Abstract
Gastrointestinal stromal tumors (GIST) are uncommon mesenchymal spindle-cell or epithelioid neoplasms, located mainly with higher frequency in the stomach and small bowel. GISTs represent the majority of primary non-epithelial neoplasms of the digestive tract, most frequently expressing the KIT protein a transmembrane tyrosine kinase receptor for stem cell factor. Extra-gastrointestinal stromal tumors tend to present In fewer than 5% of cases; they originate primarily from the mesentery, omentum or peritoneum. Furthermore, these extra-gastrointestinal tumors (EGIST) tend to be more common in patients over the age of 50 years. EGISTs are neoplasms with overlapping immunohistological features, occurring in the abdomen outside the gastrointestinal tract with no connection to the gastric or intestinal wall. We describe here a rare case of EGIST of the lesser omentum and report the clinical, macroscopic, immunohistological and radiological features of an EGIST arising in the lesser omentum of a 63-year old man. Clinical course and the prognostic factors of such lesions will also be discussed. EGISTs in the lesser omentum can grow slowly and remain silent despite a large tumor size. In most cases, a pre-operative diagnosis is not possible, and the patient undergoes a surgical operation for the generic diagnosis of abdominal mass. During the intervention it is important to achieve a complete removal of the mass and to examine every possible adhesion to the gastrointestinal wall.
Key words: medicine, oncology, tumors, EGIST, omentum, GIST.
Introduction
Gastrointestinal stromal tumors (GISTs) are mesenchymal spindle-cell or epithelioid neoplasms that arise mainly in the stomach and small intestine; they are rarely located in the colon, rectum and esophagus. They also rarely originate from other intraabdominal tissues such as the omentum and mesentery.
They are characterized by the expression of KIT (CD117), a transmembrane tyrosine kinase receptor for stem cell factor. It has become apparent that some GISTs have myogenic features (smooth muscle GISTs), neural attributes (gastrointestinal autonomic nerve tumors), characteristics of both muscle and nerve (mixed GISTs), or lack differentiation.
Their presumed cell of origin is the interstitial cell of Cajal, a pace-maker cell that controls gastrointestinal (GI) tract peristalsis and is the only GI tract cell that exhibits the CD117+/CD34+ immunophenotype, which is the diagnostic hallmark of GIST.1 The molecular pathogenesis of GISTs is usually driven by activating mutations of the KIT gene that encodes the CD117 oncoprotein.2
The clinical behavior of GISTs is variable, with tumor size and mitotic activity being the most useful parameters of metastatic risk stratification.3,4
GISTs are rarely found as primary tumors in extragastrointestinal intra-abdominal tissues such as the omentum and the mesentery (EGISTs). The origin of EGISTs is uncertain but, as a rule, their histological appearance and immunophenotype are identical to those of classical GISTs. They are, therefore, thought to represent either GISTs that have separated from the GI tract wall,5 or independent growths of mesenchymal cells of the omentum and mesentery from which they originate. Accumulating molecular genetic data may lead to improved prognostic classification and patient management.
Only 5 cases of lesser omental GISTs have been reported.6–9 Furthermore, the median age at diagnosis is about 60 years and the diagnosis is slightly more prevalent in males than in females. Currently, only 3% of GIST cases are diagnosed in patients under the age of 21 years.10–12
Our knowledge about EGISTs is based on accumulated data from individual case reports. In this paper, we describe the radiological and pathological findings in a case of EGIST while a literature review is also made.
Case Report
A male, 63-year old patient presented to the emergency department in septic condition, complaining of abdominal distention, discomfort, high fever up to 39.5°C over the previous few days. On clinical examination, there was diffuse peritonaism with a palpable mass on the epigastrium.
Blood tests revealed a severe leukocytosis (white blood cell 37300/86.2%), jaundice (Tbil 7.14 mg/dL, Dbil 6.75 mg/dL) and abnormal values of APTT (55.8 sec, INR: 1.71). Ultrasonography showed a hypoechogenic mass located between the stomach and the pancreas, adjacent to the left liver lobe, and also diffuse dilatation of the intrahepatic biliary ducts and the common bile duct. Contrast-enhanced computed tomography (CT) was performed for initial staging. CT showed a large mass (16×16×12 cm) with solid and cystic components (Figure 1). The adjacent structures including the mesenteric fat plane, the gastrohepatic ligament and the duodenum were displaced, and the mass was in contact with the stomach. The central area of the mass was of low density corresponding to cystic and necrotic components (Figure 2).
Figure 1.
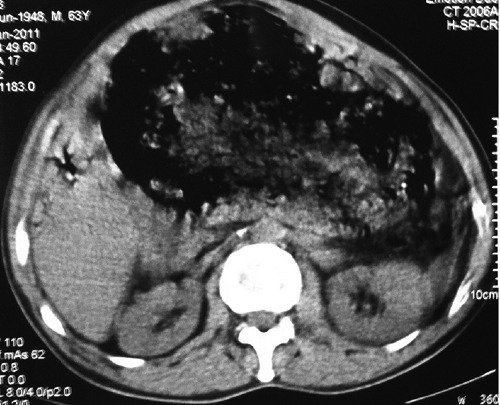
Axial computed tomography non enhanced.
Figure 2.
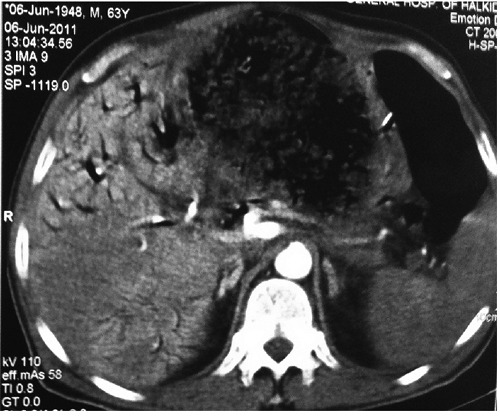
Axial computed tomography contrast ehnanced.
The patient underwent an emergency explorative laparotomy due to worsening of his clinical condition. A large mass was found in the lesser omentum in contact with the stomach. The mass could not be surgically detached from the stomach. Therefore, an enucleation of the mass was performed with simultaneous sphenoid gastrectomy of the posterior wall of the stomach in proximity to the stomach's lesser curvature using a TA 60 mm stapler. This type of resection was considered safer in terms of acquiring clear resections margins.
Postoperatively, the jaundice decreased till normalization.
Unfortunately, the patient presented peritonitis on post-surgery Day 5. An exploratory laparotomy was performed and a failure of the staplers' line of the stomach was found due to ischemic necrosis. The stomach was sutured again and a prophylactic gastro-enteroanastomosis was performed. The following postoperative period was uneventful and the patient is in good health one year after.
A macroscopic examination showed a clearly defined tumor with a white curt surface. No hemorrhage or necrosis was observed (Figure 3).
Figure 3.
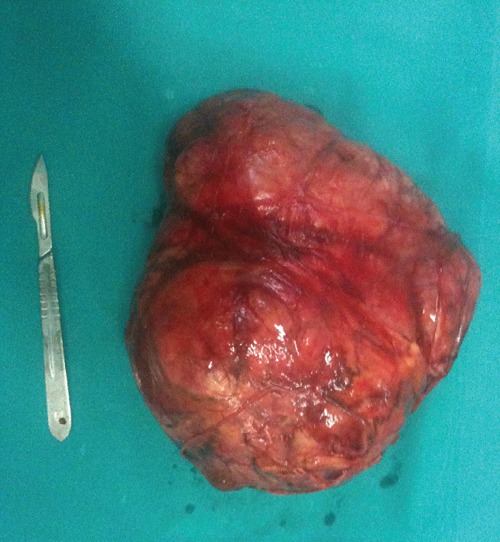
Surgical specimen of the extra-gastrointestinal tumors: macroscopic examination shows that the tumor is clearly defined and has a white curt surface without hemorrhage or necrosis.
Microscopically, the tumor was composed of spindle to ovoid cells arranged in broad fascicles. The nuclei were ovoid and focally elongated, and showed mild to moderate atypia. The mitotic figures were fewer than 5 per 50 high power fields (HPF) (Figure 4). The MIB-1 index, defined as the perentage of MIB-1 positive tumor cells seen in 500 tumor cells, was less than 10%. Immunohistochemically, the tumor cells showed diffuse and strong positivity for c-kit (CD117), smooth muscle actin (SMA) and human hematopoietic progenitor cell antigen (CD34), with negative internal control marker, as well as weak and focal positivity for caldesmon. Immunostains for desmin and S-100 protein were negative (Figure 5).
Figure 4.
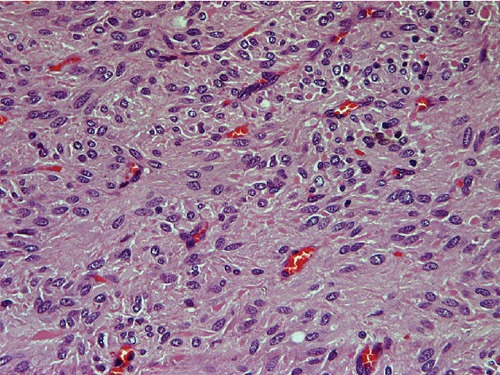
Microscopic imaging of the specimen with mitosis <5/50 high power fields (low grade). Hematoxylin and Eosin 400×.
Figure 5.
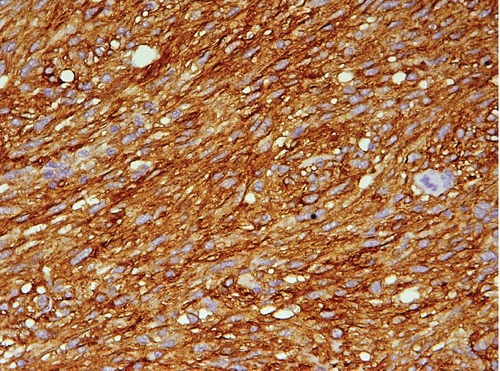
Microscopic imaging. Diffuse positive immunostain for c-kit 400×.
Based on Fletcher's classification, the tumor was classified as a low-risk GIST originating in the lesser omentum.4
Discussion
Gastrointestinal stromal tumors are currently believed to originate from the interstitial cells of Cajal, a pace-maker cell that controls GI track peristalsis and the only GI track cell that exhibits the CD117+/CD34+ immunophenotype, which is the diagnostic hallmark of GIST.13 The tumors can occur anywhere that these cells exist in the gastrointestinal tract, including the stomach (40–60%), small intestine (30–40%), ano-rectum (7%), colon, and esophagus.1,2
Sakurai et al. reported in the normal omentum CD117+/CD34+ mescenchymal cells, like Cajal cells, from which the EGIST may theoretically arise.
The median age at diagnosis of GISTS is about 60 years and is slightly more prevalent in males than in females.1,2,5 In a Medline search, we found 54 omental EGISTs reported in the form of small series or isolated cases.5,9,14–40
According to these cases, the median age at diagnosis is 65 years, with a male female ratio 1:1. Also, we ascertained that there is no difference in incidence between lesser and greater omentum.
Miettinen et al. reported that only 3% of Gists are diagnosed before the age of 21 years and GISTs arise only rarely in children.12
Omental EGISTs can remain clinically silent despite the large tumor size. The most frequent presenting complaint is an abdominal mass, but patients are often diagnosed incidentally during investigations for other medical conditions.
The omental GIST in our patient showed no myogenic or neural differentiation of myogenic features and neural attributes. Only 16 omental GISTs without myogenic or neurogenic features, including the present case, have ever been reported (Table 1).
Table 1. Reported cases of lesser omentum gastrointestinal stromal tumor.
| First Author | Year | Sex | Age (years) | Size (cm) | Necrosis |
|---|---|---|---|---|---|
| Takahashi | 1998 | F | 71 | 17 | Present |
| Fukuda | 2001 | M | 45 | 4,5 | Absent |
| Sakurai | 2001 | F | 52 | 11,5 | Absent |
| Sakurai | 2001 | F | 74 | 8 | Absent |
| Nakaya | 2004 | M | 69 | 14 | Present |
| Uchiyama Y, | 2009 | M | 24 | 18 | Absent |
| Kontopanos | 2009 | M | 68 | 15 | Absent |
| Present case | 2011 | M | 63 | 16 | Present |
The tumor arose in 7 men and 8 women ranging in age from 31 to 89 years. Tumor diameter ranged from 2.5 to 36 cm (median 15.7 cm). Macroscopically, most were large, solid masses exhibiting cystic changes.
The radiological features of omental GISTs without myogenic or neurogenic features have not been established. Generally, they may be similar to those of omental leiomyomas and leiomyosarcomas. Most GISTs with myogenic features are demonstrated as hypervascular tumors with clear margins on CT and angiography.41–43
It is difficult to differentiate a GIST in the lesser omentum from a GIST in the lesser curvature of the stomach, despite the use of advanced radiological imaging techniques. About half of all omental GISTs are misdiagnosed as extra mucosal tumors of the stomach.41,42,44 Additionally, omental EGISTs seem to be morphologically and immunohistochemically identical to their gastric and intestinal counterparts. They are cellular tumors consisting of elongated spindle and epithelioid cells that are typically positive for c-kit (CD117) and, less consistently, for CD34. They may show smooth muscle actin positivity but are negative for desmin and S-100 protein.
C-kit (CD117) may be negative in GISTs (2–5%), either due to limited sampling in the tumor with focal variation or, more rarely, due to a unique subset of CD117-negarive GIST with epithelioid morphology. CD34 strongly and diffusely stains approximately 70% of GISTs. The other 30% of GISTs have patchy weak to moderate intensity staining. In GISTs with neural differentiation, fewer cells stain postive for CD34 and the intensity is less compared to GISTS with smooth muscle differentiation. Most GISTs are desmin negative. Strong desmin staining occurs in approximately 2% of GISTs, although approximately 33% stain focally and weakly. S-100 protein stain the cytoplasm and/or nuclei focally in approximately 50% of neoplasms.45–47 Due to the rarity of omental EGISTs, there are no specific treatment data from clinical trials and surgical resection is the only effective modality, and their management follows the guidelines applicable to classical GISTs.3
It should be noted here that, according to the National Institutes of Health algorithm for assessing malignancy of classical GISTs,4 most omental EGISTs would be classified as high-risk due to their large size alone, as in at least 55% of published cases it exceeds 10 cm. However, the tumor size is not a reliable prognostic parameter in the case of omental EGISTs.
Conclusions
Gists without myogenic or neurogenic features that arise in the omentum are very rare and surgical resection is the only effective treatment approach.
Nevertheless, adjuvant therapy following resection of localized disease with imatinib has become standard of care in cases of high risk.48–50
References
- 1.Hirota S. Gastrointestinal stromal tumors: their origin and cause. Int J Clin Oncol. 2001;6:1–5. doi: 10.1007/pl00012072. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 3.Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566–78. doi: 10.1093/annonc/mdi127. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–65. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 5.Goh BK, Chow PK, Kesavan SM, et al. A single-institution experience with eight CD117-positive primary extragastrointestinal stromal tumors: critical appraisal and a comparison with their gastrointestinal counterparts. J Gastrointest Surg. 2009;13:1094–8. doi: 10.1007/s11605-009-0828-4. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Kuwano S, Yanagihara M, Kakita A. A primary solitary tumor of the lesser omentum with immunohistochemical features of gastrointestinal stromal tumors. Am J Gastroenterol. 1998;93:2269–73. doi: 10.1111/j.1572-0241.1998.00632.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda H, Suwa T, Kimura F, et al. Gastrointestinal stromal tumor of the lesser omentum: report of a case. Surg Today. 2001;31:715–8. doi: 10.1007/s005950170077. [DOI] [PubMed] [Google Scholar]
- 8.Nakaya I, Iwata Y, Abe T, et al. Malignant gastrointestinal stromal tumor originating in the lesser omentum, complicated by rapidly progressive glomerulonephritis and gastric carcinoma. Intern Med. 2004;43:102–5. doi: 10.2169/internalmedicine.43.102. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai S, Hishima T, Takazawa Y, et al. Gastrointestinal stromal tumors and KIT-positive mesenchymal cells in the omentum. Pathol Int. 2001;51:524–31. doi: 10.1046/j.1440-1827.2001.01224.x. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era. Cancer. 2005;103:821–9. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 11.Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG. Gastrointestinal stromal tumors in Iceland, 1990–2003: the Icelandic GIST, a population-based incidence and pathologic risk stratification study. Int J Cancer. 2005;117:289–93. doi: 10.1002/ijc.21167. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach; a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 13.Sicar K, Hewlett BR, Huozinga JD, et al. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377–89. doi: 10.1097/00000478-199904000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Miettinen M, Monihan JM, Sarlomo-Rikala M, et al. Gastrointestinal stromal tumors/ smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol. 1999;23:1109–18. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto H, Oda Y, Kawaguchi K, et al. c-kit and PDGFRA mutations in extragastrointestinal stromal tumor (gastrointestinal stromal tumor of the soft tissue) Am J Surg Pathol. 2004;28:479–88. doi: 10.1097/00000478-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T, Kuwao S, Yanagihara M, Kakita A. A primary solitary tumor of the lesser omentum with immunohistochemical features of gastrointestinal stromal tumors. Am J Gastroenterol. 1998;93:2269–73. doi: 10.1111/j.1572-0241.1998.00632.x. [DOI] [PubMed] [Google Scholar]
- 17.Agaimy A, Wunsch PH. Gastrointestinal stromal tumours: a regular origin in the muscularis propria, but an extremely diverse gross presentation. A review of 200 cases to critically re-evaluate the concept of so-called extra-gastrointestinal stromal tumours. Langenbecks Arch Surg. 2006;391:322–9. doi: 10.1007/s00423-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 18.Aihara R, Ohno T, Mochiki E, et al. Gastrointestinal stromal tumor of the lesser omentum in a young adult patient with a history of hepatoblastoma: Report of a case. Surg Today. 2009;39:349–52. doi: 10.1007/s00595-008-3844-1. [DOI] [PubMed] [Google Scholar]
- 19.Cai N, Morgenstern N, Wasserman P. A case of omental gastrointestinal stromal tumor and association with history of melanoma. Diagn Cytopathol. 2003;28:342–4. doi: 10.1002/dc.10292. [DOI] [PubMed] [Google Scholar]
- 20.Caricato M, Ausania F, Valeri S, et al. An omental mass: any hypothesis? Colorectal Dis. 2005;7:417–8. doi: 10.1111/j.1463-1318.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 21.Castillo-Sang M, Mancho S, Tsang AW, et al. A malignant omental extra-gastrointestinal stromal tumor on a young man: a case report and review of the literature. World J Surg Oncol. 2008;6:50–50. doi: 10.1186/1477-7819-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferchichi L, Kourda N, Zermani R, et al. Extragastrointestinal stromal tumors: a report of 4 cases. Ann Chir. 2006;131:271–5. doi: 10.1016/j.anchir.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Franzini C, Alessandri L, Piscioli I, et al. Extra-gastrointestinal stromal tumor of the greater omentum: report of a case and review of the literature. World J Surg Oncol. 2008;6:25–25. doi: 10.1186/1477-7819-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukuda H, Suwa T, Kimura F, et al. Gastrointestinal stromal tumor of the lesser omentum: report of a case. Surg Today. 2001;31:715–8. doi: 10.1007/s005950170077. [DOI] [PubMed] [Google Scholar]
- 25.Gun BD, Gun MO, Karamanoglu Z. Primary stromal tumor of the omentum: report of a case. Surg Today. 2006;36:994–6. doi: 10.1007/s00595-004-3280-9. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser AM, Kang JC, Tolazzi AR, et al. Primary solitary extragastrointestinal stromal tumor of the greater omentum coexisting with ulcerative colitis. Dig Dis Sci. 2006;51:1850–2. doi: 10.1007/s10620-006-9217-y. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Boo YJ, Jung CW, et al. Multiple malignant extragastrointestinal stromal tumors of the greater omentum and results of immunohistochemistry and mutation analysis: a case report. World J Gastroenterol. 2007;13:3392–5. doi: 10.3748/wjg.v13.i24.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshariya M, Jagad RB, Kawamoto J, et al. Gastrointestinal stromal tumor of gastrohepatic omentum in a patient with von Recklinghausen's disease (neurofibromatosis type 1) Hepatogastroenterology. 2007;54:2230–1. [PubMed] [Google Scholar]
- 29.Liu H, Li W, Zhu S. Clinical images. Extragastrointestinal stromal tumor of lesser omentum mimicking a liver tumor. Am J Surg. 2009;197:7–8. doi: 10.1016/j.amjsurg.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Llenas-Garcia J, Guerra-Vales JM, Moreno A, et al. Primary extragastrointestinal stromal tumors in the omentum and mesentery: a clinicopathological and immunohistochemical study. Hepatogastroenterology. 2008;55:1002–5. [PubMed] [Google Scholar]
- 31.Nakagawa M, Akasaka Y, Kanai T, et al. Extragastrointestinal stromal tumor of the greater omentum: case report and review of the literature. Hepatogastroenterology. 2003;50:691–5. [PubMed] [Google Scholar]
- 32.Nakaya I, Iwata Y, Abe T, et al. Malignant gastrointestinal stromal tumor originating in the lesser omentum, complicated by rapidly progressive glomerulonephritis and gastric carcinoma. Intern Med. 2004;43:102–5. doi: 10.2169/internalmedicine.43.102. [DOI] [PubMed] [Google Scholar]
- 33.Ouazzani A, Lefebvre JC, Mefire Y, et al. Electronic clinical challenges and images in GI. Cystic GISTof the lesser omentum. Gastroenterology. 2008;134:1–2. doi: 10.1053/j.gastro.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Sakurai S, Hasegawa T, Sakuma Y, et al. Myxoid epithelioid gastrointestinal stromal tumor (GIST) with mast cell infiltrations: a subtype of GIST with mutations of platelet-derived growth factor receptor alpha gene. Hum Pathol. 2004;35:1223–30. doi: 10.1016/j.humpath.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Kaneko G, Kubota K, et al. Malignant tumor, of the gastrointestinal stromal tumor type, in the greater omentum. J Gastroenterol. 2003;38:985–8. doi: 10.1007/s00535-003-1182-z. [DOI] [PubMed] [Google Scholar]
- 36.Tajima K, Fuyama S, Inaba Y, et al. Expression of embryonic-form smooth muscle myosin heavy chain in a gastrointestinal stromal tumor of the greater omentum. Dig Dis Sci. 2001;46:1629–32. doi: 10.1023/a:1010632900025. [DOI] [PubMed] [Google Scholar]
- 37.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol. 2008;14:7256–9. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dedemadi G, Georgoulis G, Kontopanos D, et al. Extragastrointestinal Stromal Tumors of the Omentum: Review Apropos of a Case with a Novel Gain-of-Function KIT Mutation. J Gastrointest Canc. 2009;40:73–8. doi: 10.1007/s12029-009-9089-4. [DOI] [PubMed] [Google Scholar]
- 39.Todoroki T, Sano T, Sakurai S, et al. Primary omental gastrointestinal stromal tumor (GIST) World J Surg Oncol. 2007;5:66–66. doi: 10.1186/1477-7819-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uchiyama Y, Matsuyama R, Suzuki K, et al. [A case of inflammatory myofibroblastic tumor of the lesser omentum with intratumoral hemorrhage] Nihon Shokakibyo Gakkai Zasshi. 2009;106:1321–6. [Article in Japanese] [PubMed] [Google Scholar]
- 41.Lee JT, Kim MJ, Yoo HS, et al. Primary leiomyosarcoma of the greater omentum: CT findings. J Comput Assist Tomogr. 1991;15:92–4. doi: 10.1097/00004728-199101000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Ikematsu Y, Usui K, Kamohara Y, et al. Leiomyoma of the lesser omentum: report of a case. Surg Today. 1996;26:46–8. doi: 10.1007/BF00311991. [DOI] [PubMed] [Google Scholar]
- 43.Kimura H, Maeda K, Konishi K, et al. Primary leiomyosarcoma arising in the lesser sac: report of a case. Surg Today. 1997;27:672–5. doi: 10.1007/BF02388230. [DOI] [PubMed] [Google Scholar]
- 44.Sasamoto A, Yamaguchi A, Isogai M, et al. [A case of primary leiomyosarcoma of the lesser omentum] Nippon Rinsyou Geka Gakkai Zasshi [Article in Japanese] 1998;59:1409–13. [Google Scholar]
- 45.Mitettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT) Mod Pathol. 2000;13:1134–42. doi: 10.1038/modpathol.3880210. [DOI] [PubMed] [Google Scholar]
- 46.Greenson JK. Gastrointestinal stromal tumors and other mesenchymal lesion of the gut. Mod Patholo. 2003;16:366–75. doi: 10.1097/01.MP.0000062860.60390.C7. [DOI] [PubMed] [Google Scholar]
- 47.Tworek JA, Goldblum JR, Weiss SW, et al. Stromal tumors of the abdominal cavity: A clinicopathologic study of 20 cases. Am J Surg Pathol. 1999;23:946–54. doi: 10.1097/00000478-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–77. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 49.Jiang Y, Ming L, Montero AJ, et al. Optimizing imatinib mesylate treatment in gastrointestinal stromal tumors. Gastrointest Cancer Res. 2008;2:245–50. [PMC free article] [PubMed] [Google Scholar]
- 50.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


