Abstract
Pathogenesis-related proteins from intercellular fluid washings of stressed barley (Hordeum vulgare L.) leaves were analyzed to determine their binding to various water-insoluble polysaccharides. Three proteins (19, 16, and 15 kD) bound specifically to several water-insoluble β-1,3-glucans. Binding of the barley proteins to pachyman occurred quickly at 22°C at pH 5.0, even in the presence of 0.5 m NaCl, 0.2 m urea, and 1% (v/v) Triton X-100. Bound barley proteins were released by acidic treatments or by boiling in sodium dodecyl sulfate. Acid-released barley proteins could bind again specifically and singly to pachyman. Water-soluble laminarin and carboxymethyl-pachyman competed for the binding of the barley proteins to pachyman. The N-terminal sequence of the 19-kD barley β-1,3-glucan-binding protein showed near identity to the barley seed protein BP-R and high homology to other thaumatin-like (TL) permatins. The 16-kD barley protein was also homologous to TL proteins, whereas the 15-kD barley protein N-terminal sequence was identical to the pathogenesis-related Hv-1 TL protein. Antifungal barley protein BP-R and corn (Zea mays) zeamatin were isolated by binding to pachyman. Two extracellular proteins from stressed pea (Pisum sativum L.) also bound to pachyman and were homologous to TL proteins.
PR proteins are induced by a large spectrum of pests (viroids, viruses, bacteria, fungi, nematodes, and mites) and stimuli (Bol et al., 1990; Lotan and Fluhr, 1990; Linthorst, 1991; Stintzi et al., 1993). Such proteins have been classified into five main affinity groups and are often characterized by their extracellular or vacuolar localization in addition to their acidic or basic nature. Only two groups, PR-2 (β-1,3-glucanases) and PR-3 (chitinases), display specific enzymatic activities. β-1,3-Glucanases (EC 3.2.1.39) hydrolyze laminarin, an oligomeric, water-soluble β-1,3-glucan most commonly used for assaying these enzymes. Chitinases (EC 3.2.1.14) degrade chitin, a β-1,4-N-acetyl-d-glucosamine polymer. Some proteins belonging to PR groups 1, 4, and 5 have been shown to display antifungal potential, despite the lack of identification of precise mechanisms of action or catalytic activities (Niederman et al., 1995). Proteins of the PR-4 group are homologous to the carboxy-terminal domains of hevein and potato Win-1 and Win-2 proteins, which display affinity for chitin (Friedrich et al., 1991). However, PR-4 proteins lack the amino-terminal Cys-rich lectin domain that allows binding to chitin.
PR-5 proteins exhibit sequence homologies to thaumatin, an intensely sweet protein from the arils of ripe Thaumatococcus daniellii fruits (Cornelissen et al., 1986). In the past 10 years several constitutive (seed permatins and fruit proteins) or stress-induced (PR-5 proteins and osmotins) proteins have been shown to have amino acid sequence similarities to thaumatin. Such proteins were identified as TL proteins. Constitutive antifungal seed TL proteins of low molecular mass (usually 19–27 kD) have been identified as permatins, because the proposed mechanism of antifungal action involves plasma membrane permeabilization of the target fungal cell (Roberts and Selitrennikoff, 1990; Vigers et al., 1991, 1992). Osmotins are osmoticum-induced TL proteins that can accumulate in large amounts in vacuoles or cytoplasmic vesicles.
The accumulation of TL proteins in ripening or ripe fruits has also been reported recently (Pressey, 1997; Tattersall et al., 1997). Several isoforms of stress-induced TL PR-5 proteins were detected after abiotic or biotic stress (Stintzi et al., 1991; Woloshuk et al., 1991). The antifungal activity of tobacco (Nicotiana tabacum) TL osmotin, like that of corn (Zea mays) zeamatin, has been recently shown to correlate with plasma membrane permeabilization of sensitive fungi (Abad et al., 1996). Some TL PR-5 proteins exhibit anti-Phytophthora infestans activity (Woloshuk et al., 1991), and transgenic potato expressing a tobacco TL osmotin has been reported to delay symptoms induced by P. infestans (Liu et al., 1994).
We provide evidence for the specific binding of several TL proteins to water-insoluble β-1,3-glucans such as pachyman, curdlan, paramylon, zymosan, alkali-insoluble bakers' yeast (Saccharomyces cerevisiae), and Pleurotus ostreatus glucan. Two well-characterized antifungal TL permatins, corn zeamatin and barley (Hordeum vulgare) BP-R seed protein, were purified by using their binding to pachyman. A similar binding to water-insoluble β-1,3-glucans was also detected with three barley and two pea (Pisum sativum) extracellular stress-related TL proteins from chemically stressed leaves. Moreover, water-soluble β-1,3-glucans such as laminarin were shown to compete for the binding of some TL proteins to pachyman. Thaumatin and some PR-5 TL proteins, such as the acidic tobacco or tomato (Lycopersicon esculentum) PR-5 proteins, did not bind to pachyman. We describe a simple procedure for rapidly enriching and recovering TL proteins able to bind to various water-insoluble β-1,3-glucans, and our findings bring new insight into the putative properties and mechanism(s) of action of some TL proteins able to interact specifically with β-1,3-glucans.
MATERIALS AND METHODS
Chemicals, Fungal Walls, Fungi, and Bacteria
All chemicals for electrophoresis and prestained protein molecular mass markers were from Bio-Rad. Laminarin from Laminaria digitata (Peat et al., 1958), β-glucan from barley (Hordeum vulgare), chitin, chitosan, lichenan, pustulan, and zymosan from bakers' yeast (Saccharomyces cerevisiae; Di Carlo and Fiore, 1957), thaumatin from Thaumatococcus daniellii, β-glucan from Pleurotus ostreatus, and purified Triton X-100 (Audy et al., 1989) were from Sigma-Aldrich. Laminaritriose, laminaritetraose, laminaripentaose, laminarihexaose, and laminariheptaose were from Seikagaku (PDI BioScience, Aurora, ON, Canada). Potato-insoluble starch was from BDH (Poole, England), and PVDF membranes (Immobilon-PSQ) were from Millipore. Sephadex G-75 and phenyl-Sepharose CL-4B were from Pharmacia. Colloidal chitin was synthesized from chitosan (Molano et al., 1977). Lentinan was purified from shiitake mushrooms bought from local markets (Chihara et al., 1970a). Curdlan and paramylon (Kiss et al., 1988) were from Wako Chemicals (Richmond, VA).
Curdlan (degree of polymerization approximately 50) was first suspended in 100 mL of distilled water at 20 mg mL−1 and heated at 60°C for 5 min with continuous mixing. The gel recovered after slow cooling to 22°C was homogenized in a Waring blender for 3 min at maximum speed and kept at 4°C. Paramylon was dissolved in 100 mL of 0.5 n NaOH at a concentration of 1% (w/v), precipitated with 2 volumes of ethanol, and recovered by centrifugation (12,000g, 10 min, 4°C). The pellet was resuspended in 40 mL of distilled water, mixed until complete dissolution, and precipitated again with ethanol. The pellet was resuspended in 30 mL of distilled water, and the pH was adjusted to neutrality by adding 2 n HCl dropwise.
The suspension was brought to 100 mL, homogenized in a Waring blender for 5 min at maximum speed at 22°C, and stored at 4°C. Bakers' yeast insoluble β-glucan was prepared by successive NaOH and acetic acid treatments, as previously described (Cabib and Bowers, 1971; Grenier et al., 1993). Pachyman and carboxymethyl-pachyman (degree of substitution 0.1–0.2) were from Megazyme (Wicklow, Ireland). Smith-degraded pachyman was prepared as described previously (Chihara et al., 1970b). Pachyman and bakers' yeast-alkali-treated walls (Manners et al., 1973) were also digested with commercial Zymolase (Sigma). Fifty milligrams of glucan suspension was incubated twice (for 72 and 24 h at 37°C) in 1 mL of 50 mm sodium acetate buffer, pH 5.0, containing 1500 units of Zymolase. Residual insoluble material was collected by centrifugation at 15,000g for 5 min at 22°C and thoroughly washed with distilled water. Micrococccus luteus (syn: lysodeikticus), Bacillus subtilis, and Escherichia coli lyophilized cells were from Sigma. Verticillium albo-atrum spores were recovered as described previously (Grenier and Asselin, 1990). Phytophthora parasitica pv nicotianae (race 1) alkali-insoluble cell walls were prepared as described above for bakers' yeast.
Induction of PR Proteins and β-1,3-Glucanase Assay
Fully expanded leaves from barley (cv Léger) and pea (Pisum sativum cv Lincoln) were floated on silver nitrate for induction of PR proteins as previously described (Grenier and Asselin, 1990). Hypersensitive tobacco (Nicotiana tabacum L. cv Xanthi nc) leaves infected with tobacco mosaic virus U2 were harvested 6 d after inoculation, and the IFW extracts (in 50 mm sodium phosphate buffer, pH 7.0) were obtained as previously described (Parent and Asselin, 1984). Tomato (Lycopersicon esculentum cv Rutgers) leaves were induced on 1 mm Ser (Grenier and Asselin, 1990). Native PAGE assay for β-1,3-glucanase activity was performed with laminarin as the substrate (Côté et al., 1989).
Binding Assays
For assays involving PAGE analysis, purified proteins or protein extracts were incubated in microfuge tubes in 0.1 mL of 50 mm sodium acetate buffer, pH 5.0, with or without 0.5 m NaCl, 0.2 m urea, and 1% (v/v) Triton X-100, with frequent or continuous vortexing at 22°C for 15 min with 5% (w/v) of various water-insoluble polysaccharides, microbial walls, or cells. Bound proteins were recovered in the insoluble pellet (15,000g, 5 min, 22°C) after the pellet was washed twice with at least the initial volume of sodium acetate buffer with or without NaCl, urea, and Triton X-100. The supernatants containing unbound proteins and the pachyman pellet were boiled in the SDS gel-loading buffer and analyzed in 15% (w/v) denaturing SDS-polyacrylamide gels under nonreducing or reducing conditions. Protein bands were visualized with Coomassie blue R-250 and then stained with aqueous silver nitrate (Grenier et al., 1993).
For protein recovery the binding assays were composed of sonicated and HCl-washed (in 1 n HCl for 1 h at 80°C) pachyman. β-1,3-Glucans (50 mg in 5 mL) were sonicated twice for 1 min each time with a cell disrupter (Virsonic digital 475, Virtis, Gardiner, NY) using the one-eighth-inch microprobe at a power setting of 3. Such treatment gave a homogenous glucan suspension that allowed reproducible results. Proteins were released from pachyman by incubating the washed pellet for 15 min at 22°C in 0.1 mL of 0.1 n HCl or 0.1 m Gly/HCl buffer, pH 2.2. Immediately after centrifugation at 15,000g for 5 min at 22°C, the supernatant containing the released proteins was neutralized with 1 n NaOH.
Purification of Corn (Zea mays) Zeamatin and Barley BP-R Protein
Food-grade cornmeal (10 g) was homogenized in 90 mL of 50 mm sodium phosphate buffer, pH 7.0. After centrifugation at 27,000g for 15 min at 4°C, the extract was subjected to ammonium sulfate fractionation and the pellet obtained at 30% to 60% saturation (90 min on ice) was dissolved in 10 mL of 50 mm sodium acetate buffer, pH 5.0. After sedimentation the supernatant was incubated with pachyman (50 mg) at 22°C for 30 min. The extract was centrifuged at 15,000g for 10 min at 22°C, and the pachyman pellet was washed with 5 mL of sodium acetate buffer and resuspended in 800 μL of the electrophoresis loading buffer for native basic proteins (Reisfeld system). Bound proteins were electrophoretically released in the first sequential native PAGE at pH 4.3 and then denatured (nonreduced) by SDS-PAGE (Trudel and Asselin, 1994). Proteins were transferred to a PVDF membrane and stained as previously described (Trudel and Asselin, 1994). For barley BP-R protein purification, a similar procedure was followed, except that the barley endosperm (6.65 g fresh weight) of 3-d-old seedlings was first ground in 20 mL of sodium phosphate buffer, pH 7.0. Bound proteins were released by boiling for 3 min in the SDS-gel loading electrophoresis buffer and purified by two successive denaturing SDS-PAGE processes (Trudel and Asselin, 1994).
Purification of Leaf Extracellular Barley and Pea Proteins
Proteins in barley or pea IFW extracts were incubated with pachyman at 22°C for 15 min in 50 mm sodium acetate buffer, pH 5.0. Pachyman-bound proteins were recovered by centrifugation at 15,000g for 5 min at 22°C, and the pellet was washed twice by resuspension in 50 mm sodium acetate buffer, pH 5.0. Proteins were released from pachyman by boiling in SDS buffer followed by SDS-PAGE (Trudel and Asselin, 1994). In some cases proteins were desorbed in 100 mm Gly/HCl, pH 2.2, recovered in the supernatant, neutralized with NaOH, and subjected to sequential native PAGE at pH 8.9 (Davis system) or pH 4.3 (Reisfeld system), followed by denaturing SDS-PAGE (Trudel and Asselin, 1994). Protein transfer to PVDF membranes and staining were as for corn zeamatin and barley BP-R protein.
Protein Microsequencing and Search for Homologies
Protein N-terminal sequencing was by automated Edman degradation using a sequencer (model 473A, Applied Biosystems) with electrophoretically purified proteins. Sequence homologies were determined using the National Center for Biotechnology Information Basic Local Alignment Search Tool (BLAST; Altschul et al., 1990).
Competition or Interaction with Water-Soluble β-1,3-Glucans
Water-soluble (either at 22°C or after heating at 100°C) β-1,3-glucans (laminarin, laminaritriose, laminaritetraose, laminaripentaose, laminarihexaose, laminariheptaose, and carboxymethyl-pachyman) and Glc were tested at pH 5.0 for their capacity to compete for the binding of proteins to pachyman. They were added at various concentrations (1–20 mg mL−1) to the binding assay mixture (500 μL) containing sonicated and acid-washed pachyman (2 mg). After the sample was incubated and sedimented, proteins in the pellet were analyzed by SDS-PAGE as described for the binding assays. Competition was performed with barley IFW-binding proteins recovered from the HCl release. In the case of carboxymethyl-pachyman, concentrations of less than 10 mg mL−1 had to be used because of the high viscosity of this polysaccharide. Laminarin (up to 25 mg mL−1) and carboxymethyl-pachyman (up to 10 mg mL−1) were also tested for their capacity to selectively precipitate proteins recovered in pellets (15,000g, 5 min, 22°C) and analyzed by SDS-PAGE.
RESULTS
Three Extracellular PR Proteins from Stressed Barley Leaves Bind to Pachyman
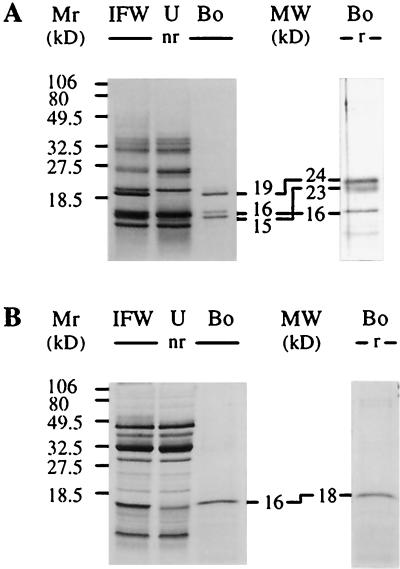
As previously reported (Grenier et al., 1993), several protein bands between 14 and 40 kD could be detected after nonreducing SDS-PAGE analysis of IFW extracts of silver-nitrate-stressed barley leaves (Fig. 1A, lane IFW). These stress (PR) proteins from barley leaf IFW extracts were incubated with suspended pachyman. When the barley IFW extract was analyzed by SDS-PAGE under nonreducing conditions, three protein bands (19, 16, and 15 kD; Fig. 1A, lane Bo [nr]) repeatedly bound to this β-1,3-glucan, whereas several other proteins did not bind to pachyman (Fig. 1A, lane U). Analysis of these binding proteins under reducing conditions showed that the migration of the 16-kD protein was unaffected, whereas the 15- and 19-kD proteins exhibited higher molecular masses (23 and 24 kD, respectively; Fig. 1A, lane Bo [r]). The binding of the three barley proteins was detected even if 0.5 m NaCl, 0.2 m urea, and 1% (v/v) Triton X-100 were included during the binding and washing of the insoluble polysaccharide (not shown).
Figure 1.
Detection of barley and pea IFW proteins binding to pachyman. Chemically stressed barley (A) and pea (B) leaf IFW extracts (lanes IFW) containing 1 mg mL−1 proteins were subjected to SDS-PAGE before and after binding to pachyman. Binding assays in acetate buffer and SDS-PAGE were performed as in Methods. Proteins bound (lanes Bo) and unbound (lanes U) to pachyman were also analyzed after SDS-PAGE. Estimated molecular masses (MW) are given for the bound proteins separated under nonreducing (nr) or reducing (r) conditions. Numbers on the left refer to molecular mass markers (Mr).
Binding Specificity and Occurrence of Other Proteins Binding to Pachyman
Several water-insoluble polysaccharides and microbial cells or walls were tested for their ability to interact with the three barley IFW proteins as analyzed by SDS-PAGE (Fig. 1A). Results are summarized in Table I. Other water-insoluble polysaccharides were able to interact with the same three IFW barley proteins (Table I). Among all of the water-insoluble β-1,3-glucans, only untreated curdlan and lentinan did not interact with the three barley IFW proteins. However, a simple heat treatment converted curdlan into a binding form. The failure to bind to lentinan was probably due to the high degree of substitution of this β-1,3-glucan by Glc residues on C-6 atoms. Unlike the other β-1,3-glucans that were able to bind the three barley proteins, lentinan displayed a regular, dense substitution pattern. On average, lentinan has a C-6-linked Glc residue every 2.5 residues (Saitô et al., 1990).
Table I.
Water-insoluble polysaccharides and microbial walls or cells assayed for specific interaction with the three barley IFW proteins
| Polysaccharides or Microbial Walls/Cells | Treatment | Structures and Linkages | Bindinga |
|---|---|---|---|
| Barley β-glucan | NTb | Polymeric β-1,4 (β-1,3)-glucan | − |
| Cellulose | NT | Polymeric β-1,4-glucan | − |
| Chitin (colloidal or not) | NT | Polymeric β-1,4-acetamido-glucan | − |
| Chitosan | NT | Polymeric β-1,4-amino-glucan | − |
| Curdlan | Heat | Linear polymeric β-1,3-glucan | + |
| NT | Linear polymeric β-1,3-glucan | − | |
| Lentinan | NT | Polymeric β-1,3-glucan with numerous β-1,6-branches | − |
| Lichenan | NT | Polymeric β-1,3 (β-1,4)-glucan | − |
| Pachyman | Smith degradation | Linear polymeric β-1,3-glucan | + |
| Sonication or not | β-1,6 Branched polymeric β-1,3 (β-1,6)-glucan (few branches) | + | |
| Zymolase digestion | Small residue devoid of β-1,3-glucan | − | |
| Paramylon | Alkalization | Linear polymeric β-1,3-glucan | + |
| NT | Linear polymeric β-1,3-glucan | + | |
| Phenyl-Sepharose CL-4B | NT | Sepharose matrix used for hydrophobic interactions (α-linkages) | − |
| P. ostreatus β-glucan | NT | β-1,6 Branched polymeric β-1,3-glucan | + |
| Pustulan | NT | Polymeric β-1,6-glucan | − |
| Sephadex G-75 | NT | Cross-linked polymeric α-1,6-glucan | − |
| Starch | NT | Polymeric α-1,4-glucan | − |
| Yeast glucan (alkali-insoluble) | NT | β-1,6 branched polymeric β-1,3 (β-1,6)-glucan | + |
| Zymosan | NT | β-1,6 branched polymeric β-1,3 (β-1,6)-glucan | + |
| B. subtilis | Lyophilize cells | Gram (+) wall with peptidoglycan (β-1,4) | − |
| E. coli | Gram (−) wall with an outer membrane | − | |
| M. luteus | Gram (+) wall with peptidoglycan (β-1,4) polymer | − | |
| S. cerevisiae (bakers' yeast) | Wall has mannan, mannoproteins, β-glucans, and chitin | − | |
| Zymolase digestion | Residue devoid of β-1,3-glucans | − | |
| P. parasitica (walls) | Alkali and acid | Wall with β-1,3-glucans | + |
| V. albo-atrum (spores) | NT | Wall has β-1,3-glucans, proteins, and chitin | − |
+, Binding; −, no binding.
NT, No treatment.
Several water-insoluble polysaccharides, different in structure and linkages from the water-insoluble β-1,3-glucans, did not interact with the three barley IFW proteins (Table I). Barley β-glucan, cellulose, chitin, colloidal chitin, chitosan, lichenan, phenyl-Sepharose CL-4B, pustulan, Sephadex G-75, and insoluble potato starch did not interact with the three barley proteins. No binding occurred with intact B. subtilis, E. coli, M. luteus, and S. cerevisiae cells or V. albo-atrum spores either (Table I). As additional controls, pachyman and S. cerevisiae walls were digested extensively with Zymolase, a bacterial extract containing primarily a β-1,3-glucanase activity. In both cases an insoluble, undigested residue (approximately 5% of the initial weight for pachyman and approximately 20% for bakers' yeast) was tested for binding of barley proteins. No binding occurred in either case, indicating that β-1,3-glucans removed with the Zymolase treatment were responsible for the binding of the barley proteins (Table I). Overall, these results demonstrate the specific binding between water-insoluble slightly branched or unbranched β-1,3-glucans and a set of barley IFW proteins. In addition to the one from barley, a pea IFW extract containing several proteins (Fig. 1B, lane IFW) yielded one protein band (16 kD after SDS-PAGE under nonreducing conditions) that bound specifically to pachyman (Fig. 1B, compare lane IFW with lane U and lanes Bo).
Interactions of Barley IFW Proteins with Water-Soluble β-1,3-Glucans
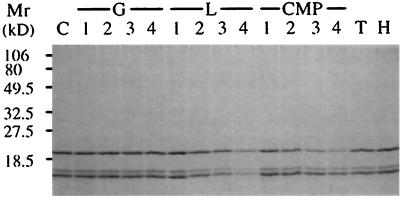
Glc, laminarin, and carboxymethyl-pachyman were assayed at pH 5.0 for their capacity to compete for the binding of the three barley IFW proteins to sonicated and HCl-treated pachyman. Glc (Fig. 2, lanes G) did not compete for the binding of the three proteins to pachyman. Laminarin (Fig. 2, lanes L) and carboxymethyl-pachyman (Fig. 2, lanes CMP) partly inhibited the binding of the barley proteins. The competing effect of laminarin was nearly equal for the three barley proteins. It is interesting to observe that carboxymethyl-pachyman was less effective than laminarin in decreasing the binding of the 16- and 15-kD barley proteins. However, it competed for the binding of the 19-kD barley protein more efficiently than laminarin at similar concentrations (Fig. 2).
Figure 2.
Binding of the three barley IFW proteins to pachyman in the presence of soluble β-1,3-glucans. Sonicated and HCl-washed pachyman (2 mg) was incubated for 15 min at 22°C in 0.5 mL of 50 mm sodium acetate buffer, pH 5.0, with the three barley IFW proteins obtained after HCl elution of IFW proteins previously bound to pachyman. The binding was tested in the presence of Glc (G), laminarin (L), carboxymethyl-pachyman (CMP), laminaritetraose (lane T), and laminarihexaose (lane H) and in the absence of a competitor as a control (lane C). After incubation the insoluble pachyman was sedimented by centrifugation at 15,000g for 5 min at 22°C and washed twice, and proteins in the pellet were analyzed by SDS-PAGE under nonreducing conditions as in Figure 1. Lanes 1, 2, 3, and 4 marked “G,” 0.5, 2.5, 5 and 10 mg of Glc, respectively; lanes 1, 2, 3, and 4 marked “L,” 0.5, 2.5, 5, and 10 mg of laminarin, respectively; lanes 1, 2, 3, and 4 marked “CMP,” 0.5, 1, 2, and 4 mg of carboxymethyl-pachyman, respectively. Ten milligrams of laminaritetraose and laminarihexaose were used. Numbers on the left refer to molecular mass markers (Mr).
Finally, laminaritetraose and laminarihexaose did not compete at 20 mg mL−1 for the binding of the barley proteins (Fig. 2, lanes T and H). The same was true for laminaritriose, laminaripentaose, and laminariheptaose (not shown). The capacity of laminarin to compete for the specific interaction between the barley proteins and pachyman suggests that the binding does not necessarily require single- or triple-helix-structured β-1,3-glucans, since laminarin is a random-coil oligomeric β-1,3-glucan. Overall, these results indicate that the barley proteins interact with some soluble oligomeric or insoluble polymeric unbranched or slightly branched β-1,3-glucans.
N-Terminal Microsequencing of the Three Purified Barley Proteins Shows Homology to TL Proteins
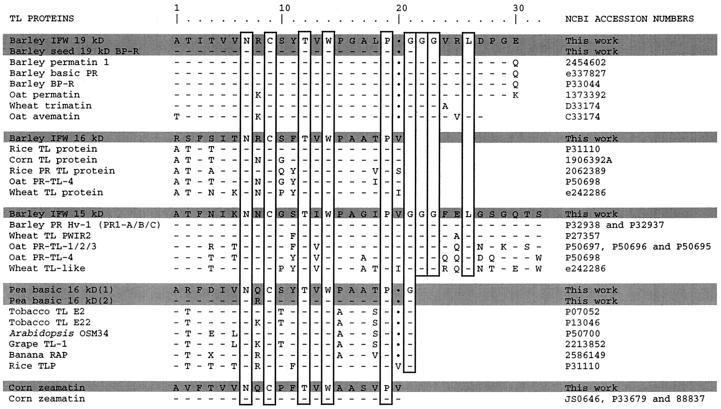
The three barley IFW proteins were first bound to pachyman, released with 0.1 n HCl, and purified electrophoretically. The electrophoretic mobility indicated that the 16- and 19-kD proteins were basic, whereas the 15-kD protein was acidic. The identification of the barley IFW proteins binding to β-1,3-glucans was determined by microsequencing of the N termini of the electrophoretically purified proteins. Unexpectedly, the three proteins showed very high similarity to several TL proteins. The 19-kD basic protein N-terminal sequence showed near identity (29 of 30 residues) to a barley grain antifungal TL permatin (protein BP-R; Hejgaard et al., 1991) and high similarity to several other cereal permatins from oat or wheat (Fig. 3, 19-kD barley IFW).
Figure 3.
Comparison of N-terminal amino acid sequences of purified pachyman-binding proteins with several TL proteins. The seven proteins microsequenced in the present study are shaded and “This work” is indicated in the far-right column. The vertical rectangles indicate amino acids identical in all sequences. Dots at residue 20 correspond to one amino acid gap. Accession numbers on the right are from the National Center for Biotechnology Information (see Methods).
The N-terminal sequence of the 16-kD basic protein was also homologous to several TL proteins from rice, corn, oat, and wheat (Fig. 3, 16-kD barley IFW). The 15-kD acidic protein N terminus was identical to stress-related barley PRHv-1 protein (also identified as PR1 A/B/C) and highly similar to several other low-molecular-mass (approximately 15 kD) TL stress proteins from wheat or oat (Fig. 3, 15-kD barley IFW). Overall, the microsequence results clearly indicate that the three β-1,3-glucan-binding proteins belong to the family of TL proteins. This large family of TL proteins is made up of diverse constitutive and stress-induced proteins: thaumatin, seed permatins, stress-related PR-5 proteins, osmotins, and fruit- and flower-associated TL proteins. Therefore, we also investigated other well-known TL proteins for their specific binding to pachyman.
Some Other TL Proteins Bind to Insoluble β-1,3-Glucans
Commercial thaumatin, tobacco, and tomato PR-5 TL proteins did not bind to pachyman (not shown). Thaumatin bound electrostatically to insoluble lichenan and was completely desorbed and recovered from insoluble lichenan with 3 m NaCl (not shown). No tobacco or tomato PR protein bound to polysaccharides, except for an approximately 20-kD protein that bound only to chitin (not shown). No binding occurred with several fruit extracts reported to contain significant amounts of constitutive fruit TL proteins (Tattersall et al., 1997; not shown). However, some TL proteins did bind to pachyman. This was the case for corn seed zeamatin, the best characterized antifungal TL permatin. Only one cornmeal protein bound selectively to pachyman. We purified this protein and identified it by microsequencing its N terminus. The N-terminal sequence of the cornmeal-purified protein was identical to corn zeamatin (Fig. 3). Zeamatin bound to pachyman in its purified form as well as in the cornmeal or seed extract. This was also the case for the three TL barley leaf proteins (not shown). Barley seeds are known to contain two antifungal TL proteins, BP-R and BP-S (Hejgaard et al., 1991). These two basic proteins of approximately 19 kD behave like corn zeamatin with regard to changes in estimated molecular mass (Hejgaard et al., 1991). Barley seed protein BP-R was purified electrophoretically (Fig. 3, 19-kD barley seed BP-R) and found to bind to pachyman in its purified form or in the crude extract (not shown). Protein BP-R was chosen for purification because it was much more abundant than BP-S in our seed extract (not shown).
Since all previous results were restricted to proteins from monocotyledonous species binding specifically to β-1,3-glucans, some proteins from dicotyledonous species were also tested for their capacity to bind to insoluble β-1,3-glucans. A pea leaf IFW extract was previously shown to bind to pachyman (Fig. 1B). Further investigation by two-dimensional PAGE analysis of the pea leaf IFW extract showed two distinct proteins binding to pachyman. Among the various pea IFW proteins (Fig. 4A), only two proteins bound specifically to pachyman (Fig. 4B). These two proteins migrated after SDS-PAGE as one protein band of 16 kD after SDS-PAGE under nonreducing conditions or 18 kD under reducing conditions (Fig. 1B). These two pea 16-kD proteins were purified electrophoretically and their N termini were microsequenced. Both proteins showed similarity to several other TL proteins from dicotyledonous species (tobacco, Arabidopsis, and grape) and even monocotyledonous species (banana and rice; Fig. 3). Proteins belonging to the TL family of proteins and binding to β-1,3-glucans are thus not restricted to monocotyledonous species.
Figure 4.
Detection of pea IFW proteins binding to pachyman after two-dimensional gel electrophoresis. Chemically stressed pea leaf IFW extract (A) and two proteins binding to pachyman (B) were subjected to two-dimensional PAGE. Binding to pachyman was as in Figure 1. The first-dimension separation (native 1st D) involved PAGE at pH 4.3 under native conditions (Reisfeld system) and was followed by a second-dimension PAGE (from top to bottom) in a denaturing (SDS) 15% polyacrylamide gel under nonreducing conditions. Proteins were stained as in Figure 1. Numbers on the left refer to molecular mass markers (Mr) and arrowheads 1 and 2 indicate the presence of the two TL proteins binding to pachyman (B) in the IFW extract (A).
No enzymatic activity has been reported for TL proteins, except for the membrane permeabilization property of some TL permatins on fungal target cells (Roberts and Selitrennikoff, 1990). Theoretically, β-1,3-glucanases could bind to insoluble β-1,3-glucans. Under the experimental conditions used in this study, β-1,3-glucanases (laminarinases) were not recovered with the insoluble β-1,3-glucan pellet. Moreover, no glucanase activity of TL proteins was detected after a native PAGE laminarinase assay (not shown).
DISCUSSION AND CONCLUSION
Overall, the present results demonstrate that seven purified constitutive or stress-induced TL proteins can bind specifically to several β-1,3-glucans. Unexpectedly, all binding proteins were shown to belong to the large family of TL proteins (Fig. 3). Proteins belonging to the large family of TL proteins are diverse. The present results clearly indicate that not all TL proteins bind specifically to pachyman. Fruit TL proteins and tobacco or tomato PR-5 stress proteins could not bind to water-insoluble β-1,3-glucans. The same was true for thaumatin. However, two well-known constitutive antifungal seed TL permatins, corn zeamatin and barley BP-R protein, bound to insoluble β-1,3-glucans in their purified form or in crude extracts. This was also the case for five extracellular acidic or basic stress-induced PR-5-like proteins homologous to TL proteins at their N termini. It will be interesting to determine whether osmotins (Singh et al., 1987; Sato et al., 1995; Yun et al., 1997) and flower-associated TL proteins (Kubogawa et al., 1997) are also able to bind to β-1,3-glucans.
The binding of some TL proteins to fungal wall β-1,3-glucans might help in explaining some previous observations about the antifungal mechanism(s) of action of TL proteins. It was reported that tobacco TL osmotin was active on cells only under hypotonic conditions (Abad et al., 1996), suggesting that cell walls could be required for or improve osmotin action. Other reports also suggest that fungal wall properties might affect TL zeamatin activity (Roberts and Selitrennikoff, 1990). The binding of some antifungal TL proteins to β-1,3-glucans at specific sites of fungal cell walls could be one prerequisite to the subsequent membrane alteration. On the other hand, binding to fungal β-1,3-glucans could also interfere with or compete with plasma membrane alteration.
Tentative results could be possibly interpreted by using a two-step mechanism of action of some antifungal TL proteins. In a first step, some TL proteins could bind to fungal wall β-1,3-glucans. A precise structure and configuration of the fungal wall β-1,3-glucans could determine the extent of binding. The second step of the mechanism of action could be membrane permeabilization (Roberts and Selitrennikoff, 1990; Abad et al., 1996), which usually requires direct insertion of the protein into fungal membranes to form transmembrane pores (Roberts and Selitrennikoff, 1990). Contrary to binding to β-1,3-glucans, membrane permeabilization seems to be sensitive to high salt concentrations. In the case of fungi resistant to TL action, the initial binding to cell wall β-1,3-glucans could be physically impeded by the presence of cell wall-associated proteins or polysaccharides. With these fungi, cell wall-degrading enzymes could be required to allow binding of TL proteins to β-1,3-glucans. The absence of wall β-1,3-glucans, as in the Mucorales, could explain at least in part the resistance of some fungi to the action of TL proteins.
There is one example of an antifungal protein reported to work in a two-step mechanism involving cell wall binding and membrane perturbation. The yeast K1 killer toxin, a heterodimeric, secreted protein, has distinct domains for binding to wall β-1,6-glucans and membrane perturbation of the target fungal cell (Hutchins and Bussey, 1983; Bussey, 1991). Binding of some TL proteins to insoluble fungal wall β-1,3-glucans could help in targeting or increasing the antifungal activity of these proteins. However, binding to β-1,3-glucans could also compete or decrease the membrane alteration by TL proteins. More work is needed to determine the relative importance of both activities for TL proteins displaying such properties. The detailed structural/conformational features of β-1,3-glucans required for their affinity with the TL β-1,3-glucan-binding proteins are still to be determined. Further studies are also necessary to determine the extent and biological significance of diverse TL proteins interacting with endogenous (such as callose) and exogenous (such as fungal wall polysaccharides) β-1,3-glucans.
Abbreviations:
- IFW
intercellular fluid washings
- PR
pathogenesis-related
- TL
thaumatin-like
Footnotes
This work was supported by a grant from the Natural Sciences and Engineering Council of Canada and the Conseil des Recherches en Pêche et Agro-Alimentaire du Québec to A.A.
LITERATURE CITED
- Abad LR, D'Urzo MP, Lin D, Narasimhan ML, Renveni M, Zhu JK, Niu X, Singh NK, Hasegawa PM, Bressan RA. Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci. 1996;118:11–23. [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local aligment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Audy P, Grenier J, Asselin A. Lysozyme activity in animal extracts after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Comp Biochem Physiol. 1989;92B:523–527. doi: 10.1016/0305-0491(89)90126-0. [DOI] [PubMed] [Google Scholar]
- Bol JF, Linthorst HJM, Cornelissen BJC. Plant pathogenesis-related proteins induced by virus infection. Annu Rev Phytopathol. 1990;28:113–138. [Google Scholar]
- Bussey H. K1 killer toxin, a pore-forming protein from yeast. Mol Microbiol. 1991;5:2339–2343. doi: 10.1111/j.1365-2958.1991.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Cabib E, Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971;246:152–159. [PubMed] [Google Scholar]
- Chihara G, Hamuro J, Maeda Y, Arai Y, Fukuoka F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom) Cancer Res. 1970a;30:2776–2781. [PubMed] [Google Scholar]
- Chihara G, Hamuro J, Maeda Y, Arai Y, Fukuoka F. Antitumour polysaccharide derived chemically from natural glucan (pachyman) Nature. 1970b;225:943–944. doi: 10.1038/225943a0. [DOI] [PubMed] [Google Scholar]
- Cornelissen BJC, Hooft Van Huijsduijnen RAM, Bol JF. A tobacco mosaic virus-induced tobacco protein is homologous to the sweet-tasting protein thaumatin. Nature. 1986;231:531–532. doi: 10.1038/321531a0. [DOI] [PubMed] [Google Scholar]
- Côté F, Letarte J, Grenier J, Trudel J, Asselin A. Detection of β-1,3-glucanase activity after native polyacrylamide gel electrophoresis: application to tobacco pathogenesis-related proteins. Electrophoresis. 1989;10:527–529. doi: 10.1002/elps.1150100714. [DOI] [PubMed] [Google Scholar]
- Di Carlo FJ, Fiore JV. On the composition of zymosan. Science. 1957;127:756–757. doi: 10.1126/science.127.3301.756-a. [DOI] [PubMed] [Google Scholar]
- Friedrich L, Moyer M, Ward E, Ryals J. Pathogenesis-related protein 4 is structurally homologous to the carboxy-terminal domains of hevein, Win-1 and Win-2. Mol Gen Genet. 1991;230:113–119. doi: 10.1007/BF00290658. [DOI] [PubMed] [Google Scholar]
- Grenier J, Asselin A. Some pathogenesis-related proteins are chitosanases with lytic activity against fungal spores. Mol Plant-Microbe Interact. 1990;3:401–407. [Google Scholar]
- Grenier J, Potvin C, Asselin A. Barley pathogenesis-related proteins with fungal cell wall lytic activity inhibit the growth of yeasts. Plant Physiol. 1993;103:1277–1283. doi: 10.1104/pp.103.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejgaard J, Jacobsen S, Svendsen I. Two antifungal thaumatin-like proteins from barley grain. Fed Eur Biochem Soc. 1991;291:127–131. doi: 10.1016/0014-5793(91)81119-s. [DOI] [PubMed] [Google Scholar]
- Hutchins K, Bussey H. Cell wall receptor for yeast killer toxin: involvement of (1→6)-β-d-glucan. J Bacteriol. 1983;154:161–169. doi: 10.1128/jb.154.1.161-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Roberts EM, Brown RM, Jr, Treimer RE. X-ray and dissolution studies of paramylon storage granules from Euglena. Protoplasma. 1988;146:150–156. [Google Scholar]
- Kubogawa T, Yoshida KT, Takeda G. An acidic 39 kDa protein secreted from stigmas of tobacco has an amino-terminal motif that is conserved among thaumatin-like proteins. Plant Cell Physiol. 1997;38:91–95. [Google Scholar]
- Linthorst HJM. Pathogenesis-related proteins of plants. Crit Rev Plant Sci. 1991;10:123–150. [Google Scholar]
- Liu D, Raghothama KG, Hasegawa PM, Bressan RA. Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci USA. 1994;91:1888–1892. doi: 10.1073/pnas.91.5.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan T, Fluhr R. Function and regulated accumulation of plant pathogenesis-related proteins. Symbiosis. 1990;8:33–46. [Google Scholar]
- Manners DJ, Masson AJ, Patterson JC. The structure of a β-(1–3)-d-glucan from yeast cell walls. Biochem J. 1973;135:19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molano J, Duran A, Cabib E. A rapid and sensitive assay for chitinase using tritiated chitin. Anal Biochem. 1977;83:648–656. doi: 10.1016/0003-2697(77)90069-0. [DOI] [PubMed] [Google Scholar]
- Niederman T, Genete Y, Bruyère T, Gees R, Stinzi A, Legrand M, Fritig B, Mösinger E. Pathogenesis-related PR-1 proteins are antifungal. Plant Physiol. 1995;108:17–27. doi: 10.1104/pp.108.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JG, Asselin A. Detection of pathogenesis-related proteins (PR or b) and other proteins in the intercellular fluid of hypersensitive tobacco plants infected with tobacco mosaic virus. Can J Bot. 1984;62:546–549. [Google Scholar]
- Peat S, Whelan WJ, Lawley HG (1958) The structure of laminarin. Part II. The minor structural features. J Chem Soc 729–737
- Pressey R. Two isoforms of NP24: a thaumatin-like protein in tomato fruit. Phytochemistry. 1997;44:1241–1245. doi: 10.1016/s0031-9422(96)00667-x. [DOI] [PubMed] [Google Scholar]
- Roberts WK, Selitrennikoff CP. Zeamatin, an antifungal protein from maize with membrane-permeabilizing activity. J Gen Microbiol. 1990;136:1771–1778. [Google Scholar]
- Saitô H, Yoshioka Y, Yokoi M, Yamada J. Distinct gelation mechanism between linear and branched (1–3)-β-d-glucans as revealed by high-resolution solid-state 13C NMR. Biopolymers. 1990;29:1689–1698. doi: 10.1002/bip.360291402. [DOI] [PubMed] [Google Scholar]
- Sato F, Koiwa H, Sakai Y, Kato N, Yamada Y. Synthesis and secretion of tobacco neutral PR-5 protein by transgenic tobacco and yeast. Biochem Biophys Res Commun. 1995;211:909–913. doi: 10.1006/bbrc.1995.1898. [DOI] [PubMed] [Google Scholar]
- Singh N, Bracker CA, Hasegawa PM, Handa AK, Buckel S, Hermodson MA, Pfankoch E, Regnier FE, Bressan RA. Characterization of osmotin. Plant Physiol. 1987;85:529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stintzi A, Heitz T, Kauffman S, Legrand M, Fritig B. Identification of a basic pathogenesis-related, thaumatin-like protein of virus-infected tobacco, as osmotin. Physiol Mol Plant Pathol. 1991;38:137–146. [Google Scholar]
- Stintzi A, Heitz T, Prasad V, Wiedemann-Merdinoglu S, Kauffmann S, Geoffroy P, Legrand M, Fritig B. Plant ‘pathogenesis-related’ proteins and their role in defense against pathogens. Biochimie. 1993;75:687–706. doi: 10.1016/0300-9084(93)90100-7. [DOI] [PubMed] [Google Scholar]
- Tattersall BD, van Heeswijck R, Bordier Hoj P. Identification and characterization of a fruit-specific, thaumatin-like protein that accumulates at very high levels in conjunction with the onset of sugar accumulation and berry softening in grapes. Plant Physiol. 1997;114:759–769. doi: 10.1104/pp.114.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel J, Asselin A. Protein purification for microsequencing by sequential native and denaturing polyacrylamide gel electrophoresis: application to one chitinase. Anal Biochem. 1994;221:214–216. doi: 10.1006/abio.1994.1404. [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Roberts WK, Selitrennikoff CP. A new family of plant antifungal proteins. Mol Plant-Microbe Interact. 1991;4:315–323. doi: 10.1094/mpmi-4-315. [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Wiedemann S, Roberts WK, Legrand M, Selitrennikoff CP, Fritig B. Thaumatin-like pathogenesis-related proteins are antifungal. Plant Sci. 1992;83:155–161. [Google Scholar]
- Woloshuk CP, Meulenhoff JS, Sela-Buurlage M, van den Elzen PJM, Cornelissen BJC. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell. 1991;3:619–628. doi: 10.1105/tpc.3.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun DJ, Zhao Y, Pardo JM, Narasimhan ML, Damsz B, Lee H, Abad LR, D'Urzo MP, Hasegawa PM, Bressan RA. Stress proteins on the yeast cell surface determine resistance to osmotin, a plant antifungal protein. Proc Natl Acad Sci USA. 1997;94:7082–7087. doi: 10.1073/pnas.94.13.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]