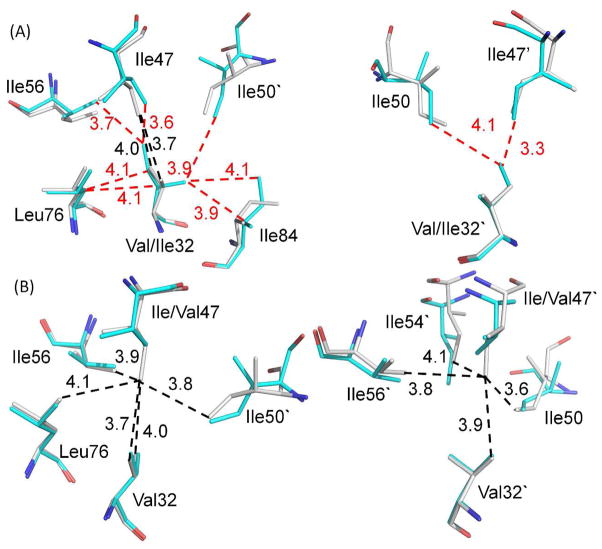
Figure 2.
The mutations alter internal hydrophobic contacts. Residues are shown from superimposed structures of PRWT with A) PRV32I and B) PRI47V. The two subunits are shown in the left and right panels, respectively. The PRWT residues are colored gray for carbon atoms, while the mutants are colored cyan. The van der Waals interactions are indicated by dashed lines in black for PRWT and in red for the mutants with interatomic distances in Å. The mutation of I47V to a smaller side chain in PRI47V eliminates hydrophobic contacts seen for Ile47 in PRWT. The opposite effect occurs with substitution of the large side chain in PRV32I.