Abstract
Background
Omalizumab (trade name Xolair) is approved by the US Food and Drug Administration for treatment of moderate-to-severe allergic asthma. Given the high acquisition cost of omalizumab, its role and cost-effectiveness in disease management require definition.
Objective
We sought to identify the clinical and economic circumstances under which omalizumab might or might not be a cost-effective option by using a mathematic model.
Methods
We merged published data on clinical and economic outcomes (including acute event incidence, frequency/severity of hospitalizations, and health-related quality of life) to project 10-year costs, quality-adjusted life years (QALYs), and cost-effectiveness of treatment with omalizumab in addition to inhaled corticosteroids. Sensitivity analyses were conducted by using input data ranges from a variety of sources (published clinical trials and observational databases).
Results
For patients with baseline acute event rates, omalizumab conferred an additional 1.7 quality-adjusted months at an incremental cost of $131,000 over a 10-year planning horizon, implying a cost-effectiveness ratio of $821,000 per QALY gained. For patients with 5 times the baseline acute event rate, the cost-effectiveness ratio was $491,000 per QALY gained. The projected cost-effectiveness ratio could fall within a range of other programs that are widely considered to be cost-effective if the cost of omalizumab decreases to less than $200.
Conclusion
Omalizumab is not cost-effective for most patients with severe asthma. The projected cost-effectiveness ratios could fall within a favorable range if the cost of omalizumab decreases significantly.
Clinical implications
Based on the high cost of omalizumab, it is especially important that clinicians explore alternative medications for asthma before initiating omalizumab.
Keywords: Omalizumab, cost-effectiveness, asthma, anti-IgE
The cost of asthma in the United States was estimated to be US $14 billion in 2002. This amount represents direct medical costs, such as hospitalizations ($3.1 billion) and pharmaceuticals ($3.7 billion), and indirect medical costs ($4.6 billion), such as time lost from work and premature death.1 For patients with severe refractory asthma, the range of available treatment options is limited. Omalizumab, a humanized mAb directed against IgE, was approved by the US Food and Drug Administration in 2003 as maintenance therapy for adults and adolescents (≥12 years of age) with moderate-to-severe allergic asthma and inadequate response to inhaled corticosteroids (ICSs). Randomized, placebo-controlled, doubleblind clinical trials demonstrate that the addition of omalizumab to patients’ drug regimens reduces the frequency of allergic asthma exacerbations compared with ICSs alone.2-4 Additional evidence suggests that omalizumab decreases the incidence of clinic visits for asthma exacerbations, emergency department (ED) visits, and hospitalizations.2,4-9
Omalizumab is more expensive than any other medication for asthma. Current US wholesale prices for omalizumab average almost $1300 per patient-month for an asthmatic patient who weighs less than 90 kg. Furthermore, because it must be administered every 2 or 4 weeks by means of slow subcutaneous injection, omalizumab requires an increase in the number of clinic visits and requires additional resources for patient education. In addition, omalizumab administration must be continued indefinitely10 because serum IgE levels appear to return when treatment is withheld.11
Viewed in the context of limited resources and competing choices, the role of omalizumab in asthma management is not well defined. Based on its price alone, many observers have summarily concluded that omalizumab will be cost-effective only in the most limited patient settings.12-14 Oba and Salzman15 conducted a cost-effectiveness analysis from a third-party payer’s perspective and found that the daily cost to achieve greater than a 0.5-point increase in the Asthma Quality of Life Questionnaire (AQLQ) score was $378 in 2003 dollars and that the cost to achieve an additional successfully controlled day was $523. They concluded that omalizumab is not cost-effective. Another cost-effectiveness analysis by Brown et al16 suggested that omalizumab is cost-effective; however, this analysis was based on a small open-label trial, and the analysis was limited to subjects who were responsive to omalizumab. This creates a strong bias in favor of omalizumab. In clinical practice we do not yet have the ability to select and then treat only those subjects who will respond to the medication. Therefore we have to use the medication in a broader population, which increases the overall costs and decreases the overall benefits (because there will be nonresponders) from the use of the therapy on a societal basis. In addition, the authors of this analysis derived preference-based measures of health outcome based on the AQLQ. Still lacking in the literature is a formal cost-effective analysis using directly obtained preference-based measures of health.17
Studies taking a societal viewpoint can better discern which group of patients should be considered for omalizumab.14 By taking a societal viewpoint and measuring effectiveness in terms of quality-adjusted life years (QALYs), a cost-effectiveness analysis can consider value for money and provide practical guidance, either to practitioners or to payers, as to the clinical and economic circumstances under which omalizumab might or might not prove to be an appropriate and cost-effective option. To our knowledge, no evaluation of omalizumab has yet been conducted in conformity with the recommendations of the US Panel on Cost-Effectiveness in Health and Medicine.18
METHODS
Analytic overview
We used the Asthma Policy Model, a previously published, computer-based mathematic model of the natural history and treatment of asthma, to simulate the use of omalizumab in a population of adult asthmatic patients with severe uncontrolled disease and to project results into 10-year outcomes, including QALYs, cost, and cost-effectiveness.19,20 In accordance with the recommendations of the Panel on Cost-Effectiveness in Health and Medicine, this analysis was performed from the societal perspective, with costs updated to 2005 US dollars by using the medical care component of the Consumer Price Index21 and using an annual discount rate of 3%. Cost-effectiveness ratios for omalizumab in addition to ICSs and quick relievers alone were calculated incrementally and reported in dollars per QALY gained. We explored the strength of our conclusions under alternative parameter estimates.
Asthma Policy Model
Details of this model, its underlying assumptions, construction, input data fields, and analysis, are described elsewhere.18,19 Briefly, this is a Markov state-transition simulation,22,23 a model that characterizes the progress of disease as a sequence of transitions through a defined set of “health states.” The model characterizes the natural history of illness as a sequence of flows into and out of 3 healthy states: chronic, acute, and death. The model assumes that patients in the same health state also share a similar clinical history and prognosis, a common perception of well-being, and a comparable pattern of health care use. Acute exacerbations are divided into 3 categories: urgent care visits, ED visits, and hospitalizations. Patients begin in an initial health state that reflects published distributions of patient age, asthma prevalence, and pulmonary function. Each month, patients’ risk profiles might change. Each transition involves a unique set of mortality risks, clinical consequences, changes in quality of life, and economic costs. After each month, patients can stay in the same health state or change to a different state. Exacerbations and hospitalizations from asthma are accounted for when a patient moves from the chronic health state to the acute state. The clinical course of a hypothetical cohort of patients is tracked for 10 years. The Online Repository at www.jacionline.org provides a detailed summary of the development of the model.20
Target population
We considered 2 treatment scenarios for patients with severe asthma: (1) ICS therapy for longer-term asthma control and quick relievers (eg, short-acting β-agonists) on an as-needed basis and (2) ICSs and quick relievers plus omalizumab therapy. We chose to focus on patients with severe persistent asthma because omalizumab is currently recommended for this population.
Input data: Effect of ICS therapy
The effect of ICS therapy was expressed in terms of change in FEV1 as a percentage of predicted normal value relative to baseline. Evidence suggests that a subset of patients with severe asthma might be insensitive to steroids.25 Thus the use of steroids in patients who are insensitive might add additional toxicity without the benefit that is realized in other subpopulations. Moreover, we assume equivalency across all ICS preparations. Although the model can be used to simulate the effects of a given agent, our approach is consistent with the most recent version of the National Asthma Education and Prevention Program guidelines (which acknowledge differences on a per-inhalation or microgram basis but which do not currently define any implications of these differences for purposes of clinical dosing recommendations). For the current analysis, we dampened the baseline efficacy assumption to reflect the fact that omalizumab is approved for use in patients who have not achieved adequate asthma control through ICS therapy. Our baseline assumption was that ICS therapy improved FEV1 percent predicted values by 17%. In addition, we explored a range of effect of ICS therapy of 9% (reflecting a 50% dampening effect of ICS therapy) to 17%.
In our previous work we have published details of these assumptions and the evidence base to support them elsewhere.20 We note, moreover, that by assuming limited disease control with inhaled steroids, we have deliberately biased the analysis in favor of omalizumab.
Input data: Effect of omalizumab therapy
We modeled the treatment effectiveness of omalizumab along 3 independent dimensions: (1) reduction in exacerbation rate, (2) reductions in the duration and intensity of an exacerbation when it occurs, and (3) improvement in health-related quality of life (HRQOL). In this section we discuss the data supporting the mechanisms of effectiveness and then describe the values we used for both our baseline and sensitivity analyses. Baseline input values and parameters for sensitivity analyses are listed in Table I.2,25-28
TABLE I.
Model parameters for base-case and sensitivity analysis
| Attribute | Baseline assumptions | Range for sensitivity analysis |
Sources |
|---|---|---|---|
| Reduction in exacerbation rates (%) | 46 | 33-92 | Walker et al25 |
| Reduction in duration/intensity of hospitalizations (%) | 63 | — | Busse et al,2 Corren et al26 |
| Increase in quality of life (%) | 0.9 | 0-7.2 | Niebauer et al28 |
| Increase in FEV1 % predicted (%) | 2.9 | — | Walker et al25 |
| Monthly cost* ($) | 1300 | 100-2500 | Drug Topics Red Book, 200527 |
Monthly cost calculated based on yearly cost of omalizumab in 2005 dollars for a 70-kg adult with an IgE level of 200 IU/mL.
Reduction in exacerbation rate
The Cochrane Airways Group’s review of omalizumab found a 46% reduction in the rate of exacerbation in subjects receiving omalizumab plus an ICS compared with that seen in the subjects receiving an ICS alone.24 Thus in our base-case analysis we assumed a 46% reduction in the exacerbation rate. In our sensitivity analyses we explored a range of 33% to 92%, based on previously published studies.26
Reduced duration/intensity of hospitalizations
Modeled as a percentage reduction in the severity of acute events, omalizumab would affect the resource use of urgent care visits, ED visits, and hospitalizations. Omalizumab treatment resulted in shorter duration of asthma exacerbations in one clinical trial, with a mean length of exacerbation of 7.8 days compared with 12.7 days in the control group (P < .001).2 Based on this difference in length of exacerbation, we chose to assume a 63% reduction in resource use intensity, which corresponds to a decrease from 12.7 days to 7.8 days.26
Change in lung function
The systematic review conducted by the Cochrane Airways Group suggests a small improvement in FEV1 percent predicted25 associated with omalizumab therapy. The systematic review finds an improvement in FEV1 percent predicted of 2.9% in subjects treated with omalizumab. Thus in our base-case analysis, we assumed omalizumab conferred an improvement in FEV1 of 2.9%.
Costs
Costs were estimated from published resource use studies29-36 and adjusted to reflect US 2005 dollars by using the Consumer Price Index.21 Baseline monthly chronic care costs (medications, routine office visits, laboratory testing) were $77 for patients with severe disease.29 Acute event costs included $75 for non-ED urgent care visits, $290 for ED visits, and $3800 for hospitalizations.29 Hospitalizations account for a large part of the expense of asthma care. ICS costs were estimated from published resource use studies.29,31-34 The wholesale price for one 150-mg vial of omalizumab in 2005 dollars is $568.31.27 Dosing for omalizumab was based on both IgE level and weight (0.016 mg/kg IgE). The 2 clinical trials of omalizumab in adolescents and adults with severe asthma reported mean IgE levels of 197 IU/mL.25 Thus a 70-kg adult would require 220 mg per dose or 2 vials. Vials of omalizumab are single use, and unused portions are discarded. The cost of 2 vials of omalizumab (150 mg each) every 4 weeks is $15,000 per year, which is a conservative estimate of the yearly cost of omalizumab because many adults weigh more than 70 kg. The maximum recommended dose according to the manufacturer is 375 mg every 2 weeks, which would entail use of 3 vials every 2 weeks at a cost of $44,000 per year. Recognizing the variation of costs based on IgE level, body weight, and costs of office visits, we explored the effects of varying costs by 10% to 200% of their baseline values.
Improved quality of life
The US Panel on Cost-effectiveness in Health and Medicine recommends that morbidity consequences be valued by community preferences for health states be used, whenever feasible.18 The panel, however, acknowledges the difficulties of adhering to this recommendation and suggests various practical approximations. In the case of the relationship between lung function and values (utilities), published evidence is limited.37,38 No studies have considered a sufficiently rich set of symptomatic health states to be suitable for our use. Moreover, we know of no studies that have directly collected community-based preferences in asthma. As described elsewhere, we previously collected our own preference weights for use by the Asthma Policy Model through direct utility assessments by using the time-tradeoff elicitation technique.39 For each patient, information was collected on FEV1 percent predicted values. In addition, several preference elicitation measures, including time tradeoff, standard gamble questions, the Health Utilities Index, and the Asthma Symptom Utility Index, were administered.39 The relationship between FEV1 percent predicted and preference scores was estimated by using ordinary least-squares regression.39 Cognizant of the small size and demographic homogeneity of our sample, we have previously explored results using preferences obtained through the other elicitation instruments (most notably, the community-weighted Health Utilities Index40 and the Asthma Symptom Utility Index).38,39 Generally, our cost-effectiveness findings have been robust in the face of plausible variation in baseline quality-of-life assumptions.
Based on the systematic review that suggests a 2.9% improvement in FEV1 with omalizumab,41 we used the Asthma Policy Model to infer the increase in utilities associated with omalizumab (a 2.9% increase in FEV1) and found that omalizumab produced an improvement in HRQOL of 0.9%. This is consistent with previous findings.25 Thus in our base-case analysis we assumed that omalizumab conferred an improvement of 0.9% of HRQOL.
Prior reports suggest that the correlation between health status measures and preference-based HRQOL measures is modest at best, with health status measures explaining less than one third of the variance in utility.42,43 A systematic review by Niebauer et al28 found a significant improvement in asthma-related HRQOL in the omalizumab- and steroid-treated groups compared with those receiving steroids alone in adults and children. A weighted average taken from the 3 adult trials of subjects with asthma estimated an improvement in health status (AQLQ) of 7.2%.28 In a report focusing on asthma, Molken et al44 observed that an 8.8% relative change in health status (AQLQ) was associated with a 0.9% change in preference based HRQOL (standard gamble). Thus our base-case analysis assumption of a preference-based HRQOL improvement of 0.9% in association with omalizumab treatment is consistent with the published literature.
RESULTS
ICSs with quick relievers alone
For patients with severe asthma receiving ICSs and quick relievers, the model predicts a population average of 0.63 hospitalizations, 0.93 ED visits, and 4.4 urgent care visits per patient-year (undiscounted).
On the basis of our model, over a 10-year horizon, patients aged 18 years and older are expected to live an average of 9.17 years (7.95 discounted life-years), with virtually all deaths attributable to non–asthma-related causes. Adjusted for both HRQOL and the time value of outcomes, this equates to 78.33 discounted quality-adjusted months (QAMs). Discounted asthma-related costs are expected to total $16,000.
Omalizumab
Under baseline assumptions, the addition of omalizumab is expected to reduce hospitalizations to 0.31, ED visits to 0.46, and urgent care visits to 2.17 per patient-year (undiscounted as shown in Table II). Although life expectancies barely change over a 10-year planning horizon, quality-adjusted survival increases to 80.01 QAMs. With the addition of omalizumab, discounted asthma-related costs increase to $131,000. Thus compared with ICSs alone, we estimate that over a 10-year planning horizon, omalizumab confers an additional 1.7 QAMs at an incremental cost of $821,000 per QALY gained.
TABLE II.
Base-case analysis over 10-year time horizon
| Omalizumab + ICS | |
|---|---|
| Asthma-related events (per person-year) | |
| Hospitalizations | 0.31 |
| ED visits | 0.46 |
| Urgent care visits | 2.17 |
| Discounted QAM | 80.01 |
| Costs, discounted | |
| Chronic asthma cost without drug ($) | 7300 |
| Acute asthma cost ($) | 380 |
| Drug cost ($) | 123,000 |
| Total cost ($) | 131,000 |
| Cost-effectiveness ratios* | |
| Cost per QALY gained ($/QALY) | 821,000 |
| Cost per SFD gained ($/SFD) | 120 |
SFD, Symptom-free day.
All cost-effectiveness ratios are incremental compared with the nextleast-costly, undominated alternative. Reported ratios might not be precisely equal to the ratio of costs and effects because of rounding.
Sensitivity analysis
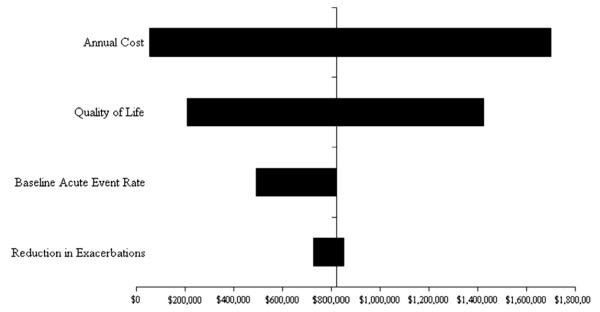
In sensitivity analyses assumptions regarding cost of omalizumab, reduction in exacerbation rate, reduction in duration and intensity of hospitalizations, improvement in HRQOL, and increase in baseline acute event rates were varied over plausible ranges (Table I). The monthly cost of omalizumab and improvement in HRQOL had the greatest effect on the incremental cost-effectiveness ratios (Fig 1). Changing assumptions regarding the baseline rate of acute exacerbations had significantly less effect on the incremental cost-effectiveness ratios.
FIG 1.
Sensitivity analysis. This tornado diagram summarizes a series of 1-way sensitivity analyses. Each horizontal bar represents a given model parameter. The vertical axis represents the base-case scenario, as listed in Table II. The length and position of each horizontal bar represents the range of cost-effectiveness outcomes produced by varying that specific parameter over its plausible range, also listed in Table II.
Reduction in exacerbations with omalizumab
We explored the cost-effectiveness ratios for omalizumab if the efficacy, measured in reduction in exacerbation rate, varied from 33% to 92%. We found that the cost per QALY gained varied from $853,000 and $727,000, respectively.
Higher acute event rates
The baseline acute event rate was multiplied by a factor of 5 to simulate a high-risk group with frequent exacerbations; all exacerbation-related events and their associated costs and HRQOL effects were increased. For example, the number of hospitalizations over a 10-year horizon increased to 1.54 per patient-year (undiscounted), and discounted asthma-related costs increased to $134,000. Given such a scenario, the cost-effectiveness ratio was more favorable. With increased acute event rates, omalizumab confers an additional 2.6 QAMs at an incremental cost of $491,000 per QALY gained.
Quality-of-life improvement
We explored the cost-effectiveness ratios for omalizumab if the therapy-related improvement in HRQOL varied from 0% to 7.2% while holding other inputs at the base-case scenario. The results suggest that the cost-effectiveness of omalizumab is sensitive to omalizumab’s effect on HRQOL. When we assumed no improvement in HRQOL with omalizumab, the cost-effectiveness ratio increased to $1.42 million. On the other hand, when improvement in HRQOL was assumed to be 7.2%, the cost-effectiveness ratio decreased to $207,000 per QALY.
Cost of omalizumab
Given the baseline assumption for acute event rates, monthly drug costs of less than $200 resulted in a cost-effectiveness ratio of $100,000 per QALY. Similar results were obtained when we assumed an acute event rate 5 times the baseline value: drug costs of $450 per month resulted in a cost-effectiveness ratio of $100,000 per QALY.
DISCUSSION
At $821,000 per QALY gained, omalizumab is not cost-effective. Although no consensus defines the threshold that represents acceptable value for money, cost-effectiveness ratios are often placed in context by comparisons with other interventions, such as hemodialysis for chronic renal failure.45 The “dialysis threshold” that is frequently quoted is $93,500 per QALY in 2002 US dollars. Accordingly, an intervention costing $821,000 per QALY is not considered attractive value for money.
Not surprisingly, the cost-effectiveness of omalizumab is highly sensitive to price assumptions. Holding all other model parameters at their baseline value, monthly drug costs of less than $100 and $200 per month would be required to clear $50,000 and $100,000 per QALY gained, respectively. This would translate into annual drug costs of $1200 to $2400 for a 70-kg person with an IgE level of 200 IU/mL. In addition, the cost-effectiveness of omalizumab is sensitive to assumptions in improvement in HRQOL. Based on existing clinical trials, omalizumab improved HRQOL. When effects were modeled assuming HRQOL is improved by 7.2% more than when taking an ICS alone, the cost-effectiveness ratio is close to $207,000. The cost-effectiveness ratio was less sensitive to other input data assumptions, such as reduction in exacerbations, reduction in hospital resource use, increase in baseline acute event rate, and dampening of the ICS effect.
Similar to Oba and Salzman’s cost-effectiveness study of omalizumab,15 we find that the incremental cost associated with omalizumab is higher than is commonly used as a benchmark for an acceptable incremental cost. Because our analysis measures cost per QALY rather than cost to improve the AQLQ score by 0.5 points for an entire year, it is easier to compare the cost of omalizumab with the costs of other therapeutics and interventions. The analysis by Brown et al does use the unit of cost per QALY but has other shortcomings. Although the study used for analysis includes a total of 206 subjects in the omalizumab group, the analysis by Brown et al only includes the 68 subjects who responded to omalizumab, defined as having a 0.5-point or greater improvement in the Mini-AQLQ (Brown et al16 and Ayres et al46). Thus this analysis is a narrow analysis and does not represent the real-world setting in which omalizumab response cannot be predicted before treatment. In addition, this analysis used utilities that might not be accurate because they were transformed from values from the Mini-AQLQ, and the utilities were based on an open-label trial that could result in biased responses to the AQLQ.16,46
Our findings have significant implications for the allocation of health care resources in the treatment of severe asthma. Because of the substantial clinical benefits seen in randomized control trials, omalizumab is potentially a valuable therapeutic strategy in populations whose prognosis is otherwise poor and for whom few satisfactory alternatives exist. For these individuals, the usual parameters by which cost-effectiveness is judged might be different from those used in healthier populations. For example, studies of cost-effectiveness in the treatment of advanced cancer have demonstrated that society is willing to pay more to prolong and improve the life of very ill patients, regardless of whether the interventions are less cost-effective than those used in healthier patients.47-50 Nevertheless, based on our analysis, the incremental cost-effectiveness ratio for omalizumab falls far outside the range considered reasonable for all but the most severely ill patients.
A few limitations to this study deserve mention. Perhaps most notable is the fact that this analysis is based on a model. The logistic and financial obstacles, however, to conducting a clinical trial comparing omalizumab with placebo and comparing long-term costs and outcomes would be difficult to surmount. A model-based approach permits the extrapolation of costs and health effects beyond the time horizon of a single clinical study. Second, a model-based approach can be used to anticipate the results of new clinical investigations and to guide clinicians and policymakers in judging the quality and interpreting the policy relevance of the outcomes. In addition to relating biologic and clinical information, a model can provide quantitative insight into the relative importance of different components of a treatment strategy and investigate how results will change if values of key parameters are affected. By identifying the most important sources of uncertainty, a model can also be used to help prioritize and guide data collection efforts.
Another limitation of the Asthma Policy Model is that there might be concern that FEV1 percent predicted might not fully predict prognosis. Nevertheless, based on previously analyses, we found that FEV1 percent predicted is associated with symptoms, acute exacerbations, costs, and quality of life. In addition, using FEV1 percent predicted as an asthma severity index has several advantages of objectivity and reproducibility and is an underlying physiologic driver of clinical events. Furthermore, the rates of exacerbation predicted in our model are lower than the rates in the clinical trials, likely because our model predicts rates of exacerbation of a general population rather than a population in a randomized control trial that is selected for being more sick; however, we addressed this concern by examining the effect of changing our assumptions for the baseline exacerbation rates for the population in sensitivity analyses. Moreover, we do not include indirect costs, such as lost productivity, in our analysis. The Panel on Cost-Effectiveness in Health and Medicine recommends not to do so. Instead, indirect costs are incorporated in the measure of QALYs.
To summarize, omalizumab does not provide sufficient clinical benefit and resource savings to justify its current price in a general population of patients with severe asthma. The projected cost-effectiveness ratio might fall within an accepted value range for interventions within the United States if the cost for omalizumab decreases significantly.
This analysis and the development of the model on which it is based were supported by the National Heart, Lung, and Blood Institute. Although we gratefully acknowledge its support and scientific advice, the agency played no significant role in the design or conduct of the study. No limitations on publication were imposed, nor was any prepublication review required.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (R01 HL68201-01A1).
Abbreviations used
- AQLQ
Asthma Quality of Life Questionnaire
- ED
Emergency department
- HRQOL
Health-related quality of life
- ICS
Inhaled corticosteroid
- QALY
Quality-adjusted life year
- QAM
Quality-adjusted month
Footnotes
Disclosure of potential conflict of interest: S. T. Weiss has consulting arrangements with Glaxo-Wellcome, Roche Pharmaceuticals, Millennium Pharmaceuticals, Genentech, Schering-Plough, Variagenics, Genome Therapeutics, and Merck Frost and has received grant support from AstraZeneca and Pfizer. A. L. Fuhlbrigge has consulting arrangements with Merck and GlaxoSmithKline, has received grant support from Boehringer Ingelheim and Merck, is on the speakers’ bureau for Merck and GlaxoSmithKline, and is a member of the Data Safety Monitoring Board for an industry-sponsored clinical trial by Sepracor. The other authors have declared that they have no conflict of interest.
REFERENCES
- 1.National Heart, Lung, and Blood Institute . Morbidity and mortality: 2002. National Heart, Lung, and Blood Institute; Bethesda (MD): 2002. [Google Scholar]
- 2.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–90. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 3.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108:e36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 4.Soler M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 5.Lanier BQ, Corren J, Lumry W, Liu J, Fowler-Taylor A, Gupta N. Omalizumab is effective in the long-term control of severe allergic asthma. Ann Allergy Asthma Immunol. 2003;91:154–9. doi: 10.1016/S1081-1206(10)62170-9. [DOI] [PubMed] [Google Scholar]
- 6.Buhl R, Hanf G, Soler M, Bensch G, Wolfe J, Everhard F, et al. The anti-IgE antibody omalizumab improves asthma-related quality of life in patients with allergic asthma. Eur Respir J. 2002;20:1088–94. doi: 10.1183/09031936.02.00016502. [DOI] [PubMed] [Google Scholar]
- 7.Lemanske RF, Jr, Nayak A, McAlary M, Everhard F, Fowler-Taylor A, Gupta N. Omalizumab improves asthma-related quality of life in children with allergic asthma. Pediatrics. 2002;110:e55. doi: 10.1542/peds.110.5.e55. [DOI] [PubMed] [Google Scholar]
- 8.Buhl R, Soler M, Matz J, Townley R, O’Brien J, Noga O, et al. Omalizumab provides long-term control in patients with moderate-to-severe allergic asthma. Eur Respir J. 2002;20:73–8. doi: 10.1183/09031936.02.00278102. [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, Wenzel S, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60:302–8. doi: 10.1111/j.1398-9995.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 10.Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006;354:2689–95. doi: 10.1056/NEJMct055184. [DOI] [PubMed] [Google Scholar]
- 11.Saini SS, MacGlashan DW, Jr, Sterbinsky SA, Togias A, Adelman DC, Lichtenstein LM, et al. Down-regulation of human basophil IgE and FC epsilon RI alpha surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162:5624–30. [PubMed] [Google Scholar]
- 12.Belliveau PP, Lahoz MR. Evaluation of omalizumab from a health plan perspective. J Manag Care Pharm. 2005;11:735–45. doi: 10.18553/jmcp.2005.11.9.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davydov L. Omalizumab (Xolair) for treatment of asthma. Am Fam Physician. 2005;71:341–2. [PubMed] [Google Scholar]
- 14.Miller TP, Reeves MJ. Lack of cost-effectiveness of omalizumab. J Allergy Clin Immunol. 2005;115:429–31. doi: 10.1016/j.jaci.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 15.Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114:265–9. doi: 10.1016/j.jaci.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 16.Brown R, Turk F, Dale P, Bousquet J. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy. 2007;62:149–53. doi: 10.1111/j.1398-9995.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 17.Asche CV, Brixner DI, Oderda GM. Has the cost-effectiveness of Xolair (omalizumab) been underestimated? J Allergy Clin Immunol. 2005;115:1095–6. doi: 10.1016/j.jaci.2004.12.1136. [DOI] [PubMed] [Google Scholar]
- 18.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 19.Fuhlbrigge AL, Bae SJ, Weiss ST, Kuntz KM, Paltiel AD. Cost-effectiveness of inhaled steroids in asthma: impact of effect on bone mineral density. J Allergy Clin Immunol. 2006;117:359–66. doi: 10.1016/j.jaci.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Paltiel AD, Fuhlbrigge AL, Kitch BT, Liljas B, Weiss ST, Neumann, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. J Allergy Clin Immunol. 2001;108:39–46. doi: 10.1067/mai.2001.116289. [DOI] [PubMed] [Google Scholar]
- 21.United States Bureau of Labor Statistics . Consumer Price Index (CPI) detailed report. US Department of Labor; Washington (DC): 2006. [Google Scholar]
- 22.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3:419–58. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 23.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 24.Adcock IM, Ito K. Steroid resistance in asthma: a major problem requiring novel solutions or a non-issue? Curr Opin Pharmacol. 2004;4:257–62. doi: 10.1016/j.coph.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Walker S, Monteil M, Phelan K, Lasserson TJ, Walters EH. Anti-IgE for chronic asthma in adults and children. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003559.pub3. CD003559. [DOI] [PubMed] [Google Scholar]
- 26.Corren J, Casale T, Deniz Y, Ashby M. Omalizumab, a recombinant humanized anti-IgE antibody, reduces asthma-related emergency room visits and hospitalizations in patients with allergic asthma. J Allergy Clin Immunol. 2003;111:87–90. doi: 10.1067/mai.2003.49. [DOI] [PubMed] [Google Scholar]
- 27.Drug topics red book. Medical Economics Company; Montvale (NJ): 2005. [Google Scholar]
- 28.Niebauer K, Dewilde S, Fox-Rushby J, Revicki DA. Impact of omalizumab on quality-of-life outcomes in patients with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol. 2006;96:316–26. doi: 10.1016/S1081-1206(10)61242-2. [DOI] [PubMed] [Google Scholar]
- 29.Serra-Batlles J, Plaza V, Morejon E, Comella A, Brugues J. Costs of asthma according to the degree of severity. Eur Respir J. 1998;12:1322–6. doi: 10.1183/09031936.98.12061322. [DOI] [PubMed] [Google Scholar]
- 30.Cisternas MG, Blanc PD, Yen IH, Katz PP, Earnest G, Eisner MD, et al. A comprehensive study of the direct and indirect costs of adult asthma. J Allergy Clin Immunol. 2003;111:1212–8. doi: 10.1067/mai.2003.1449. [DOI] [PubMed] [Google Scholar]
- 31.Stanford R, McLaughlin T, Okamoto LJ. The cost of asthma in the emergency department and hospital. Am J Respir Crit Care Med. 1999;160:211–5. doi: 10.1164/ajrccm.160.1.9811040. [DOI] [PubMed] [Google Scholar]
- 32.Lewis CE, Rachelefsky G, Lewis MA, de la Sota A, Kaplan M. A randomized trial of A.C.T. (asthma care training) for kids. Pediatrics. 1984;74:478–86. [PubMed] [Google Scholar]
- 33.Ross RN, Morris M, Sakowitz SR, Berman BA. Cost-effectiveness of including cromolyn sodium in the treatment program for asthma: a retrospective, record-based study. Clin Ther. 1988;10:188–203. [PubMed] [Google Scholar]
- 34.Weiss KB, Gergen PJ, Hodgson TA. An economic evaluation of asthma in the United States. N Engl J Med. 1992;326:862–6. doi: 10.1056/NEJM199203263261304. [DOI] [PubMed] [Google Scholar]
- 35.Coventry JA, Weston MS, Collins PM. Emergency room encounters of pediatric patients with asthma: cost comparisons with other treatment settings. J Ambul Care Manage. 1996;19:9–21. doi: 10.1097/00004479-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Segal R, Ried LD, Mackowiak J. Cost of asthma illnesses: emergency department visits without admission. Pharm Pract Manag Q. 1995;15:72–82. [PubMed] [Google Scholar]
- 37.Rutten-van Molken MP, Van Doorslaer EK, Jansen MC, Kerstjens HA, Rutten FF. Costs and effects of inhaled corticosteroids and bronchodilators in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;151:975–82. doi: 10.1164/ajrccm.151.4.7697275. [DOI] [PubMed] [Google Scholar]
- 38.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment: the multiattribute Asthma Symptom Utility Index. Chest. 1998;114:998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 39.Moy ML, Fuhlbrigge AL, Blumenschein K, Chapman RH, Zillich AJ, Kuntz KM, et al. Association between preference-based health-related quality of life and asthma severity. Ann Allergy Asthma Immunol. 2004;92:329–34. doi: 10.1016/S1081-1206(10)61570-0. [DOI] [PubMed] [Google Scholar]
- 40.Torrance GW, Feeny DH, Furlong WJ, Barr RD, Zhang Y, Wang Q. Multiattribute utility function for a comprehensive health status classification system. Health Utilities Index Mark 2. Med Care. 1996;34:702–22. doi: 10.1097/00005650-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Omalizumab (Xolair): an anti-IgE antibody for asthma. Med Lett Drugs Ther. 2003;45:67–8. [PubMed] [Google Scholar]
- 42.Revicki DA. Relationship between health utility and psychometric health status measures. Med Care. 1992;30(suppl):MS274–82. doi: 10.1097/00005650-199205001-00027. [DOI] [PubMed] [Google Scholar]
- 43.Revicki DA, Kaplan RM. Relationship between psychometric and utility-based approaches to the measurement of health-related quality of life. Qual Life Res. 1993;2:477–87. doi: 10.1007/BF00422222. [DOI] [PubMed] [Google Scholar]
- 44.Molken MP, Van Doorslaer EK, Rutten FF. Economic appraisal of asthma and COPD care: a literature review 1980-1991. Soc Sci Med. 1992;35:161–75. doi: 10.1016/0277-9536(92)90163-k. [DOI] [PubMed] [Google Scholar]
- 45.Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–28. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- 46.Ayres JG, Higgins B, Chilvers ER, Ayre G, Blogg M, Fox H. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with poorly controlled (moderate-to-severe) allergic asthma. Allergy. 2004;59:701–8. doi: 10.1111/j.1398-9995.2004.00533.x. [DOI] [PubMed] [Google Scholar]
- 47.Weeks JC, Tierney MR, Weinstein MC. Cost effectiveness of prophylactic intravenous immune globulin in chronic lymphocytic leukemia. N Engl J Med. 1991;325:81–6. doi: 10.1056/NEJM199107113250202. [DOI] [PubMed] [Google Scholar]
- 48.Liberato NL, Quaglini S, Barosi G. Cost-effectiveness of interferon alfa in chronic myelogenous leukemia. J Clin Oncol. 1997;15:2673–82. doi: 10.1200/JCO.1997.15.7.2673. [DOI] [PubMed] [Google Scholar]
- 49.Lee SJ, Anasetti C, Kuntz KM, Patten J, Antin JH, Weeks JC. The costs and cost-effectiveness of unrelated donor bone marrow transplantation for chronic phase chronic myelogenous leukemia. Blood. 1998;92:4047–52. [PubMed] [Google Scholar]
- 50.Earle CC, Chapman RH, Baker CS, Bell CM, Stone PW, Sandberg EA, et al. Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18:3302–17. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]