Abstract
Abundant myxoid stroma rarely occurs in urothelial carcinomas, and may cause diagnostic challenges when cells with eosinophilic cytoplasm forming nests and cords in a myxoid background are seen, particularly in the absence of typical carcinomatous appearance. Microscopic examination of transurethral resection specimen of a 71-year-old male patient revealed non-cohesive oval or elongated tumor cells with eosinophilic cytoplasm arranged in cord-like filigree pattern in an abundant myxoid stroma. Immunohistochemically the tumor was positive for cytokeratin 7, cytokeratin 20, and 34BE12. About 90 to 100% nuclear staining was observed with p63, p53, and Ki-67. A second neoplasm with a flat overlying urothelial epithelium and a complete inverted cellular growth pattern was also noted. The neoplasm exhibited less than 2% and 10% nuclear staining with Ki-67 and p53, respectively. Considering histological, histochemical, and immunohistochemical findings, a diagnosis of synchronous urothelial carcinoma with abundant myxoid stroma and inverted papilloma was made.
Key words: urinary bladder, urothelial carcinoma, transitional cell carcinoma, myxoid, inverted papilloma.
Introduction
Diagnosis is straightforward for most of the urothelial neoplasias with their noninvasive character and typical histological appearance. However, some uncommon histological patterns in invasive urothelial carcinomas may cause diagnostic challenges. Many subtypes have also been defined for urothelial carcinomas in addition to those lesions with typical morphological features.1–3 As such, urothelial carcinoma with abundant myxoid stroma has been proposed to represent a new diagnostic entity due to its chordoma-like morphology and prognostic features.4 The proposed terminologies for these new entities include myxoid urothelial carcinoma with abundant myxoid stroma or urothelial carcinoma with abundant myxoid stroma showing chordoid-like features.4,5 Although rare, abundant myxoid stroma may occur in urothelial carcinomas and in its subtypes such as micropapillary or sarcomatoid urothelial carcinomas.6–8 It may also be observed in mucinous carcinomas of the urinary bladder and in prostate or colon cancer invading the urinary bladder. Presence of cord, nest, filigree and single cell patterns with oval cells having a narrow eosinophilic cytoplasm reminiscent of chordoma and myoepithelioma may lead to diagnostic challenges. On the other hand, inverted papilloma is a rare benign neoplasia with only 450 cases reported in the literature.9–17 Malignant transformation is very rare and only very rare occurrences of synchronous tumors have been reported.9–17 In the 1980s several reported cases suggest the malignant potential of inverted papilloma including those indicating malignant evidence.12,13 Recent article and review shows that malignant tranformation is absent.14–17 Some authors consider most atypical inverted papillomas previously described as urothelial carcinoma with inverted growth.14–18 In this case report, we discuss the histological features and diagnostic challenges associated with urothelial carcinoma with abundant myxoid stroma in conjunction with a discussion regarding the appropriateness of the terminology and its coincidental simultaneous occurrence with inverted papilloma.
Case Report
In a 71-year-old male patient presenting with hematuria and dysuria, a 3-cm polypoid mass with ulceration in the right lateral wall of the urinary bladder was removed transurethrally. Microscopy showed abundant acellular faintly basophilic myxoid stroma with inter-dispensed carcinoma cells in all fields. Carcinoma comprised mostly of oval and sometimes elongated single cells with non-cohesive eosinophilic cytoplasm and occasionally their cord-like filigree patterns (Figures 1 and 2). In addition, areas of polymorph leukocyte, lymphocyte and plasmocyte infiltration mostly surrounding vascular structures were found. The surface was entirely ulcerated with no discernible epithelium. Focal areas of necrosis were observed. No glandular, sarcomatoid or micropapillary carcinomatous growth could be detected. Biopsy material consisted of only invasive carcinoma and myxoid stroma. Lamina propria and muscularis propria could not be distinguished. Within the biopsy material, a second neoplasia different from the myxoid tumor was also found with flat urothelial epithelium in the surface and complete inverted cellular growth (Figure 3). There were oval and spindle-like cells without pleomorphism but with thick cordon and trabeculae formation (Figure 4). Peripherally cellular palisade, and parallel spindle-like nuclei causing a central flow-effect were present. Cyst-like structures with eosinophilic material were also present. No mitosis was found.
Figure 1.
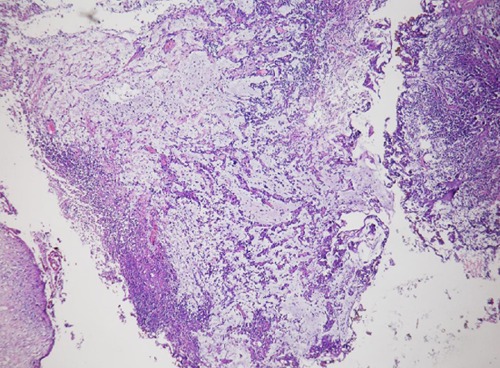
The low magnification, demonstrates abondant myxoid stroma with cord-like and individual tumor cells.
Figure 2.
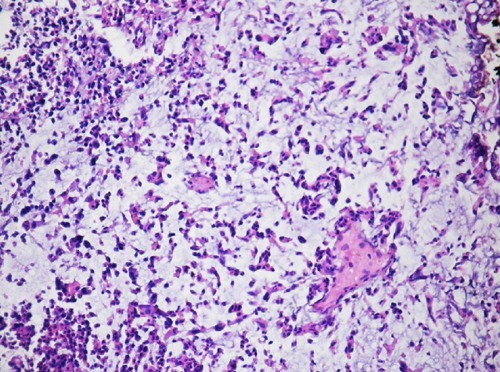
The high magnification, urothelial carcinoma composed of individual cells anf irregular agregates associated with myxoid stroma.
Figure 3.
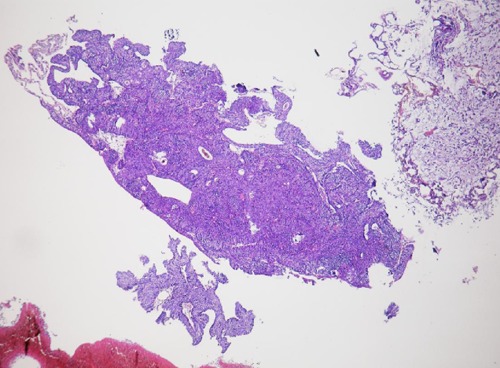
The high magnification, inverted urothelial papilloma and carcinomas mass.
Figure 4.
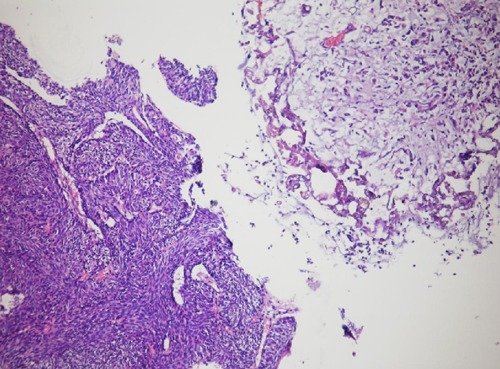
The high magnification, inverted urothelial papilloma and carcinomas mass.
Intense staining with alcian blue pH 2.5 (Figure 5) periodic acid-schiff, and mucicarminewas found in the myxoid areas of the stroma. The cellular structures within the myxoid stroma exhibited 90 to 100% nuclear staining with p63 (Figure 6), p53 and Ki-67; strong cytoplasmic staining with cytokeratin 20 (CK20), cytokeratin 7 (CK7) and high molecular weight keratin (34BE12) (Figure 7); and no staining with S-100, calponin, Glial fibrillary acidic protein (GFAP), CDX2, prostate specific antigen (PSA), and leukocyte common antigen (LCA).
Figure 5.
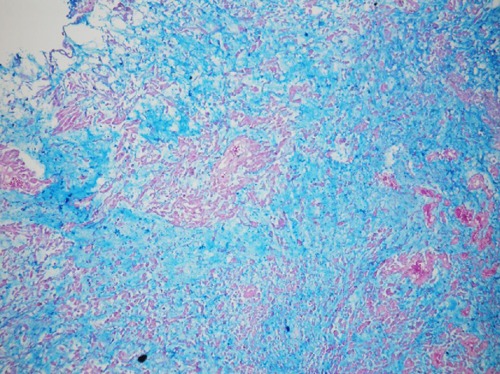
Alcian blue positivity in the stroma.
Figure 6.
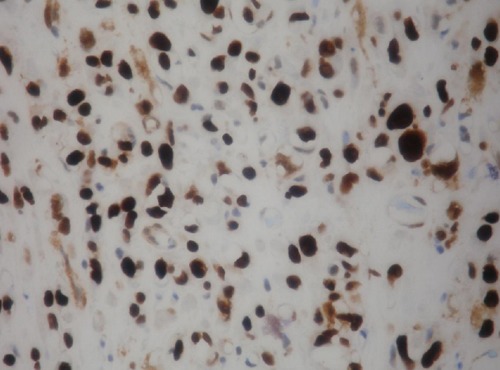
Urothelial carcinoma with abondant myxoid stroma diffuse nuclear p63 immunoreactivity.
Figure 7.
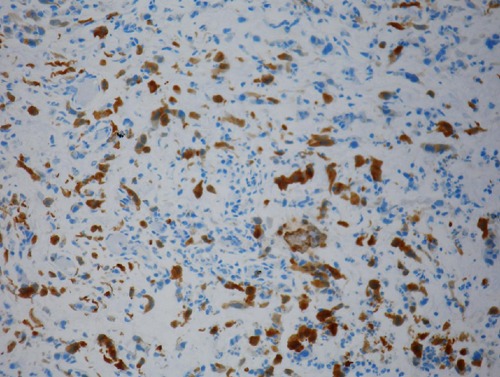
Urothelial carcinoma with abondant myxoid stroma diffuse cytoplasmic cytokeratin 34BE12 immunoreactivity.
The neoplasia with inverted growth pattern showed less than 2% staining with Ki-67 and less than 10% nuclear staining with p53. Taken together, these findings were considered to provide evidence for the synchronous diagnosis of two neoplasias, i.e. invasive myxoid urothelial carcinoma with chordoid features and inverted papilloma. Cystectomy was performed in another center 3 months after the diagnosis. In cystectomy, material perivesical; adipose tissue and prostate were infiltrated, there was no lymphoid involvement. On whole body scanning, there was no metastasis. The patient did not accept the chemotherapy. Six months after surgery he had widespread recurrence. The patient died of the disease at 13 months following surgery.
Discussion
Although uncommon, stromal myxoid degeneration can sometimes occur in invasive or non-invasive urothelial carcinomas, particularly in sarcomatoid and micropapillary subtypes.6–8 It may also be observed in mucinous adenocarcinomas of the urinary bladder and in prostate or colon cancer invading the urinary bladder. Presence of nest or cord like structures in an urothelial carcinoma with abundant myxoid stroma may resemble chordomas or myoepitheliomas leading to diagnostic challenges that require additional immunohistochemical markers for differential diagnosis. However, other features such as the presence of typical urothelial carcinoma fields; positive staining for 34BE12, p63, p53, CK7 and CK20; and negative staining for S-100, GFAP, and calponin assist in differential diagnosis. Except for the absence of areas with histological findings for urothelial carcinoma, our case exhibited the typical immunohistochemical properties of the urothelial carcinoma. In such cases, primary urothelial carcinoma should be given diagnostic precedence over other alternatives, since myoepithelioma and chordoma almost never develop at this site. Another very important consideration in the differential diagnosis of such lesions is myxoid cystitis, which may present with an epithelioid appearance and chordoid-like lymphocytic morphology, thus easily misleading to a diagnosis of urothelial carcinoma.19 Correspondingly, histology showed a bland appearance, myxoid stroma, necrosis, and abundance of inflammatory cells in our case, resembling cystitis in hematoxylin eosin stained preparations. Only after keratin positivity and LCA negativity were established through immunohistochemical studies, a differential diagnosis could be made.
Although urothelial carcinoma with abundant myxoid stroma described above has only been reported in a limited number of studies in the literature, many different names have been given to this pathology including myxoid urothelial carcinoma, urothelial carcinoma with abundant myxoid stroma, and urothelial carcinoma with chordoid features.4,5,7 Neoplasias with an abundant myxoid stroma (e.g. myxoid liposarcoma, chordoma, myoepithelioma, mucinous carcinoma) usually demonstrate eosinophilic cytoplasms, single and elongated cellular patterns, cords, and nests microscopically. It is expected that histological features similar to other neoplasia with myxoid stroma may readily be exhibited by the urothelial carcinomas of the urinary bladder with an abundant myxoid stroma. However, in our view, terms like myxoid or chordoid may lead to some diagnostic challenges, since histological criteria based on the ratio of myxoid stroma and chordoid features have not yet been specified in detail and these two attributes may also accompany non-invasive and low-grade urothelial carcinomas. Thus, appropriateness of their introduction as new and separate diagnostic entities is debated.
Considering the fact that inverted papilloma is an uncommon benign neoplasia of the urinary bladder, its co-existence with urothelial carcinoma in a patient is an interesting observation.9–17 Follow up studies in inverted papilloma does not suggest a significantly increased risk of urothelial carcinoma, and its synchronous occurrence with urothelial carcinoma is rare.9–18 Generally, different pathways of tumorigenesis are thought to be involved in these two lesions.18
Conclusions
In line with this information, no direct causative role for the inverted papilloma could be determined in our case with urothelial carcinoma as suggested by the fact that papilloma exhibited similar microscopic characteristics throughout the lesion with a low nuclear Ki-67 and p53 staining in all fields. We believe that this synchronous occurrence of the two tumors was coincidental.
Myxoid stroma and chordoid features are expected findings in urothelial carcinomas. Differential diagnosis is usually not problematic due to typical histological features in tissue preparations. However, presence of myxoid stroma in all fields of urothelial carcinoma without the typical features may complicate the diagnosis such as in our case. Furthermore, presence of asynchronous inverted papilloma in the same patient may be even more confusing.
References
- 1.Lopez-Beltran A, Sauter G, Gasser T, et al. Eble JN, Sauter G, Epstein JI, et al. Pathology and genetics tumors of the urinary system and male genital organs. Lyon: IARC Press; 2004. Infiltrating urothelial carcinoma; pp. 93–109. [Google Scholar]
- 2.Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009;22(suppl2):96–118. doi: 10.1038/modpathol.2009.26. [DOI] [PubMed] [Google Scholar]
- 3.Hameed O, Humphrey PA. Humphrey PA, Dehner LP, Pfeifer JD. The Washington manual of surgical pathology. Philadelphia: Lippincott Williams & Wilkins; 2008. The urinary bladder; pp. 304–320. [Google Scholar]
- 4.Cox RM, Schneider AG, Sangoi AR, et al. Invasive urothelial carcinoma with chordoid features: a report of 12 distinct cases characterized by prominent myxoid stroma and chordlike epithelial architecture. Am J Surg Pathol. 2009;33:1213–9. doi: 10.1097/PAS.0b013e3181a8ffbe. [DOI] [PubMed] [Google Scholar]
- 5.Tavaro F, Epstein JI. Urothelial carcinoma with abundant myxoid stroma. Human Pathol. 2009;40:1391–8. doi: 10.1016/j.humpath.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Amin MB, Ro JY, el-Sharkawy T, et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol. 1994;18:1224–32. doi: 10.1097/00000478-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Chetty R, Clarke B. Myxoid invasive papillary urothelial carcinoma of the bladder and urethra. Pathology. 2001;33:515–8. doi: 10.1080/00313020152635829. [DOI] [PubMed] [Google Scholar]
- 8.Torenbeek R, Blomjous CE, de Bruin PC, et al. Sarcomatoid carcinoma of the urinary bladder. Clinicopathologic analysis of 18 cases with immunohistochemical and electron microscopic findings. Am J Surg Pathol. 1994;18:241–9. [PubMed] [Google Scholar]
- 9.Cheng CW, Chan LW, Chan CK, et al. Is surveillance necessary for inverted papilloma in the urinary bladder and urethra? ANZ J Surg. 2005;75:213–7. doi: 10.1111/j.1445-2197.2005.03327.x. [DOI] [PubMed] [Google Scholar]
- 10.Sung MT, Maclennan GT, Lopez-Beltran A, et al. Natural history of urothelial inverted papilloma. Cancer. 2006;107:2622–7. doi: 10.1002/cncr.22311. [DOI] [PubMed] [Google Scholar]
- 11.Yu J, Sun ZX, Kong CZ, Du SQ. [The clinical analysis of 62 cases of the urothelial inverted papilloma] Zhonghua Wai Ke Za Zhi. 2009;47:1400–2. [Article in Chinese] [PubMed] [Google Scholar]
- 12.Aubert J, Dore B, Touchard G. Inverted papilloma of the bladder: A report of 10 cases. Eur Urol. 1984;10:24–7. doi: 10.1159/000463505. [DOI] [PubMed] [Google Scholar]
- 13.Mattelaer J, Leonard A, Goddeeris P, et al. Inverted papilloma of bladder: clinical significance. Urology. 1988;22:192–7. doi: 10.1016/0090-4295(88)90383-4. [DOI] [PubMed] [Google Scholar]
- 14.Cheville JC, Wu K, Sebo TJ, et al. Inverted urothelial papilloma: Is ploidy, MIB-1 proliferative activity, or p53 protein accumulation predictive of urothelial carcinoma? Cancer. 2000;88:632–6. doi: 10.1002/(sici)1097-0142(20000201)88:3<632::aid-cncr21>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 15.Asano K, Miki J, Maeda S, et al. Clinical studies on inverted papilloma of the urinary tract: Report of 48 cases and review of the literature. J Urol. 2003;170:1209–12. doi: 10.1097/01.ju.0000085342.15918.d7. [DOI] [PubMed] [Google Scholar]
- 16.Picozzi S, Casellato S, Bozzini G, et al. Inverted papilloma of the bladder: A review and an analysis of the recent literature of 365 patients. Urol Oncol. 2012:18–18. doi: 10.1016/j.urolonc.2012.03.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Brown AL, Cohen RJ. Inverted papilloma of the urinary tract. BJU Int. 2011;107(suppl3):24–6. doi: 10.1111/j.1464-410X.2011.10046.x. [DOI] [PubMed] [Google Scholar]
- 18.Lott S, Wang M, Zhang S, et al. FGFR3 and TP53 mutation analysis in inverted urothelial papilloma: incidence and etiological considerations. Mod Pathol. 2009;22:627–32. doi: 10.1038/modpathol.2009.28. [DOI] [PubMed] [Google Scholar]
- 19.Hameed O. Myxoid cystitis with chordoid lymphocytes. Another mimic of invasive urothelial carcinoma. Am J Surg Pathol. 2010;34:1061–5. doi: 10.1097/PAS.0b013e3181d68e95. [DOI] [PubMed] [Google Scholar]


