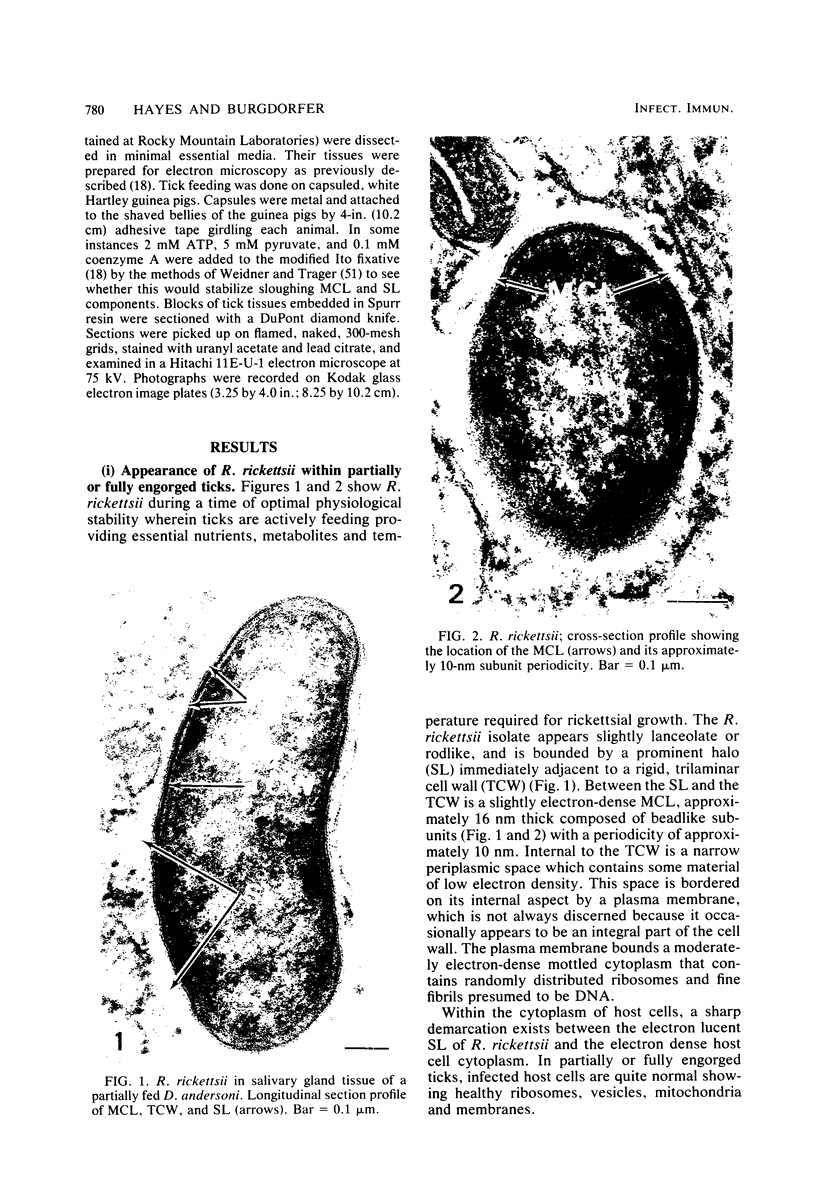
Abstract
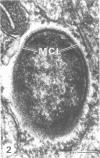
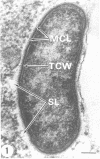
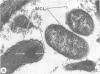
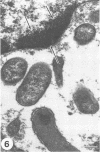
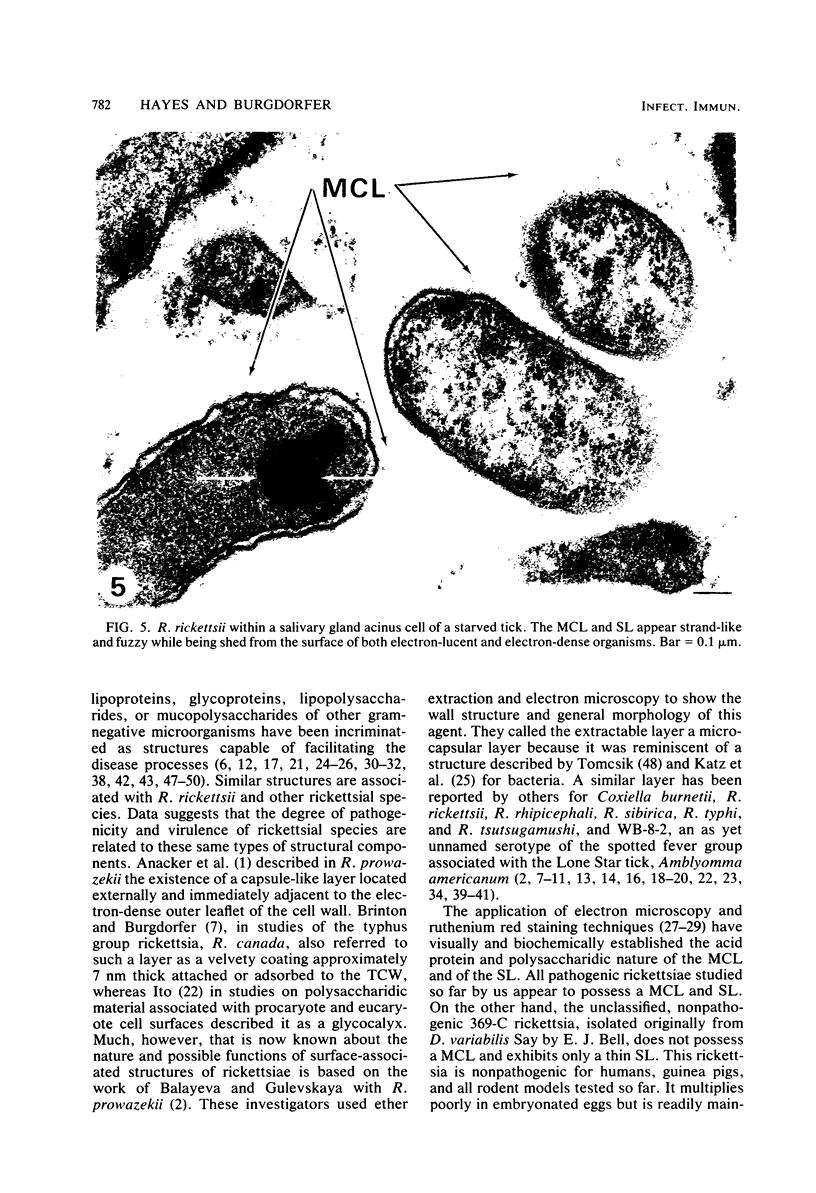
Virulent Rickettsia in Dermacentor andersoni lose their pathogenicity and virulence for guinea pigs when subjected to physiological stresses, such as starvation (overwintering), of its tick vector. However, incubation of infected ticks at an elevated temperature (37 degrees C) for 24 to 48 h or feeding for a time (usually greater than 10 h) induces R. rickettsii to revert to a virulent state, a phenomenon defined as "reactivation." Electron microscopy reveals that the microcapsular and slime layers of R. rickettsii undergo changes dependent upon the physiological conditions within the tick vector. In engorged ticks, the microcapsular layer is readily identified as a discrete layer, approximately 16 nm thick, composed of globular subunits that have a periodicity of approximately 10 nm. The slime layer external to the microcapsular layer forms a discrete electron-lucent zone around the rickettsia. In starved ticks, neither the microcapsular layer nor slime layer remains a discrete entity. Instead, they are shed and form stringy, shredded, and somewhat flocculent strands of low electron density without periodicity. Incubation at 37 degrees C or feeding of starved infected ticks results in the restoration of a discrete microcapsular and slime layer. These reversible structural modifications are linked to physiological changes in the tick host and correlate with reactivation, i.e., restoration of pathogenicity and virulence of R. rickettsii.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Pickens E. G., Lackman D. B. Details of the ultrastructure of Rickettsia prowazekii grown in the chick yolk sac. J Bacteriol. 1967 Jul;94(1):260–262. doi: 10.1128/jb.94.1.260-262.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of the toxicity and hemolytic activity of typhus rickettsiae by starvation. J Bacteriol. 1957 Nov;74(5):637–645. doi: 10.1128/jb.74.5.637-645.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus Rickettsiae. I. Inactivation by freezing. J Gen Physiol. 1954 Nov 20;38(2):169–179. doi: 10.1085/jgp.38.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus rickettsiae at O C. J Bacteriol. 1957 Jan;73(1):56–62. doi: 10.1128/jb.73.1.56-62.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balayeva N. M., Gulevskaya S. A. Comparative characteristics of "ether" and "non-ether" soluble ricketssia prowazekii antigens and electron microscopy findings on the morphology of the rickettsiae during isolation of the antigens. J Hyg Epidemiol Microbiol Immunol. 1972;16(1):92–100. [PubMed] [Google Scholar]
- Bradley S. G. Cellular and molecular mechanisms of action of bacterial endotoxins. Annu Rev Microbiol. 1979;33:67–94. doi: 10.1146/annurev.mi.33.100179.000435. [DOI] [PubMed] [Google Scholar]
- Brinton L. P., Burgdorfer W. Fine structure of Rickettsia canada in tissues of Dermacentor andersoni Stiles. J Bacteriol. 1971 Mar;105(3):1149–1159. doi: 10.1128/jb.105.3.1149-1159.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Aeschlimann A., Peter O., Hayes S. F., Philip R. N. Ixodes ricinus: vector of a hitherto undescribed spotted fever group agent in Switzerland. Acta Trop. 1979 Dec;36(4):357–367. [PubMed] [Google Scholar]
- Burton P. R., Stueckemann J., Paretsky D. Electron microscopy studies of the limiting layers of the rickettsia Coxiella burneti. J Bacteriol. 1975 Apr;122(1):316–324. doi: 10.1128/jb.122.1.316-324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. H., Elberg S. S., Boyles J., Velez M. A. Yersinia pestis: correlation of ultrastructures and immunological status. Infect Immun. 1975 Jun;11(6):1382–1390. doi: 10.1128/iai.11.6.1382-1390.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasch G. A. Isolation of species-specific protein antigens of Rickettsia typhi and Rickettsia prowazekii for immunodiagnosis and immunoprophylaxis. J Clin Microbiol. 1981 Sep;14(3):333–341. doi: 10.1128/jcm.14.3.333-341.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl P. A., Rehacek J., Bazlikova M. The ultrastructure of Rickettsia slovaca in naturally infected females of the tick Dermacentor marginatus. Ann Parasitol Hum Comp. 1980 May-Jun;55(3):259–270. doi: 10.1051/parasite/1980553259. [DOI] [PubMed] [Google Scholar]
- Golinevitch H. M., Voronova Z. A. The superficial protective antigen of R. prowazeki. J Hyg Epidemiol Microbiol Immunol. 1968;12(4):413–419. [PubMed] [Google Scholar]
- Goodwin C. S., Tyrrell D. A., Head B., Rees R. J. Inhibition of haemaggregation by lepromin and other mycobacterial substances. Nature. 1967 Dec 9;216(5119):1019–1020. doi: 10.1038/2161019a0. [DOI] [PubMed] [Google Scholar]
- Hall P. J., Yang G. C., Little R. V., Brubaker R. R. Effect of Ca2+ on morphology and division of Yersinia pestis. Infect Immun. 1974 Jun;9(6):1105–1113. doi: 10.1128/iai.9.6.1105-1113.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S. F., Burgdorfer W., Aeschlimann A. Sexual transmission of spotted fever group rickettsiae by infected male ticks: detection of rickettsiae in immature spermatozoa of Ixodes ricinus. Infect Immun. 1980 Feb;27(2):638–642. doi: 10.1128/iai.27.2.638-642.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S. F., Burgdorfer W. Ultrastructure of Rickettsia rhipicephali, a new member of the spotted fever group rickettsiae in tissues of the host vector Rhipicephalus sanguineus. J Bacteriol. 1979 Jan;137(1):605–613. doi: 10.1128/jb.137.1.605-613.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Brennan P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981 Sep;147(3):728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Vinson J. W., McGuire T. J., Jr Murine typhus Rickettsiae in the Oriental rat flea. Ann N Y Acad Sci. 1975;266:35–60. doi: 10.1111/j.1749-6632.1975.tb35087.x. [DOI] [PubMed] [Google Scholar]
- Kats L. N., Solov'ev N. N., Volkova N. A. Metody tsitologicheskogo issledovaniia bakterial'nykh kapsul. Zh Mikrobiol Epidemiol Immunobiol. 1966 Apr;43(4):95–98. [PubMed] [Google Scholar]
- Kay W. W., Buckley J. T., Ishiguro E. E., Phipps B. M., Monette J. P., Trust T. J. Purification and disposition of a surface protein associated with virulence of Aeromonas salmonicida. J Bacteriol. 1981 Sep;147(3):1077–1084. doi: 10.1128/jb.147.3.1077-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- McCoy E. C., Doyle D., Burda K., Corbeil L. B., Winter A. J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect Immun. 1975 Mar;11(3):517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy E. C., Wiltberger H. A., Winter J. Major outer membrane protein of Campylobacter fetus: physical and immunological characterization. Infect Immun. 1976 Apr;13(4):1258–1265. doi: 10.1128/iai.13.4.1258-1265.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Wu H. C. Proteins of the outer membrane of gram-negative bacteria. Annu Rev Microbiol. 1980;34:369–422. doi: 10.1146/annurev.mi.34.100180.002101. [DOI] [PubMed] [Google Scholar]
- PRICE W. H. The epidemiology of Rocky Mountain spotted fever. I. The characterization of strain virulence of Rickettsia rickettsii. Am J Hyg. 1953 Sep;58(2):248–268. doi: 10.1093/oxfordjournals.aje.a119604. [DOI] [PubMed] [Google Scholar]
- Popov V. L., Ignatovich V. F. Electron microscopy of surface structures of Rickettsia prowazeki stained with ruthenium red. Acta Virol. 1976 Oct;20(5):424–428. [PubMed] [Google Scholar]
- Public Health Weekly Reports for FEBRUARY 23, 1923. Public Health Rep. 1923 Feb 23;38(8):333–381. [PMC free article] [PubMed] [Google Scholar]
- Public Health Weekly Reports for MARCH 12, 1926. Public Health Rep. 1926 Mar 12;41(11):461–503. [PMC free article] [PubMed] [Google Scholar]
- Sharon N. Glycoproteins. Sci Am. 1974 May;230(5):78–86. doi: 10.1038/scientificamerican0574-78. [DOI] [PubMed] [Google Scholar]
- Silverman D. J., Boese J. L., Wisseman C. L., Jr Ultrastructural studies of Rickettsia prowazeki from louse midgut cells to feces: search for "dormant" forms. Infect Immun. 1974 Jul;10(1):257–263. doi: 10.1128/iai.10.1.257-263.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr Comparative ultrastructural study on the cell envelopes of Rickettsia prowazekii, Rickettsia rickettsii, and Rickettsia tsutsugamushi. Infect Immun. 1978 Sep;21(3):1020–1023. doi: 10.1128/iai.21.3.1020-1023.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Wisseman C. L., Jr, Waddell A. D., Jones M. External layers of Rickettsia prowazekii and Rickettsia rickettsii: occurrence of a slime layer. Infect Immun. 1978 Oct;22(1):233–246. doi: 10.1128/iai.22.1.233-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977 Jun;41(2):475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J. Regularly arranged protein on the surfaces of Gram-negative bacteria. Biol Rev Camb Philos Soc. 1977 May;52(2):219–234. doi: 10.1111/j.1469-185x.1977.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Weidner E., Trager W. Adenosine triphosphate in the extracellular survival of an intracellular parasite (Nosema michaelis, Microsporidia). J Cell Biol. 1973 May;57(2):586–591. doi: 10.1083/jcb.57.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]