Abstract
Background
The epidemiology of high-risk (hr) HPV infections in mid-adult women with new sex partners is undefined.
Methods
We analyzed baseline data from 518 25–65 year old female online daters. Women were mailed questionnaires and kits for self-collecting vaginal specimens for PCR-based hrHPV testing. Risk factors for infection were identified using Poisson regression models to obtain prevalence ratios (PRs).
Results
The prevalence of hrHPV infection was 35.9%. In multivariate analysis restricted to sexually active women, the likelihood of hrHPV infection was associated with abnormal Pap test history (PR=1.42, 95% CI:1.10–1.84), lifetime number of sex partners >14 (relative to 1–4; PR=2.13, 95% CI:1.13–4.02 for 15–24 partners and PR=1.91, 95% CI:1.00–3.64 for ≥25 partners), male partners with ≥1 concurrent partnership (PR=1.34, 95% CI:1.05–1.71) and male partners whom the subject met online (PR=1.39, 95% CI:1.08–1.79). Age was inversely associated with infection only in women who were sexually inactive (PR=0.67 per 5-year age difference, adjusted for Pap history and lifetime number of partners). Compared to sexually inactive women, the likelihood of infection increased with increasing risk level, (from low-risk to high-risk partners) (p<.0001 by trend test). In multivariate analysis, infection with multiple versus single hrHPV types was inversely associated with ever having been pregnant (PR=0.64, 95% CI:0.46–0.90) and recent consistent condom use (PR=0.56, 95% CI:0.32–0.97), and positively associated with genital wart history (PR=1.43, 95% CI:1.03–1.99).
Conclusions
Measures of both cumulative and recent sexual history were associated with prevalent hrHPV infection in this high-risk cohort of mid-adult women.
Keywords: HPV, human papilloma virus, mid-adult, prevalence, risk factors
INTRODUCTION
While much is now known about the acquisition and natural history of human papillomavirus (HPV) infections in sexually active young women, less is known about HPV infections in mid-adult women.1 In particular, the risk of acquiring new high-risk (hr) infections from new sex partners is undefined and the frequency of re-activating infections that were acquired in adolescence or young adulthood is unknown. Whether mid-adult women are susceptible to acquiring new hrHPV infections from new sex partners has important implications for prophylactic vaccine implementation guidelines in mid-adult women. Prophylactic HPV vaccines are not currently licensed for women >26 years of age in the U.S.2 It is debatable whether it would be beneficial or cost-effective to vaccinate mid-adult women, or if there are certain high-risk groups that might be targeted for vaccination.2–4
Reflective of the marital status of the general population, the majority of mid-adult female participants in HPV studies have been married or in long-term monogamous relationships with male partners. To our knowledge, no studies of HPV infections have specifically targeted mid-adult women with recent new sex partners. In this study, we specifically recruited 25–65 year old U.S. women who reported recent use of online dating websites (i.e., women likely to report recent new sex partners), targeting women past the recommended age for ‘catch-up’ vaccination through the recommended age for cervical cancer screening cessation. For one year, women were longitudinally followed with tri-annual at-home vaginal self-collections for hrHPV testing and detailed health and sexual behavior questionnaires. In this report, we present the baseline prevalence and predictors of hrHPV infections in this high-risk cohort of mid-adult women.
METHODS
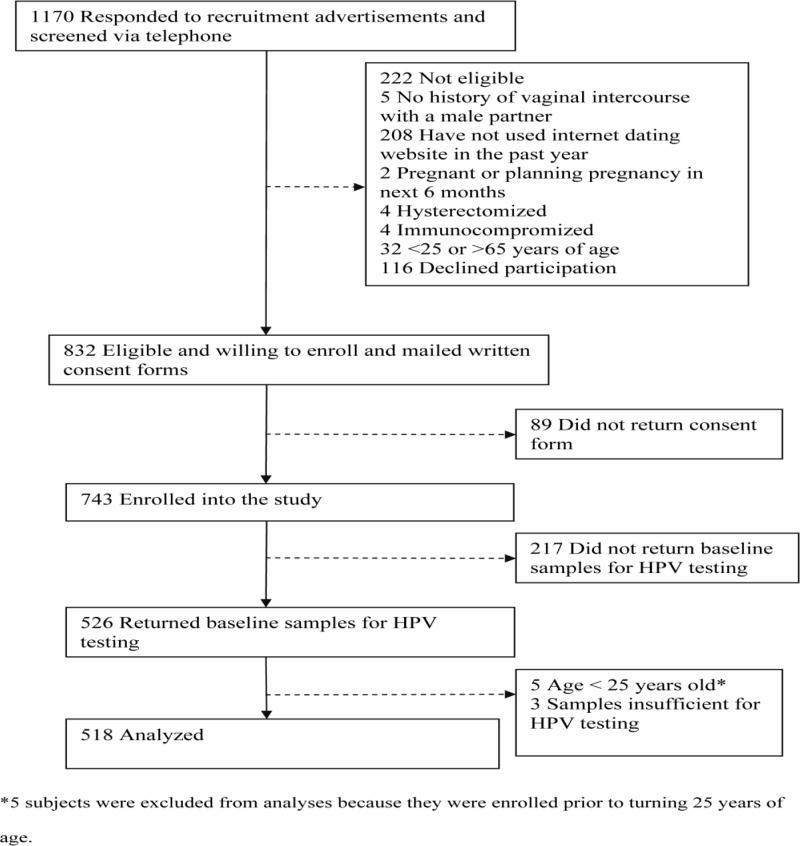
Between 2007–2010, 25–65 year old women were recruited through the Internet and enrolled into a longitudinal study of genital HPV infections. Recruitment advertisements were posted primarily on Craigslist.org (a community-based website offering free classified advertisements in over 700 cities worldwide). Advertisements were posted weekly in the “volunteer opportunities” section of one city on a rotating basis (37 major U.S. cities were targeted). Advertisements were also posted on University of Washington research volunteer recruitment websites and other national websites offering free classified advertisements. Advertisements targeted 25–65 year old women who reported using the Internet to search for romantic partners within the previous 12 months. Women who were pregnant or breastfeeding or planning a pregnancy in the next 6 months, hysterectomized, immunocompromized, or reported no history of sex with men were not eligible to participate. 1170 women responding to the recruitment advertisements were screened over the telephone, of whom 832 (71.1%) women were eligible. 743 (63.5% of women screened) returned a mailed consent form and were enrolled. (Figure 1) The protocol was reviewed and approved by the University of Washington Institutional Review Board.
Figure 1.
Enrollment and status of subjects.
Every four months for up to one year, enrolled women were mailed demographic, sexual behavior, and health history questionnaires and materials for self-collecting vaginal samples for HPV DNA testing. Self-collection materials included illustrated instructions, two Dacron-tipped swabs (two sequential self-collected swabs increases sensitivity for HPV detection5), a capped tube containing 1.5 ml of Specimen Transport Medium (Qiagen, Gaithersburg, MD), nitrile gloves, and packing and shipping materials. The kit also contained written instructions to refrain from douching, vaginal intercourse, and the use of vaginal medications or preparations for 48 hours prior to collecting the vaginal samples, and to collect the samples at least two days after the end of the last menses. Women were instructed to complete the questionnaire and collect the samples on the same day, and to return the materials in the provided pre-paid standard overnight Federal Express envelope.
Vaginal samples were tested for HPV DNA using polymerase chain reaction (PCR)-based methods. Exfoliated cell samples were digested with 20 μg/ml protease K at 37°C for one hour. DNA was isolated using the QIAamp DNA blood mini column (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol. Two μl purified DNA (equivalent to one 250th of each sample) was amplified in 50 μl PCR reaction and 10 μL of PCR products were dotted onto nylon filters and probed with both a biotin-labeled HPV generic probe and a biotin-labeled β-globin probe. Samples negative for β-globin DNA were deemed insufficient. Specimens determined to be HPV positive by generic probe were typed using the Roche Linear Array HPV genotyping test (Roche Molecular Systems, Inc., Alameda, CA) for 37 HPV types.
The current analyses focused on the baseline visit only. We evaluated risk factors for prevalent hrHPV infection (positive for any of the following types classified as carcinogenic, probably carcinogenic, or possibly carcinogenic): 16/18/26/31/33/35/39/45/51/52/53/56/58/59/66/68/73/82/IS396, 7) using Poisson regression models with robust variance estimates to obtain prevalence ratios (PRs). Potential risk factors included demographics, smoking history, women’s health history (e.g. pregnancy history, history of an abnormal Pap test or genital warts, menopausal status, and hormone use) and cumulative and recent sexual behaviors. Age was divided by 5 and included in the model as a continuous variable to reflect the relative change in the likelihood of hrHPV detection associated with a 5-year difference in age (we evaluated a linear trend using a likelihood ratio test to compare a categorical variable based on 5-year age groups to the linear model based on age divided by 5; the likelihood ratio test was not significant, indicating a linear trend). Cumulative sexual behavior variables included age at first intercourse with a male partner and lifetime number of male sex partners. Recent sexual behavior variables included characteristics of male sex partners and partnerships in the past 6 months. If multiple partners were reported in the past 6 months, the characteristic was summarized across partners/partnerships. (Table 3) Variables found to be statistically significant (p<.10) in univariate analyses were entered into multivariate models. Forward stepwise regression was used to construct the final multivariate models, with p<.10 used as the criterion for entering and removing variables. Because the final multivariate model included partner/partnership variables restricted to women who reported sex with male partners in the past 6 months, women without recent male sex partners were excluded. While effect modification by recent sexual activity was not formally explored (due to a limited number of women reporting no male sex partners in the past 6 months; n=87), we compared the final multivariate model to a second multivariate model that excluded recent partner/partnership variables to explore whether the magnitude of association between hrHPV infection and any other variables in the model differed between recently sexually active women and the full dataset of women who were and were not recently sexually active.
TABLE 3.
Prevalence Ratios (PR) for the Associations Between Selected Risk Factors and High-Risk (hr) HPV Infection in 25- to 65-Year-Old Women Who Date Online (n = 518)
| Risk Factor | Total | hrHPV Positive (%) | Univariate PR (95% CI) | Adjusted PR* (95% CI) |
|---|---|---|---|---|
| Age5† | 518 | 186 (35.9) | 0.94 (0.88 – 1.00) | |
| Race | ||||
| White | 330 | 119 (36.1) | 1.00 | |
| African American | 85 | 31 (36.5) | 1.01 (0.74 – 1.39) | |
| Asian | 38 | 14 (36.8) | 1.02 (0.66 – 1.59) | |
| Other | 59 | 19 (32.2) | 0.89 (0.60 – 1.33) | |
| Hispanic ethnicity | ||||
| No | 451 | 162 (35.9) | 1.00 | |
| Yes | 52 | 19 (36.5) | 1.02 (0.70 – 1.49) | |
| Highest level of education completed | ||||
| High school diploma or less | 33 | 11 (33.3) | 1.00 | |
| Some postsecondary education | 180 | 61 (33.9) | 1.02 (0.60 – 1.72) | |
| Bachelor’s degree or higher | 301 | 110 (36.5) | 1.10 (0.66 – 1.82) | |
| Annual household income | ||||
| <$35,000 | 181 | 68 (37.6) | 1.00 | |
| $35,000–$49,999 | 124 | 46 (37.1) | 0.99 (0.73 – 1.33) | |
| ≥$50,000 | 189 | 63 (33.3) | 0.89 (0.67 – 1.17) | |
| Geographic region | ||||
| Midwest | 80 | 24 (30.0) | 1.00 | |
| Northeast | 123 | 38 (30.9) | 1.03 (0.67 – 1.58) | |
| South | 110 | 43 (39.1) | 1.30 (0.87 – 1.96) | |
| West | 203 | 79 (38.9) | 1.30 (0.89 – 1.89) | |
| Urbanization‡ | ||||
| Urban | 495 | 178 (36.0) | 1.00 | |
| Rural | 18 | 6 (33.3) | 0.93 (0.48 – 1.80) | |
| Ever married | ||||
| No | 321 | 123 (38.3) | 1.00 | |
| Yes | 196 | 63 (32.1) | 0.84 (0.66 – 1.07) | |
| Ever divorced | ||||
| No | 388 | 139 (35.8) | 1.00 | |
| Yes | 129 | 47 (36.4) | 1.02 (0.78 – 1.32) | |
| Current marital status | ||||
| Married | 62 | 17 (27.4) | 1.00 | |
| Unmarried, living with partner | 66 | 23 (34.9) | 1.27 (0.75 – 2.14) | |
| Unmarried | 365 | 142 (38.9) | 1.42 (0.93 – 2.17) | |
| Separated | 23 | 4 (17.4) | 0.63 (0.24 – 1.69) | |
| Smoking status | ||||
| Never | 327 | 113 (34.6) | 1.00 | |
| Former | 114 | 42 (36.8) | 1.07 (0.80 – 1.42) | |
| Current | 77 | 31 (40.3) | 1.17 (0.85 – 1.59) | |
| Ever pregnant | ||||
| No | 220 | 81 (36.8) | 1.00 | |
| Yes | 296 | 105 (35.5) | 0.96 (0.76 – 1.21) | |
| Current hormonal contraceptive user | ||||
| No | 402 | 141 (35.1) | 1.00 | |
| Yes | 96 | 37 (38.5) | 1.10 (0.83 – 1.46) | |
| Menopausal status§ | ||||
| Premenopausal | 46 | 12 (26.1) | 1.00 | |
| Perimenopausal/menopausal/post-menopausal | 52 | 16 (30.8) | 1.18 (0.62 – 2.23) | |
| History of an abnormal Pap test | ||||
| No | 270 | 73 (27.0) | 1.00 | 1.00 |
| Yes | 232 | 107 (46.1) | 1.71 (1.34 – 2.17) | 1.42 (1.10 – 1.84) |
| Ever had a procedure after an abnormal Pap test¶ | ||||
| Never had abnormal Pap | 270 | 73 (27.0) | 1.00 | |
| No | 62 | 29 (46.8) | 1.73 (1.24 – 2.41) | |
| Yes | 170 | 78 (45.9) | 1.70 (1.31 – 2.19) | |
| History of genital warts | ||||
| No | 433 | 151 (34.9) | 1.00 | |
| Yes | 76 | 34 (44.7) | 1.28 (0.97 – 1.70) | |
| Age at first intercourse (yr)|| | ||||
| ≤15 | 132 | 47 (35.6) | 1.00 | |
| 16 | 75 | 22 (29.3) | 0.82 (0.54 – 1.25) | |
| 17 | 87 | 31 (35.6) | 1.00 (0.70 – 1.44) | |
| 18–19 | 116 | 45 (38.8) | 1.09 (0.79 – 1.51) | |
| ≥20 | 104 | 39 (37.5) | 1.05 (0.75 – 1.48) | |
| Lifetime no. male sex partners|| | ||||
| 1–4 | 82 | 16 (19.5) | 1.00 | 1.00 |
| 5–8 | 105 | 39 (37.1) | 1.90 (1.15 – 3.16) | 1.77 (0.92 – 3.41) |
| 9–14 | 108 | 34 (31.5) | 1.61 (0.96 – 2.72) | 1.75 (0.92 – 3.35) |
| 15–24 | 96 | 44 (45.8) | 2.35 (1.44 – 3.84) | 2.13 (1.13 – 4.02) |
| ≥25 | 106 | 44 (41.5) | 2.13 (1.30 – 3.49) | 1.91 (1.00 – 3.64) |
| Sexual behaviors in the past 6 mo | ||||
| Time since most recent sex with a male partner | ||||
| <2 d | 114 | 47 (41.2) | 1.00 | |
| ≥2 d | 375 | 132 (35.2) | 0.85 (0.66 – 1.11) | |
| Sex with a male partner | ||||
| No | 87 | 20 (23.0) | 1.00 | |
| Yes | 431 | 166 (38.5) | 1.68 (1.12 – 2.51) | |
| New male sex partner**†† | ||||
| No | 190 | 73 (38.4) | 1.00 | |
| Yes | 241 | 93 (38.6) | 1.00 (0.79 – 1.28) | |
| Casual male sex partner**†† | ||||
| No | 248 | 83 (33.5) | 1.00 | |
| Yes | 177 | 80(45.2) | 1.35 (1.06 – 1.72) | |
| ≥2 concurrent male sex partners**†† | ||||
| No | 338 | 123 (36.4) | 1.00 | |
| Yes | 71 | 32 (45.1) | 1.24 (0.92 – 1.66) | |
| Younger male sex partner**†† | ||||
| No | 264 | 95 (36.0) | 1.00 | |
| Yes | 161 | 70 (43.5) | 1.21 (0.95 – 1.53) | |
| Male sex partner with ≥1 concurrent partnership**†† | ||||
| No or unknown | 253 | 84 (33.2) | 1.00 | 1.00 |
| Yes | 161 | 76 (47.2) | 1.42 (1.12 – 1.81) | 1.34 (1.05 – 1.71) |
| Male sex partner whom the subject met online**†† | ||||
| No | 213 | 69 (32.4) | 1.00 | 1.00 |
| Yes | 215 | 95 (44.2) | 1.36 (1.07 – 1.74) | 1.39 (1.08 – 1.79) |
| Condom use with male sex partners**‡‡ | ||||
| Always | 95 | 35 (36.8) | 1.00 | |
| Not always | 333 | 130 (39.0) | 1.06 (0.79 – 1.42) | |
| Circumcision status of male sex partners**§§ | ||||
| Uncircumcised or unknown | 107 | 45 (42.1) | 1.00 | |
| Circumcised | 310 | 118 (38.1) | 0.91 (0.69 – 1.18) |
The final multivariate model was restricted to women who reported sex with male partners in the past 6 months and had nonmissing data for all included variables (n = 383). The following variables were included: history of an abnormal Pap test, lifetime number of male sex partners, report of a recent male sex partner with ≥1 concurrent partnership, and report of a recent male sex partner whom the subject met online.
Age divided by 5. The relative risk estimate reflects the relative change in the likelihood of hrHPV detection associated with a 5-year difference in age. Example: A 30-year-old woman was 6% less likely to have hrHPV detected than a 25-year-old woman.
Based on zip codes.
Restricted to women ≥45 years of age.
Procedures included colposcopy, biopsy, or treatment of a cervical lesion.
Approximate quintiles.
Restricted to women reporting sex with a male partner in the past 6 months.
The variable was coded as “yes” if at least 1 partner/partnership during the past 6 months fit the characteristic of interest.
The variable was coded as “always” if the subject reported always using condoms with all male partners during the past 6 months. If a subject reported not always using condoms with at least 1 male partner, the variable was coded as “not always.” If condom use data were missing for at least 1 partner, the variable was set to missing.
If the circumcision status of 1 partner was reported as uncircumcised or unknown, the variable was coded as “uncircumcised or unknown.” If all partners were reported as circumcised, the variable was coded as “circumcised.”
HPV indicates human papillomavirus; CI, confidence interval.
Separately, we evaluated a composite variable constructed to characterize additive effects of partner/partnership risk factors on the likelihood of hrHPV detection. Partner or partnership characteristics found to be significantly associated (p<.10) with hrHPV detection in the univariate analyses described above were selected as risk factors and incorporated into this variable (3 risk factors were selected: report of ≥1 casual male sex partner, report of ≥1 male sex partner with a concurrent partnership, and sex with ≥1 male partner whom the subject met online). The composite variable was categorized as a five-level variable: (1) not sexually active with a male partner in the past 6 months; or, sexually active with male partner(s) who (2) did not have any identified risk factors, had (3) 1 risk factor, (4) 2 risk factors, (5) or all 3 risk factors. We evaluated potential confounding by variables that were associated with hrHPV detection in the univariate analyses described above (excluding individual sexual behavior variables over the past 6 months). Each variable was tested individually with the composite variable; those that changed any of the composite variable point estimates by >10% were considered confounders and retained in the final model.
Finally, among women testing positive for hrHPV, we evaluated whether any of the variables described above were predictive of testing positive for multiple hr types versus a single hr type. All variables were tested univariately, and forward stepwise regression was used to construct one final multivariate model, with p<.10 used as the criterion for entering and removing variables.
Most analyses were pre-specified; we explicitly note post-hoc analyses.
RESULTS
526 of 743 (70.8%) enrolled subjects returned their first self-sampling kit and questionnaire. Five were enrolled prior to turning 25 years of age and were excluded from all analyses. Three (0.6% of 521) samples were insufficient for HPV testing. (Figure 1) The mean age of the remaining 518 subjects at the time of first sample collection was 35.7 (SD,9.5) years, and the median reported lifetime number of sex partners was 11 (range 1–300). Other demographic, health, and sexual behavior characteristics are described in Table 1.
TABLE 1.
Demographic, Health, and Sexual History Characteristics of 25- to 65-Year-Old Women Who Date Online (n = 518)
| Characteristic | n* (%) |
|---|---|
| Age (yr) | |
| 25–29 | 199 (38.4) |
| 30–34 | 96 (18.5) |
| 35–39 | 77 (14.9) |
| 40–44 | 46 (8.9) |
| 45–49 | 45 (8.7) |
| 50–54 | 31 (6.0) |
| 55–65 | 24 (4.6) |
| Race | |
| White | 330 (64.4) |
| African American | 85 (16.6) |
| Asian | 38 (7.4) |
| American Indian/Alaska Native | 5 (1.0) |
| Other | 54 (10.6) |
| Hispanic ethnicity | |
| Yes | 52 (10.3) |
| No | 451 (89.7) |
| Geographic region | |
| Midwest | 80 (15.5) |
| Northeast | 123 (23.9) |
| South | 110 (21.3) |
| West | 203 (39.4) |
| Urbanization† | |
| Urban | 495 (96.5) |
| Rural | 18 (3.5) |
| Highest level of education completed | |
| High school diploma or less | 33 (6.4) |
| Some postsecondary education | 180 (35.0) |
| Bachelor’s degree or higher | 301 (58.6) |
| Annual household income | |
| <$35,000 | 181 (36.6) |
| $35,000–$49,999 | 124 (25.1) |
| ≥$50,000 | 189 (38.3) |
| Ever married | |
| Yes | 196 (37.9) |
| No | 321 (62.1) |
| Ever divorced | |
| Yes | 129 (25.0) |
| No | 388 (75.1) |
| Current marital status | |
| Unmarried | 365 (70.7) |
| Unmarried, living with partner | 66 (12.8) |
| Married | 62 (12.0) |
| Separated | 23 (4.5) |
| Current smoker | |
| Yes | 77 (15.2) |
| No | 431 (84.8) |
| Ever pregnant | |
| Yes | 296 (57.4) |
| No | 220 (42.6) |
| Current hormonal contraceptive user | |
| Yes | 96 (19.3) |
| No | 402 (80.7) |
| History of an abnormal Papanicolaou test result | |
| Yes | 232 (46.2) |
| No | 270 (53.8) |
| History of genital warts | |
| Yes | 76 (14.9) |
| No | 433 (85.1) |
| Age at first intercourse‡ | |
| ≤15 | 132 (25.7) |
| 16 | 75 (14.6) |
| 17 | 87 (17.0) |
| 18–19 | 115 (22.4) |
| ≥20 | 104 (20.3) |
| Lifetime no. male sex partners‡ | |
| 1–4 | 82 (16.5) |
| 5–8 | 105 (21.1) |
| 9–14 | 108 (21.7) |
| 15–24 | 96 (19.3) |
| ≥25 | 106 (21.3) |
Numbers may not add up to totals due to missing data.
Based on zip code.
Approximate quintiles.
The prevalence of high-risk HPV was 35.9%. 41.4% of all high-risk positive samples were positive for multiple high-risk HPV types (range 1–7 types), representing 14.9% of all samples tested. The most common high-risk HPV type detected was HPV-16 (Table 2).
TABLE 2.
Prevalence of Type-Specific High-Risk (hr) HPV in Self-Collected Vaginal Samples Collected From Women Aged 25 to 65 Years Who Date Online (n = 518)
| HPV Type | n (%) |
|---|---|
| Any hr | 186 (35.9) |
| 16 | 42 (8.1) |
| 18 | 25 (4.8) |
| 26 | 3 (0.6) |
| 31 | 13 (2.5) |
| 33 | 5 (1.0) |
| 35 | 12 (2.3) |
| 39 | 21 (4.1) |
| 45 | 10 (1.9) |
| 51 | 28 (5.4) |
| 52 | 27 (5.2) |
| 53 | 34 (6.6) |
| 56 | 14 (2.7) |
| 58 | 18 (3.5) |
| 59 | 20 (3.9) |
| 66 | 25 (4.8) |
| 68 | 12 (2.3) |
| 73 | 10 (1.9) |
| 82 | 2 (0.4) |
| IS39* | 2 (0.4) |
IS39 is now HPV82.
HPV indicates human papillomavirus.
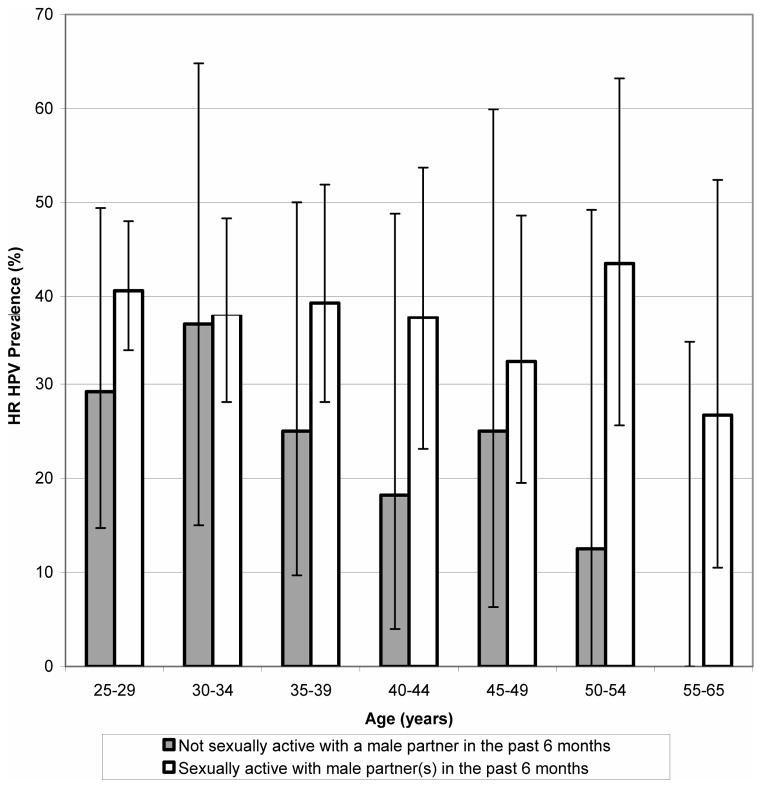
In univariate analyses, increasing age was negatively associated with hrHPV positivity. History of an abnormal Pap test, having ever had a follow-up test or procedure after an abnormal Pap test result, and history of genital warts were all positively associated with hrHPV infection. (Compared to never having had an abnormal Pap test, the PR for having ever had a follow-up test or procedure after an abnormal Pap test (e.g. colposcopy, biopsy, or treatment for a cervical lesion) was similar to the PR for never having had a procedure; therefore, the latter was not entered into the multivariate model.) Lifetime number of sex partners was categorized into approximate quintiles a priori; women reporting a lifetime number of sex partners >4 were more likely to be positive than those reporting ≤4 partners. Women reporting having had sex with a male partner in the past 6 months were more likely to be hrHPV positive than women who were not sexually active. Among those who were sexually active in the past 6 months, male partner characteristics associated with an increased likelihood of positivity included casual partners, partners reported to have ≥1 concurrent partnership, and partners whom the subject met online. In the final multivariate model (restricted to women who were sexually active in the past 6 months), a history of an abnormal Pap test (PR=1.42,95%CI:1.10–1.84) and a lifetime number of sex partners >14 (relative to 1–4 partners) (PR for 15–24 partners=2.13, 95%CI:1.13–4.02 and PR for >24 partners=1.91, 95% CI:1.00–3.64) were both positively associated with hrHPV infection. In addition, reports of partners with ≥1 concurrent partnership (PR=1.34,95%CI:1.05–1.71) and partners whom the subject met online (PR=1.39,95%CI:1.08–1.79) were both positively associated with hrHPV infection. (Table 3) When we excluded partner/partnership variables from the multivariate model (expanding the dataset to include women reporting no male sex partners in the past 6 months), increasing age was statistically significantly negatively associated with hrHPV positivity (PR=0.92,95%CI:0.86–0.99 for each 5-year age difference, adjusting for history of an abnormal Pap test and lifetime number of sex partners). (Risk estimates for the other variables evaluated in multivariate analyses were similar between the restricted and full models.) We then stratified the risk estimates for the relationship between age and hrHPV infection by recent sexual activity (a post hoc analysis) (Figure 2); a statistically significant relationship between age and hrHPV positivity was observed among women reporting no male sex partners in the past 6 months (adjusted PR=0.67,95%CI:0.49–0.91 for each 5-year age difference), but not among women who were sexually active in the past 6 months (adjusted PR=0.96,95%CI: 0.90–1.03 for each 5-year age difference).
Figure 2.
Age-specific high-risk (hr) HPV prevalence, by sexual activity in the 6 months prior to enrollment. 87 women reported no sexual activity with male partners in the past 6 months, and 431 women reported sexual activity with one or more male partners. 95% confidence intervals for hrHPV prevalence are represented by error bars. No hrHPV infections were detected in 9 women aged 55 to 65 years who reported no sexual activity with male partners in the past 6 months.
Compared to women who were not sexually active, those who reported sex with male partners with ≥1 identified risk factor (including partners who were casual, reported to have ≥1 concurrent partnership, and partners whom the subject met online) were more likely to be hrHPV positive. (Table 4) A linear categorical dose-response effect was observed, as the likelihood of hrHPV positivity increased with increasing level of risk (from no recent sexual activity to partners with all three identified risk factors) (p<.0001 by the test for trend). No confounding by any non-partner/partnership variables was identified.
TABLE 4.
Prevalence Ratios (PR) for the Association Between Additive Partner or Partnership Risk Factors and High-Risk (hr) HPV Infection in 25- to 65-Year-Old Women Who Date Online (n = 518)
| Composite Variable for Additive Partner or Partnership Risk Factors*† | Total | hrHPV Positive (%) | PR (95% CI) |
|---|---|---|---|
| Not sexually active with a male partner in the past 6 mo | 87 | 20 (23.0) | 1.00 |
| Sexually active with male partner(s) in the past 6 mo and No risk factors | 110 | 31 (28.2) | 1.23 (0.75 – 2.00) |
| 1 risk factor | 149 | 55 (36.9) | 1.61 (1.04 – 2.49) |
| 2 risk factors | 112 | 44 (39.3) | 1.71 (1.09 – 2.68) |
| 3 risk factors | 60 | 36 (60.0) | 2.61 (1.69 – 4.04) |
Risk factors included report of ≥1 casual male sex partner, report of ≥1 male sex partner with a concurrent partnership, and sex with ≥1 male partner whom the subject met online. For example, the composite variable was coded as “sexually active with male partner(s) in the past 6 mo and 2 risk factors” if a subject reported 1 casual partner whom the subject met online or if a subject reported 1 partner who was casual and another partner whom the subject met online.
The likelihood of hrHPV positivity increased with increasing level of risk, from no recent sexual activity to partners with all 3 identified risk factors (P < 0.0001 by the test for trend).
HPV indicates human papillomavirus; CI, confidence interval.
Among women who were hrHPV positive, women who reported having ever been pregnant (PR=0.64,95%CI:0.46–0.90, adjusted for recent condom use and genital wart history) and women reporting having always used condoms with all male partners in the past 6 months (PR=0.56,95%CI:0.32–0.97, adjusted for pregnancy and genital wart history) were less likely to test positive for multiple hrHPV types versus a single hrHPV type. Women with a history of genital warts were more likely to test positive for multiple hrHPV types (PR=1.43,95%CI:1.03–1.99, adjusted for pregnancy history and recent condom use). (Table 5) In a post hoc analysis, a negative linear dose response effect was observed between increasing number of births and likelihood of testing positive for multiple hrHPV types (p=.015 by the test for trend, adjusted for recent condom use and genital warts history).
TABLE 5.
Prevalence Ratios (PR) for the Associations Between Selected* Risk Factors and Multiple-Type (hr) HPV Infection in hrHPV-Positive 25- to 65-Year-Old Women Who Date Online (n = 186)
| Risk Factor | Total† | Multiple-Type hrHPV Positive (%) | Univariate PR (95% CI) | Adjusted‡ PR (95% CI) |
|---|---|---|---|---|
| Current marital status | ||||
| Married | 17 | 6 (35.3) | 1.00 | |
| Unmarried, living with partner | 23 | 14 (60.9) | 1.72 (0.84 – 3.56) | |
| Unmarried | 142 | 54 (38.0) | 1.08 (0.55 – 2.12) | |
| Separated | 4 | 3 (75.0) | 2.13 (0.90 – 5.02) | |
| Ever pregnant | ||||
| No | 81 | 42 (51.9) | 1.00 | 1.00 |
| Yes | 105 | 35 (33.3) | 0.64 (0.46 – 0.91) | 0.64 (0.46 – 0.90) |
| History of genital warts | ||||
| No | 151 | 57 (37.8) | 1.00 | 1.00 |
| Yes | 34 20 (58.8) | 1.56 (1.10–2.21) | 1.43 (1.03 – 1.99) | |
| ≥2 concurrent male sex partners§ | ||||
| No or unknown | 123 | 50 (40.7) | 1.00 | |
| Yes | 32 18 (56.3) | 1.38 (0.95–2.01) | ||
| Male sex partner with ≥1 concurrent partnership§ | ||||
| No or unknown | 84 | 31 (36.9) | 1.00 | |
| Yes | 76 | 39 (51.3) | 1.39 (0.97 – 1.99) | |
| Condom use with male sex partners§ | ||||
| Not always | 130 | 63 (48.5) | 1.00 | 1.00 |
| Always | 35 9 (25.7) | 0.53 (0.29–0.96) | 0.56 (0.32 – 0.97) |
Only risk factors that were statistically significant (P < 0.10) are included in the table.
Total number of hrHPV-positive women.
The final multivariate model was restricted to women who reported sex with male partners in the past 6 months and had nonmissing data for all included variables (n = 164). The following variables were included: ever pregnant, history of genital warts, and condom use with male sex partners.
Restricted to women reporting sex with a male partner in the past 6 months.
HPV indicates human papillomavirus; CI, confidence interval.
DISCUSSION
To our knowledge, this is the first study of HPV infections to specifically target mid-adult women with recent new sex partners (46.5% of women in this online dating cohort reported at least one new male sex partner in the past 6 months). The overall prevalence of hrHPV infection in this high-risk cohort of 25 to 65 year old women was 36%. Population-based data from the U.S. show a peak in hrHPV prevalence in the early 20s, followed by an age-related decline (in some non-U.S. populations, a second prevalence peak is observed in older women8). Among women self-sampling for HPV in the 2003–2006 population-based National Health and Nutrition Examination Survey (NHANES), hrHPV prevalence peaked in women aged 20–24 years (43%), and then declined with increasing age, ranging from 31% in 25–29 year old women down to 24% in those aged 50–59 years (compared to our study, the NHANES included four additional types classified by the International Agency for Research on Cancer as possibly carcinogenic: 64, 67, 69 and 70)9 In our cohort, the relationship between age and hrHPV prevalence varied by recent sexual activity with male partners. Whereas hrHPV prevalence declined with age in sexually inactive women, age was unrelated to hrHPV prevalence in sexually active women.
Measures of both cumulative (e.g. lifetime number of male sex partners and history of an abnormal Pap test) and recent (e.g. report of recent male sex partners with concurrent partnerships, recent casual male partners, and recent male partners whom the subject met online) risk behavior were associated with an increased likelihood of infection. A dose-response relationship was observed, whereby compared to sexually inactive women, the likelihood of hrHPV positivity increased with increasing level of risk, from low-risk partners to high-risk partners. Previous studies in average-risk populations of mid-adult women have also reported associations between HPV infection and recent markers of risk behavior (e.g. marital status10, 11, 12 and recent new partners11–16) in addition to cumulative risk behaviors (e.g. lifetime number of partners11, 14, 16). Self-reported sexual behavior can be challenging to interpret, as behaviors may correlate over time. While we measured some cumulative risk behaviors (e.g. lifetime number of partners), residual confounding by past high-risk sexual behavior may partially account for the observed associations between recent risk behavior and prevalent infection (e.g. women who choose riskier partners in young adulthood may also be more likely to choose riskier partners in mid-adulthood, and also more likely to harbor persistent or reactivated infections from young adulthood). This could explain our finding that report of recent high-risk partners (e.g. those with concurrent partners), but not recent new partners, was associated with HPV infection. Nonetheless, a role for new hrHPV acquisition in high-risk mid-adult women cannot be ruled out.
Women who reported recent sex with a male partner whom they met online were more likely to test positive for hrHPV (but not more or less likely to test positive for multiple versus single hrHPV types). The literature on the association between seeking sex partners via Internet dating or social networking websites and sexually transmitted infection (STI) risk has been mixed. Several studies have reported an increased likelihood of high-risk sexual behaviors associated with online sex-seeking or online dating,17–19 but studies linking online dating/sex-seeking to sexually transmitted infections (STIs) have been equivocal.20–22 One study aiming to correlate self-reported history of HPV infection with online sex-seeking behavior reported no association (in either men or women).21 To our knowledge, our study is the first to directly evaluate online dating in relation to current HPV infection, via HPV testing.
In our high-risk cohort of mid-adult women, coinfection with multiple hrHPV types was common (representing 41% of hrHPV infections) and unrelated to age. In contrast, studies in average-risk populations tend to report an inverse relationship between age and multiple type HPV infections.23–25 For reasons that are unclear, we identified risk factors for multiple versus single hr type infections that differed from those associated with testing hrHPV positive versus negative. Among hrHPV positive women, those reporting a history of genital warts were more likely to test positive for multiple hr types, whereas those reporting always using condoms with male partners in the past 6 months or past pregnancy were less likely to test positive for multiple hrHPV types (in a post hoc analysis, a linear dose response relationship was observed between number of births and likelihood of single type hrHPV infection). Parity and genital wart history may reflect cumulative lifetime sexual risk behavior. The inverse association between parity and multiple hr type infection is in agreement with previous reports linking increasing number of births to decreased likelihood of infection with multiple HPV types.26, 27 While consistent condom use has been shown to be protective against newly acquired HPV infection in newly sexually active young women, cross-sectional studies have been equivocal, likely due to the transient nature of HPV infections and difficulty in establishing a temporal relationship between condom use and HPV infection.28 While recent condom use was unsurprisingly unrelated to hrHPV positivity in this cohort of high-risk mid-adult women, the association with multiple versus single type infection is of interest. In a randomized trial, Hogewoning et al29 reported that condom use promoted regression of cervical intraepithelial neoplasia and clearance of HPV infection, and postulated that condom use might contribute to lowered HPV viral load and clearance by blocking repetitive transmission of shed HPV virus between partners. Applying this theory to our findings, condom use may have the effect of limiting the number of type-specific infections. With longitudinal data, we will be able to further test this theory by exploring the relationship between condom use and persistent type-specific detection.
Limitations to our study should be noted. Given the self-selected nature of our cohort, the results may not be generalizable to all mid-adult women who seek or have had recent new partners. Women reported higher lifetime numbers of male sex partners than reported in national surveys (for example, the median lifetime number of partners reported by 25 to 44 year old women in our study was 10 versus 3.6 in the 2006–2008 National Survey of Family Growth30). Furthermore, in this cross-sectional analysis, we were unable to distinguish between persistent versus newly acquired or reactivated infections. In future analyses, we will use longitudinal data from this cohort of high-risk mid-adult women to identify risk factors for newly detected and persistently detected infections. Such longitudinal data are critical for understanding the natural history of HPV infections in mid-adult women and elucidating the relative contribution of reactivation versus new acquisition associated with new sex partners. Additional studies are needed to further evaluate the carcinogenic progression of infections that are acquired in mid-adulthood. Information on new acquisition, persistence, and progression could be used to further inform decisions surrounding prophylactic HPV vaccination recommendations in mid-adult women.
Acknowledgments
Source of Funding: This work was supported by the Developmental Awards Program of the National Institutes of Health NIAID Sexually Transmitted Infections and Topical Microbicide Cooperative Research Centers (STI-TM CRC) grant to the University of Washington (AI 31448), and by a National Institutes of Health K01 grant (AI 079270) to RLW.
Footnotes
Conflicts of Interest: LAK has received honoraria from Roche Diagnostics and Dana-Farber Cancer Institute.
References
- 1.Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24 (Suppl 3):S52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Grant LA, Dunne EF, Chesson H, Markowitz LE. Considerations for human papillomavirus (HPV) vaccination of mid-adult women in the United States. Vaccine. 2011;29:2365–70. doi: 10.1016/j.vaccine.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Castellsague X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol Oncol. 2009;115:S15–23. doi: 10.1016/j.ygyno.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Kim JJ, Ortendahl J, Goldie SJ. Cost-effectiveness of human papillomavirus vaccination and cervical cancer screening in women older than 30 years in the United States. Ann Intern Med. 2009;151:538–45. doi: 10.7326/0003-4819-151-8-200910200-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harper DM, Longacre MR, Noll WW, Belloni DR, Cole BF. Factors affecting the detection rate of human papillomavirus. Ann Fam Med. 2003;1:221–7. doi: 10.1370/afm.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz N, Castellsague X, de Gonzalez AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine. 2006;24S3:S1–S10. doi: 10.1016/j.vaccine.2006.05.115. [DOI] [PubMed] [Google Scholar]
- 7.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 8.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 9.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204:566–73. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 10.Lindau ST, Drum ML, Gaumer E, Surawska H, Jordan JA. Prevalence of high-risk human papillomavirus among older women. Obstet Gynecol. 2008;112:979–89. doi: 10.1097/AOG.0b013e31818b0df2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velicer C, Zhu X, Vuocolo S, Liaw KL, Saah A. Prevalence and incidence of HPV genital infection in women. Sex Transm Dis. 2009;36:696–703. doi: 10.1097/OLQ.0b013e3181ad25ff. [DOI] [PubMed] [Google Scholar]
- 12.Althoff KN, Paul P, Burke AE, Viscidi R, Sangaramoorthy M, Gravitt PE. Correlates of cervicovaginal human papillomavirus detection in perimenopausal women. J Womens Health (Larchmt) 2009;18:1341–6. doi: 10.1089/jwh.2008.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke MA, Gage JC, Ajenifuja KO, et al. A population-based cross-sectional study of age-specific risk factors for high risk human papillomavirus prevalence in rural Nigeria. Infect Agent Cancer. 2011;6:12. doi: 10.1186/1750-9378-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez P, Hildesheim A, Rodriguez AC, et al. Behavioral/lifestyle and immunologic factors associated with HPV infection among women older than 45 years. Cancer Epidemiol Biomarkers Prev. 2010;19:3044–54. doi: 10.1158/1055-9965.EPI-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munoz N, Mendez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190:2077–87. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 16.Almonte M, Silva Idos S, Asare A, et al. Sexual behavior and HPV infection in British women, by postal questionnaires and telephone interviews. J Med Virol. 2011;83:1238–46. doi: 10.1002/jmv.22085. [DOI] [PubMed] [Google Scholar]
- 17.Bolding G, Davis M, Hart G, Sherr L, Elford J. Heterosexual men and women who seek sex through the Internet. Int J STD AIDS. 2006;17:530–4. doi: 10.1258/095646206778145695. [DOI] [PubMed] [Google Scholar]
- 18.McFarlane M, Bull SS, Rietmeijer CA. Young adults on the Internet: risk behaviors for sexually transmitted diseases and HIV(1) J Adolesc Health. 2002;31:11–6. doi: 10.1016/s1054-139x(02)00373-7. [DOI] [PubMed] [Google Scholar]
- 19.McFarlane M, Kachur R, Bull S, Rietmeijer C. Women, the Internet, and sexually transmitted infections. J Womens Health (Larchmt) 2004;13:689–94. doi: 10.1089/jwh.2004.13.689. [DOI] [PubMed] [Google Scholar]
- 20.McFarlane M, Bull SS, Rietmeijer CA. The Internet as a newly emerging risk environment for sexually transmitted diseases. Jama. 2000;284:443–6. doi: 10.1001/jama.284.4.443. [DOI] [PubMed] [Google Scholar]
- 21.Ross MW, Daneback K, Mansson SA, Berglund T, Tikkanen R. Reported sexually transmitted infections in Swedish Internet-using men and women. J Eur Acad Dermatol Venereol. 2008;22:696–703. doi: 10.1111/j.1468-3083.2008.02634.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Tayyib AA, McFarlane M, Kachur R, Rietmeijer CA. Finding sex partners on the internet: what is the risk for sexually transmitted infections? Sex Transm Infect. 2009;85:216–20. doi: 10.1136/sti.2008.032631. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen A, Kjaer SK, Munk C, Iftner T. Type-specific HPV infection and multiple HPV types: prevalence and risk factor profile in nearly 12,000 younger and older Danish women. Sex Transm Dis. 2008;35:276–82. doi: 10.1097/OLQ.0b013e31815ac5c7. [DOI] [PubMed] [Google Scholar]
- 24.Wentzensen N, Schiffman M, Dunn T, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer. 2009;125:2151–8. doi: 10.1002/ijc.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rousseau MC, Pereira JS, Prado JC, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508–17. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 26.Soto-De Leon S, Camargo M, Sanchez R, et al. Distribution patterns of infection with multiple types of human papillomaviruses and their association with risk factors. PLoS One. 2011;6:e14705. doi: 10.1371/journal.pone.0014705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molano M, Posso H, Weiderpass E, et al. Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer. 2002;87:324–33. doi: 10.1038/sj.bjc.6600442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354:2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 29.Hogewoning CJ, Bleeker MC, van den Brule AJ, et al. Condom use promotes regression of cervical intraepithelial neoplasia and clearance of human papillomavirus: a randomized clinical trial. Int J Cancer. 2003;107:811–6. doi: 10.1002/ijc.11474. [DOI] [PubMed] [Google Scholar]
- 30.Chandra A, Mosher WD, Copen C, Sionean C. Sexual behavior, sexual attraction, and sexual identity in the United States: data from the 2006–2008 National Survey of Family Growth. Natl Health Stat Report. 2011:1–36. [PubMed] [Google Scholar]