Abstract
In this issue of Molecular Cell, Raman et al. and Franz et al. establish the AAA ATPase p97/CDC48 as an essential regulator of eukaryotic DNA replication that coordinates the release of chromatin-bound CDT1 with its degradation by the proteasome.
Keywords: p97/VCP/CDC48, CDT1, DNA replication, ubiquitin, proteasome, ERAD
To restrict DNA replication to a single round per cell cycle, eukaryotic cells employ several strategies to inhibit certain replication initiation factors termed “licensing factors” once replication has initiated. One important mechanism is to target CDT1, a crucial component of the prereplicative complex that has been assembled at the potential replication sites on the G1 chromosome, for proteasomal degradation at the onset of S-phase by a Cullin Ring-based ubiquitin ligase (CRL4CDT2). The timely removal of CDT1 prevents re-firing of the replication origins and thus ensures accurate DNA duplication (Arias and Walter, 2007). Importantly, CDT1 is also rapidly turned over upon DNA damage, which halts DNA replication to allow repair of damaged DNA. Despite being a key node in an important biological regulatory circuit, the mechanism that targets chromosome-bound CDT1 to the proteasome has been elusive. In this issue of Molecular Cell, two research groups independently identify the AAA (ATPase associated with various cellular activities) ATPase p97 as a crucial modulator that couples the extraction of chromosome-bound CDT1 with its proteasomal targeting (Franz et al., 2011; Raman et al., 2011).
p97 (also termed CDC48 in yeast and C. elegans) is an abundant, conserved, hexameric ATPase that acts with distinct adaptors to function in various biological pathways such as endoplasmic reticulum-associated degradation (ERAD), membrane fusion, endocytic trafficking and autophagy (Schuberth and Buchberger, 2008; Ye, 2006). The activity of p97 is best characterized in ERAD in which it functions with the dimeric cofactor Ufd1-Npl4 to extract polyubiquitinated substrates from the membrane for proteasomal degradation (Figure 1). Interestingly, p97 can interact with DNA replication and repair factors such as the human Werner RecQ helicase and BRCA1, and its deficiency in C. elegans causes severe DNA replication defects in developing embryos (Mouysset et al., 2008). However, it is unclear through which substrate(s) p97 acts to regulate DNA replication or repair.
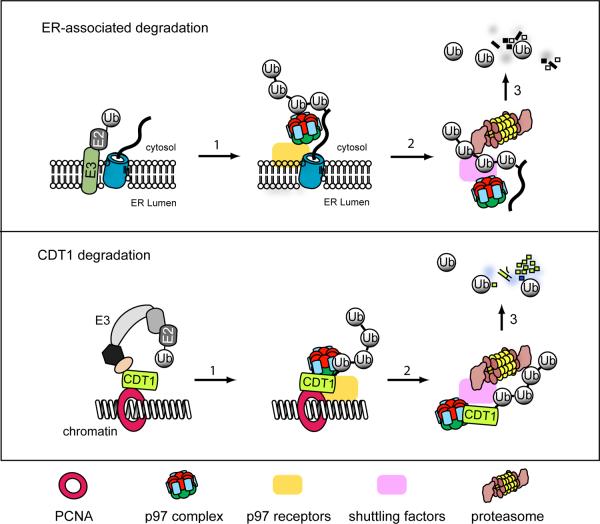
Figure 1. Comparison of CDT1 degradation to the ERAD pathway.
In ERAD, misfolded proteins undergo retrotranslocation through one of the few membrane complexes postulated to form retrotranslocation channels (blue cylinder). The process can be divided into three steps. 1) A ubiquitin ligase within a retrotranslocation complex conjugates ubiquitin chains to the substrate. 2) The p97/CDC48 complex that is recruited to the membrane via interacting with membrane receptors such as Derlin-1, VIMP, UbxD8, gp78 extracts ubiquitinated substrates from the membrane. 3) The p97/CDC48 complex acts with shuttling factors such as Ufd2, ataxin3, and ubiquilin to target dislocated substrates to the proteasome for degradation. The elimination of PCNA-bound CDT1 from the chromatin appears to follow the same suit, but the p97 receptors on the chromatin and the shuttling factors are unclear.
Raman et al. identify CDT1 as a new p97 substrate using a genome-wide siRNA screening approach. Their studies show that depletion of p97 or knockdown of the p97 cofactor UFD1 stabilizes CDT1 in UV-irradiated cells. Moreover, expression of wild type p97, but not of an ATPase defective mutant, in cells lacking endogenous p97, restores UV-induced CDT1 turnover, indicating that the p97 ATPase activity is required for CDT1 turnover. Because CDT1 accumulated in p97 or UFD1 deficient cells is entirely bound to the chromosome, Raman et al. postulate that p97 may use the energy from ATP hydrolysis to extract CDT1 from the chromatin, which facilitates its targeting to the proteasome. This idea is tested by a set of elegant in vitro experiments using Xenopus egg extracts (HSS) and artificially damaged DNA. The results demonstrate that damaged DNA in control HSS, but not in p97-depleted HSS, triggers CRL4-dependent degradation of ubiquitinated CDT1 and that chromatin-bound CDT1 can be released from DNA in an ATP dependent manner by purified p97-UFD1-NPL4 complex under conditions that uncouple the extraction and degradation reactions. Together, the findings from Raman and colleagues support a model in which p97 acts on chromatin-bound, ubiquitinated CDT1 upon DNA damage to release it for proteasomal degradation (Figure 1).
In the second study, Franz and colleagues follow up on their recent observation that CDC48-depleted C.elegans embryos have reduced DNA content due to defects in S-phase progression and replication check point activation (Mouysset et al., 2008). Since similar phenotypes were observed with depletion of the licensing factors CDT1 and CDC6, Franz et al. hypothesize that CDC48 may act through these factors to regulate S-phase progression. Indeed, time-lapse microscopy shows that embryos lacking the CDC48 cofactor UFD1 or NPL4 accumulate CDT1 on mitotic chromatin, a finding that echoes those described by Raman et al. The fact that the CDT1 protein levels, but not its mRNA transcript, are increased in embryos deficient in CDC48, UFD1 or NPL4, similarly indicates a function for the CDC48 complex in regulating CDT1 stability in C. elegans. Based on both genetic and biochemical evidence, Franz et al. propose that the CDC48 complex is specifically involved in a CDT1 degradation pathway that is linked to dissociation of the CDC45/GINS complex from the chromatin during mitosis, an event required for accurate DNA replication and cell cycle progression.
Although both studies designate p97/CDC48 as a crucial regulator of CDT1 turnover, they differ in describing the timing of p97/CDC48 engagement and the physiological consequences of CDT1 turnover. Raman et al. show that depletion of p97 stabilizes CDT1 in both UV-treated and untreated cells, pinpointing a role for p97 in both DNA damage-induced as well as S-phase turnover of CDT1 in human cells. Downregulation of CDT1 in these contexts revokes its licensing activity and thereby prevents undesired DNA replication. By contrast, Franz et al. show that CDC48 deficiency in C. elegans causes accumulation of CDT1 in mitotic but not S-phase cells. They propose a unique mitotic CDT1 turnover pathway that differs from the S-phase CDT1 degradation in its requirement for CDC48. Their genetic studies indicate that mitotic destruction of CDT1 helps cells avoid check point activation, which ensures proper initiation of the following round of DNA replication. This model provides a plausible explanation for the DNA replication deficiency associated with loss of CDC48 function in C. elegans. Whether p97-dependent elimination of mitotic CDT1 occurs in mammals is unclear because CDT1 is thought to be stabilized by binding to Geminin and thus not subject to proteasomal degradation during mitosis in human cells (Ballabeni et al., 2004). It is also unclear why in C. elegans CDC48 is dispensable for the S-phase turnover of CDT1, but becomes essential for the same process in mitosis. Lastly, the two stories also report on distinct requirement for the p97 cofactor NPL4 in CDT1 turnover, further differentiating the two events.
Despite the discordances, both studies establish CDT1 as the first DNA replication relevant substrate for p97 and highlight the potential impact that p97 can have on eukaryotic DNA replication by governing the destruction of this critical licensing factor. The findings also corroborate the idea that p97 could be a potential anti-cancer drug target. Additionally, the studies expand the list of the chromosomal proteins that are released by p97 (Ramadan et al., 2007; Verma et al., 2011; Wilcox and Laney, 2009) and therefore strengthen the concept that modulating the localization/activity of chromosomal proteins is a general and potentially major function for the nuclear pool of p97. Given the apparent similarity between the turnover of CDT1 and ERAD substrates (Figure 1), it is anticipated that questions perplexing the ERAD field over the years will be applicable to future studies on p97-mediated CDT1 turnover. For example, what is the receptor that recruits p97 to the chromatin? Could all ubiquitinated chromosomal proteins be p97 substrates? Does p97 extract chromosomal substrates by threading them through its central pore? What is the role of ubiquitin recognition in the extraction reaction? Does this process involve p97-associated deubiquitinases? Moreover, given that certain chromatin proteins released by p97 are apparently not degraded (Ramadan et al., 2007; Wilcox and Laney, 2009), how the fates of the released proteins are determined should be investigated. Undoubtedly, answers to these questions should shed important insights into the general mechanism that permits p97 to release polypeptides from large immobile subcellular structures in various biological settings.
REFERENCES
- Arias EE, Walter JC. Genes Dev. 2007;21:497–518. doi: 10.1101/gad.1508907. [DOI] [PubMed] [Google Scholar]
- Ballabeni A, Melixetian M, Zamponi R, Masiero L, Marinoni F, Helin K. Embo J. 2004;23:3122–3132. doi: 10.1038/sj.emboj.7600314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz A, Orth M, Pirson PA, Sonneville R, Julian Blow J, Gartner A, Stemmann O, Hoppe T. Molecular Cell. 2011 doi: 10.1016/j.molcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouysset J, Deichsel A, Moser S, Hoege C, Hyman AA, Gartner A, Hoppe T. Proc Natl Acad Sci U S A. 2008;105:12879–12884. doi: 10.1073/pnas.0805944105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan K, Bruderer R, Spiga FM, Popp O, Baur T, Gotta M, Meyer HH. Nature. 2007;450:1258–1262. doi: 10.1038/nature06388. [DOI] [PubMed] [Google Scholar]
- Raman M, Havens CG, Walter JC, Harper JW. Molecular Cell. 2011 doi: 10.1016/j.molcel.2011.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuberth C, Buchberger A. Cell Mol Life Sci. 2008;65:2360–2371. doi: 10.1007/s00018-008-8072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Oania R, Fang R, Smith GT, Deshaies RJ. Mol Cell. 2011;41:82–92. doi: 10.1016/j.molcel.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ, Laney JD. Nat Cell Biol. 2009;11:1481–1486. doi: 10.1038/ncb1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y. J Struct Biol. 2006;156:29–40. doi: 10.1016/j.jsb.2006.01.005. [DOI] [PubMed] [Google Scholar]