Left surface 1.7 cm | Lung | None | 1 |
| Friedlander, 197814 | 84/M | Apex 2 cm | Lung | Excision | 3 |
| Fitzgerald, 198215 | 63/M | Right dorsal surface | Brain | Radiation therapy | 3 |
| Kitao, 19863,12 | 57/M | Base 2.1 cm | Bones | Excision | Patient alive at publication |
| Inai, 198716 | 42/M | Left base 3 cm | Lung, bones | Radiation therapy | 7 |
| Kapoor, 198717 | 70/M | Not known | None | Excision | Not known |
| Matsumoto,19873,12 | 77/M | Left surface 3 cm | Lung | Chemotherapy | 2 |
| Madison, 198818 | 63/M | Right ventral surface 2.5 cm | Lung, liver | Not reported | Not reported |
| Ishikawa, 199119 | 59/F | Left base 2.5 cm | Lung, bones | Excision | 6 |
| Okabe, 199220 | 58/M | Not known | Lung, brain | Excision | 3 |
| Shibayama,199321 | 41/M | Not known | Brain, lung, liver | Immunotherapy | 6 |
| Aguirre, 199622 | 82/F | Not known | Brain | Excision | 35 (Patient alive at publication) |
| Airoldi, 199523 | 51/M | Left margin | Lung | Excision | 2 |
| Tomita, 199624 | 50/M | Left surface 2.5 cm | Lung, brain, skin | Radiation therapy | 12 |
| Goel, 200312 | 62/M | Base 4 cm | Lung | Excision | 12 (Patient alive at publication) |
| Hsiang-CheHuang, 20053,12 | 76/F | Left margin 3.5 cm | Lung, liver | Excision | 1 |
| Kancheria, 20086 | 60/M | Not known | Lung, bones, skin | Excision | 8 (Patient alive at publication) |
| Present case, 2008 | 70/M | Left hemi-tongue | Lung, adrenals, skin | Excision | Less than 1 month |
Prognosis is very poor in these patients, with the latest estimates indicating 5.8 months between the diagnosis of metastasis and death.6
Conclusions
The major clinical problem linked to the presence of lingual lesions, apart from the poor prognosis with which they seem to be linked, is the host of symptoms that can cause: pain, bleeding, halitosis, obviously dysgeusia, and difficulty swallowing and pronouncing words. These symptoms usually worsen and may cause a rapid deterioration in the patient's clinical condition, as well as having a devastating impact on their quality of life.3,9
Surgery or radiation therapy appear to be reasonable treatment option and treatment should be selected case by case, while metastasectomy in RCC patients, in whom it certainly cannot have a curative purpose, can represent a valid option in selected cases, such as isolated skeletal, and especially pulmonary, lesions.
Lingual metastasis should be immediately evaluated for surgical excision, trying to preserve organ function as far as possible, in order to prevent progressive metastasis enlargement from causing airway obstruction. The sequelae of partial glossectomy, such as susceptibility to infections and bleeding, or difficulty swallowing and speaking, are usually not of primary importance.6 If we extrapolate some data on the treatment of primary lingual tumors, we find that the surgical removal of T1 and T2 cancers (up to 4 cm) by partial glossectomy allowed long-term local control of the disease in 90% and 70% of patients, respectively.5,25,26
Radiation therapy, with doses greater than 40 Gy,27 can be used for palliation. In our patient, we took into consideration the typical resistance to irradiation of RCC,4 even though a couple of trials demonstrated the macroscopic disappearance of lingual metastasis from clear-cell RCC with radiation doses of about 50 Gy.16,24
In selected cases, embolization through super-selective catheterization of the tongue vascular district, as performed in our patient, can provide good local control in terms of mass debulking, palliation of painful symptoms, and decrease in the number of bleeding episodes that patients may experience. Newer and improved embolization techniques are becoming available for primary tongue cancers but are likely to be suitable also for metastatic lesions. Experimental trials with cisplatin, albumin microspheres, and carboplatin microspheres have been carried out on primary cancers of the tongue.28,29
Our patient could also have benefited from antiangiogenic drugs, but this treatment option was considered unfeasible mainly due to severe kidney impairment. On the other hand, the highly aggressive nature of the lesion and the impact of the specific localization in terms of natural feeding, induction of pain and risk of bleeding made it a priority to perform palliative interventions aimed at improving the patient's quality of life.
References
- 1. [Accessed on 08 Aug 2012];UK National Statistics, Cancer incidence and mortality in the United Kingdom, 2006–2008. Available from: http://www.ons.gov.uk/ons/publications/re-referenceta-bles.html?edition=tcm%3A77–218569.
- 2.Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J. 2008;14:288–301. doi: 10.1097/PPO.0b013e3181867628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zegarelli DJ, Tsukada Y, Pickren JW, Greene GW., Jr. Metastatic tumor to the tongue. Report of twelve cases. Oral Surg Oral Med Oral Pathol. 1973;35:202–11. doi: 10.1016/0030-4220(73)90286-7. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol. 1994;12:206–12. doi: 10.1200/JCO.1994.12.1.206. [DOI] [PubMed] [Google Scholar]
- 5.Spiro RH, Strong EW. Discontinuous partial glossectomy and radical neck dissection in selected patients with epidermoid carcinoma of the mobile tongue. Am J Surg. 1973;126:544–6. doi: 10.1016/s0002-9610(73)80047-9. [DOI] [PubMed] [Google Scholar]
- 6.Kancherla K, Hall P, Sastry K, Brown J. Lingual metastasis from renal cell carcinoma. Kidney Cancer J Int. 2008;2:5–9. [Google Scholar]
- 7.Marioni G, Gaio E, Poletti A, et al. Uncommon metastatic site of renal adenocarcnoma: the oral tongue. Acta OtolaryngoI. 2004;124:197–201. doi: 10.1080/00016480310016929. [DOI] [PubMed] [Google Scholar]
- 8.Azam F, Abubakerr M, Gollins S. Tongue metastasis as an initial presentation of renal cell carcinoma: a case report and literature review. J Med Case Rep. 2008;2:249–249. doi: 10.1186/1752-1947-2-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boles R, Cerny J. Head and neck metastasis from renal carcinomas. Michigan Med. 1971;70:616–8. [PubMed] [Google Scholar]
- 10.Nahum AM, Bailey BJ. Malignant tumors metastatic to the paranasal sinuses: case report and review of the literature. Laryngoscope. 1963;73:942–53. doi: 10.1288/00005537-196307000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Lang EE, Patl N, Walsh RM, et al. A case of renal cell carcinoma metastatic to the nose and tongue. Ear Nose Throat J. 2003;82:382–3. [PubMed] [Google Scholar]
- 12.Goel MC, Williams DW, Evans H, Roberts JG. Lingual metastasis from renal cell carcinoma: management and review of the literature. Urol Int. 2003;7:418–21. doi: 10.1159/000074097. [DOI] [PubMed] [Google Scholar]
- 13.Del Carmen BV, Kobitz BC. Oral metastasis from hypernephroma. J Am Geriatr Soc. 1970;18:743–6. doi: 10.1111/j.1532-5415.1970.tb02822.x. [DOI] [PubMed] [Google Scholar]
- 14.Friedlander AH, Singer R. Renal adenocarcinoma of the kidney with metastasis to the tongue. J Am Dent Assoc. 1978;97:989–91. doi: 10.14219/jada.archive.1978.0420. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald RH, Jr, McInnes BK, Manry HC. Renal cell carcinoma involving oral soft tissues. J Oral Maxillofac Surg. 1982;40:604–6. doi: 10.1016/0278-2391(82)90294-4. [DOI] [PubMed] [Google Scholar]
- 16.Inai T, Kagawa S, Aga Y, Akiyama K. [A renal cell carcinoma with metastasis to the tongue] Hinyokika Kiyo. 1987;33:1240–43. [Article in Japanese] [PubMed] [Google Scholar]
- 17.Kapoor VK, Mukhopadhyay AK, Chatto-padhyay TK, Sharma LK. Renal cell carcinoma metastatic to the tongue. J Indian Med Assoc. 1987;85:119–20. [PubMed] [Google Scholar]
- 18.Madison JF, Frierson HF., Jr. Pathologic quiz case 2. Clear cell carcinoma, consistent with metastatic renal cell carcinoma. Arch Otolaryngol Head Neck Surg. 1988;114:570–3. [PubMed] [Google Scholar]
- 19.Ishikawa J, Morisue K, Imanishi O, Kamidono S. Renal cell carcinoma metastatic to the tongue: a case report. Hinyokika Kiyo. 1991;37:263–5. [PubMed] [Google Scholar]
- 20.Okabe Y, Ohoka H, Miwa T, et al. View from beneath: pathology in focus. Renal cell carcinoma metastasis to the tongue. J Laryngol Otol. 1992;106:282–4. doi: 10.1017/s0022215100119280. [DOI] [PubMed] [Google Scholar]
- 21.Shibayama T, Hasegawa S, Nakamura S, et al. Disappearance of metastatic renal cell carcinoma to the base of the tongue after systemic administration of interferon-alpha. Eur Urol. 1993;24:297–9. doi: 10.1159/000474313. [DOI] [PubMed] [Google Scholar]
- 22.Aguirre A, Rinaggio J, Diaz-Ordaz E. Lingual metastasis of renal cell carcinoma. J Oral Maxillofac Surg. 1996;5:344–6. doi: 10.1016/s0278-2391(96)90757-0. [DOI] [PubMed] [Google Scholar]
- 23.Airoldi M, Succo G, Valente G, et al. Head and neck metastasis of renal cancer after nephrectomy: a report of 2 cases. Tumori. 1995;81:213–4. doi: 10.1177/030089169508100313. [DOI] [PubMed] [Google Scholar]
- 24.Tomita T, Inouye T, Shinden S, et al. Palliative radiotherapy for lingual metastasis of renal cell carcinoma. Auris Nasus Larynx. 1998;25:209–209. doi: 10.1016/s0385-8146(97)10037-2. [DOI] [PubMed] [Google Scholar]
- 25.Marks JE, Lee F, Freeman RB, et al. Carcinoma of the oral tongue: a study of patient selection and treatment result. Laryngoscope. 1981;91:1548–59. [PubMed] [Google Scholar]
- 26.Lam KH, Wong J, Lim ST, Ong GB. Carcinoma of the tongue: factors affecting the results of surgical treatment. Br J Surg. 1980;67:101–5. doi: 10.1002/bjs.1800670210. [DOI] [PubMed] [Google Scholar]
- 27.Vaeth JM. Proceedings: cancer of the kidney-radiation therapy and its indications in non-Wilms' tumors. Cancer. 1973;32:1053–55. doi: 10.1002/1097-0142(197311)32:5<1053::aid-cncr2820320504>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Wen Y, Wang C. [Study on drug release after arterial embolism with cisplatin-loaded albumin microsphere in treating carcinoma of tongue] Hua Xi Kou Qiang Yi Xue Za Zhi. 2003;21:112–3. [Article in Chinese]. [PubMed] [Google Scholar]
- 29.Lam KH, Wong J, Lim ST, Ong GB. Carcinoma of the tongue: factors affecting the results of surgical treatment. Br J Surg. 1980;67:101–5. doi: 10.1002/bjs.1800670210. [DOI] [PubMed] [Google Scholar]


