Abstract
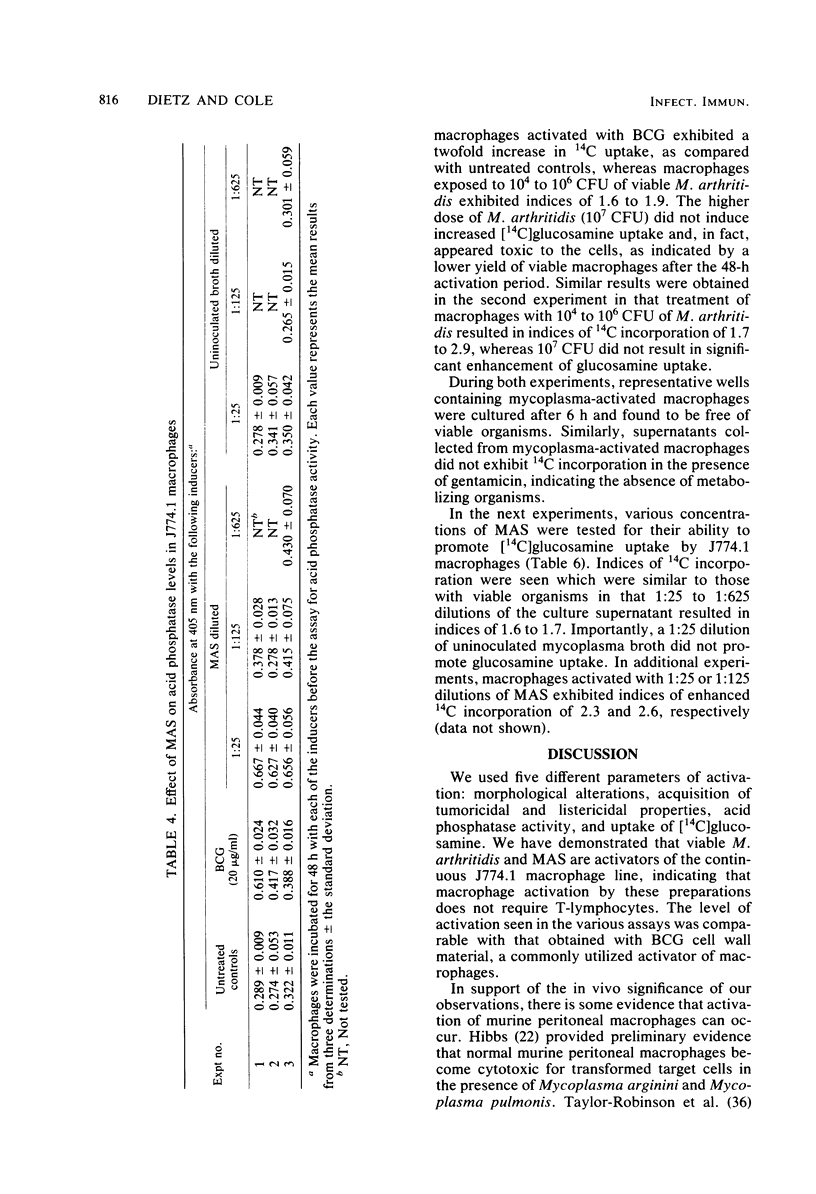
Viable Mycoplasma arthritidis and supernatants from M. arthriditis cultures produced marked morphological changes in the J774.1 continuous macrophage line similar to those seen during activation of these cells by Mycobacterium bovis BCG cell walls. The mycoplasma-treated macrophages developed pronounced tumoricidal activity against syngenic 3T12-3 target cells and developed an enhanced capacity for the killing of intracellular listeriae, including both virulent and laboratory-maintained strains. Increased acid phosphatase levels and [14C]glucosamine uptake were also seen. We conclude that mycoplasmas can profoundly alter the functions of macrophages, an event which may not only have in vivo significance with regard to disease pathogenesis, but which may pose considerable problems for in vitro work when unsuspected mycoplasma contamination is present.
Full text
PDF
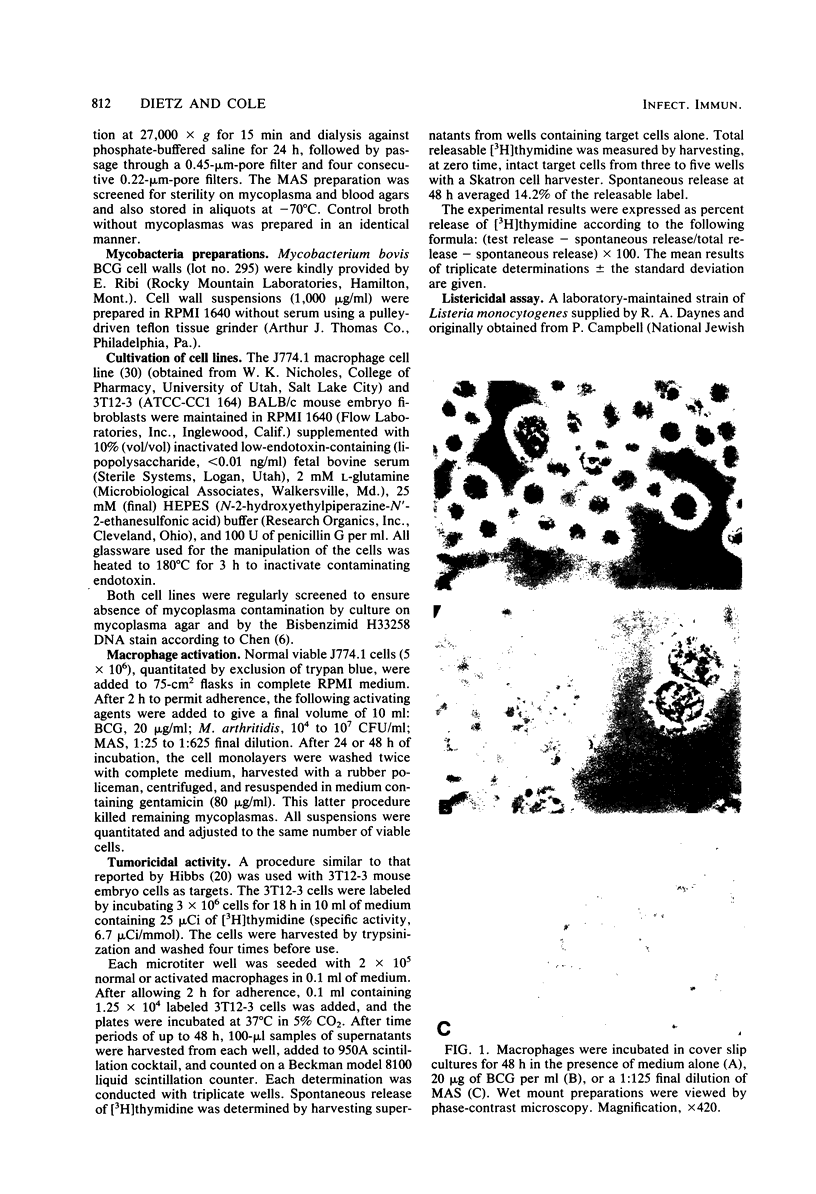

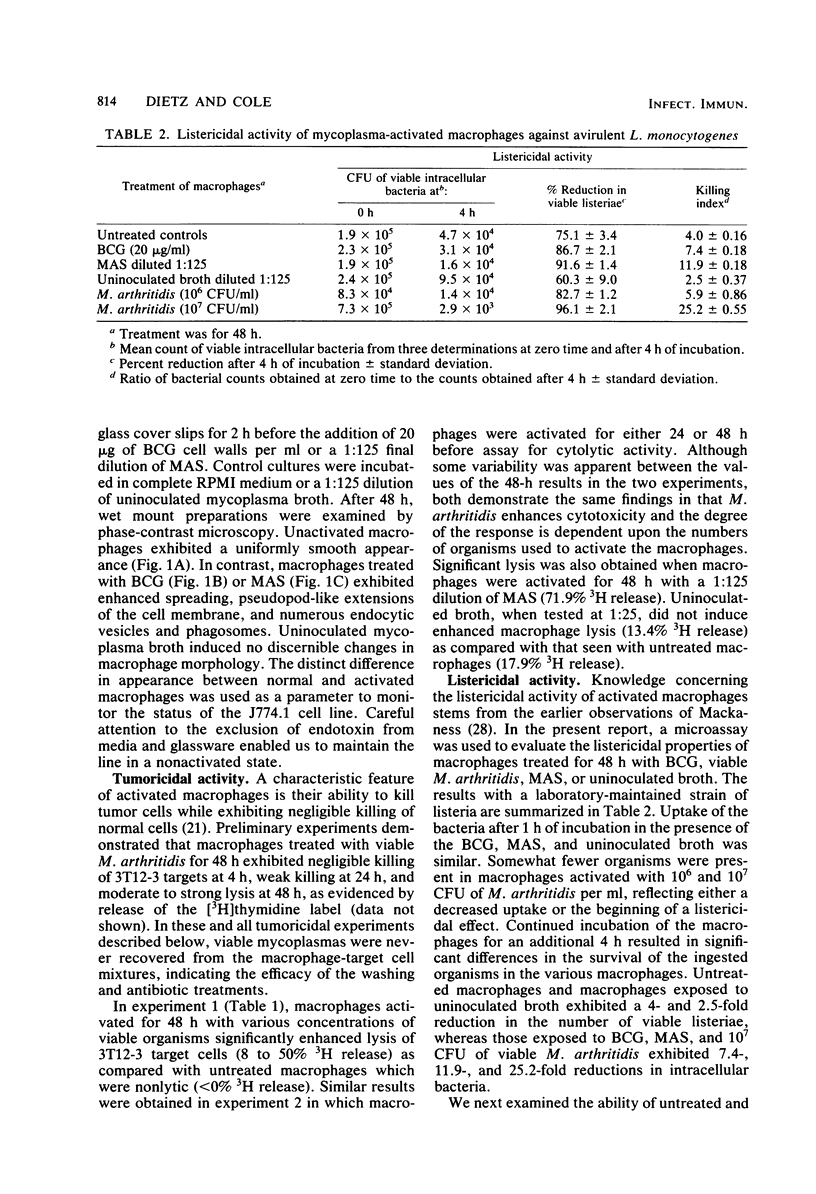
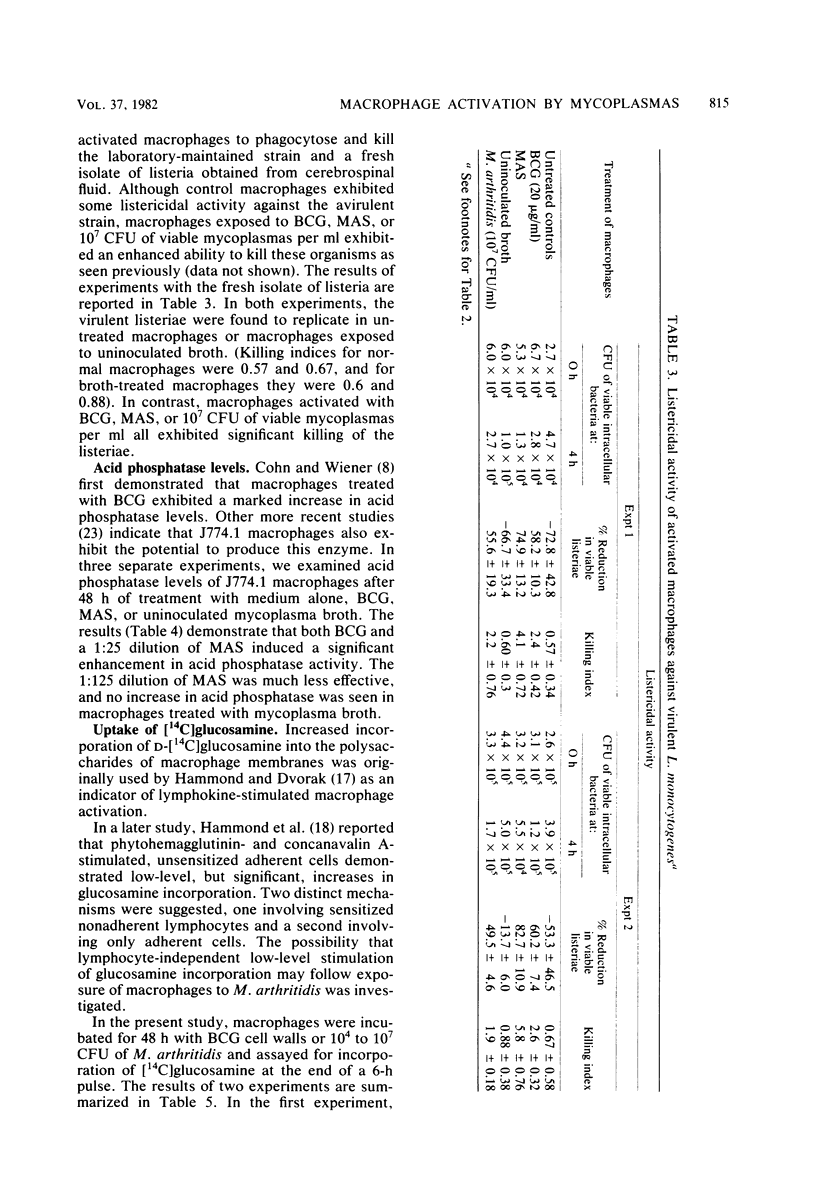



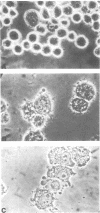
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Cole B. C., Ward J. R. Mycoplasma-dependent activation of normal lymphocytes: induction of a lymphocyte-mediated cytotoxicity for allogeneic and syngeneic mouse target cells. Infect Immun. 1977 Nov;18(2):377–385. doi: 10.1128/iai.18.2.377-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., Leventhal B. G. Possible mechanism for Mycoplasma inhibition of lymphocyte transformation induced by phytohaemagglutinin. Nature. 1968 Aug 17;219(5155):750–752. doi: 10.1038/219751a0. [DOI] [PubMed] [Google Scholar]
- Beck J., Engler H., Brunner H., Kirchner H. Interferon production in cocultures between mouse spleen cells and tumor cells: possible role of mycoplasmas in interferon induction. J Immunol Methods. 1980;38(1-2):63–73. doi: 10.1016/0022-1759(80)90331-2. [DOI] [PubMed] [Google Scholar]
- Birke C., Peter H. H., Langenberg U., Müller-Hermes W. J., Peters J. H., Heitmann J., Leibold W., Dallügge H., Krapf E., Kirchner H. Mycoplasma contamination in human tumor cell lines: effect on interferon induction and susceptibility to natural killing. J Immunol. 1981 Jul;127(1):94–98. [PubMed] [Google Scholar]
- Brooks C. G., Rees R. C., Leach R. H. High nonspecific reactivity of normal lymphocytes against mycoplasma-infected target cells in cytotoxicity assays. Eur J Immunol. 1979 Feb;9(2):159–165. doi: 10.1002/eji.1830090213. [DOI] [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Aldridge K. E., Sullivan G. J., Ward J. R. Mycoplasma-dependent activation of normal mouse lymphocytes: requirement for functional T lymphocytes in the cytotoxicity reaction mediated by Mycoplasma arthritidis. Infect Immun. 1980 Oct;30(1):90–98. doi: 10.1128/iai.30.1.90-98.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Aldridge K. E., Ward J. R. Mycoplasma-dependent activation of normal lymphocytes: mitogenic potential of mycoplasmas for mouse lymphocytes. Infect Immun. 1977 Nov;18(2):393–399. doi: 10.1128/iai.18.2.393-399.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Mycoplasma-mediated activation of normal mouse lymphocytes: induction of cytotoxic lymphocytes and lymphocyte transformation by Mycoplasma arthritidis are under Ir gene control. J Immunol. 1981 Mar;126(3):922–927. [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. I. Transformation is associated with an H-2-linked gene that maps to the I-E/I-C subregion. J Immunol. 1981 Nov;127(5):1931–1936. [PubMed] [Google Scholar]
- Cole B. C., Sullivan G. J., Daynes R. A., Sayed I. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. II. Cellular requirements for T cell transformation mediated by a soluble Mycoplasma mitogen. J Immunol. 1982 May;128(5):2013–2018. [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Interaction of Mycoplasma arthritidis and other mycoplasmas with murine peritoneal macrophages. Infect Immun. 1973 May;7(5):691–699. doi: 10.1128/iai.7.5.691-699.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond M. E., Dvorak H. F. Antigen-induced stimulation of glucosamine incorporation by guinea pig peritoneal macrophages in delayed hypersensitivity. J Exp Med. 1972 Dec 1;136(6):1518–1532. doi: 10.1084/jem.136.6.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond M. E., Selvaggio S. S., Dvorak H. F. Antigen-enhanced glucosamine incorporation by peritoneal macrophages in cell-mediated hypersensitivity. I. Studies on biology and mechanism. J Immunol. 1975 Oct;115(4):914–921. [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Control of carcinogenesis: a possible role for the activated macrophage. Science. 1972 Sep 15;177(4053):998–1000. doi: 10.1126/science.177.4053.998. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr Macrophage nonimmunologic recognition: target cell factors related to contact inhibition. Science. 1973 May 25;180(4088):868–870. doi: 10.1126/science.180.4088.868. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Mørland B. Properties of a murine monocytic tumor cell line J-774 in vitro. I. Morphology and endocytosis. Exp Cell Res. 1978 Aug;115(1):53–61. doi: 10.1016/0014-4827(78)90401-9. [DOI] [PubMed] [Google Scholar]
- Kelly M. T. Activation of guinea pig macrophages by cell walls of Mycobacterium bovis, strain BCG. Cell Immunol. 1976 Oct;26(2):254–263. doi: 10.1016/0008-8749(76)90369-5. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Pelus L. M., Ralph P., Bockman R. S., Moore M. A. Induction of prostaglandin E synthesis in normal and neoplastic macrophages: role for colony-stimulating factor(s) distinct from effects on myeloid progenitor cell proliferation. Proc Natl Acad Sci U S A. 1979 May;76(5):2326–2330. doi: 10.1073/pnas.76.5.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman L. B., Hacker M. P., Blyden G. T., Handschumacher R. E. Preparation of lymphocyte-activating factor from continuous murine macrophage cell lines. Cell Immunol. 1977 Dec;34(2):416–419. doi: 10.1016/0008-8749(77)90263-5. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. 1975 Oct 2;257(5525):393–394. doi: 10.1038/257393a0. [DOI] [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Simberkoff M. S., Elsbach P. The interaction in vitro between polymorphonuclear leukocytes and mycoplasma. J Exp Med. 1971 Dec 1;134(6):1417–1430. doi: 10.1084/jem.134.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simberkoff M. S., Thorbecke G. J., Thomas L. Studies of PPLO infection. V. Inhibition of lymphocyte mitosis and antibody formation by mycoplasmal extracts. J Exp Med. 1969 Jun 1;129(6):1163–1181. doi: 10.1084/jem.129.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. F., Langworthy T. A., Mayberry W. R. Distribution and composition of lipopolysaccharides from mycoplasmas. J Bacteriol. 1976 Mar;125(3):916–922. doi: 10.1128/jb.125.3.916-922.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Schorlemmer H. U., Furr P. M., Allison A. C. Macrophage secretion and the complement cleavage product C3a in the pathogenesis of infections by mycoplasmas and L-forms of bacteria and in immunity to these organisms. Clin Exp Immunol. 1978 Sep;33(3):486–494. [PMC free article] [PubMed] [Google Scholar]
- Washburn L. R., Cole B. C., Gelman M. I., Ward J. R. Chronic arthritis of rabbits induced by mycoplasmas. I. Clinical microbiologic, and histologic features. Arthritis Rheum. 1980 Jul;23(7):825–836. doi: 10.1002/art.1780230709. [DOI] [PubMed] [Google Scholar]
- Wilton J. M., Rosenstreich D. L., Oppenheim J. J. Activation of guinea pig macrophages by bacterial lipopolysaccharide requires bone marrow-derived lymphocytes. J Immunol. 1975 Jan;114(1 Pt 2):388–393. [PubMed] [Google Scholar]