Abstract
The primary cause of mortality in breast cancer is tumor aggressiveness, characterized by metastases to regional lymph nodes, bone marrow, lung, and liver. C-X-C chemokine receptor type 4 (CXCR4) has been shown to mobilize breast cancer cells along chemokine gradients. Quantification of CXCR4 surface expression may predict the efficacy of anti-CXCR4 labeled liposomal therapeutics to target and kill breast cancer cells. We evaluated gene and surface receptor expression of CXCR4 on breast cancer cell lines distinguished as having low and high invasiveness, MDA-MB-175VII and HCC1500, respectively. CXCR4 surface expression did not correlate with invasiveness. MDA-MB-175VII exhibited more binding to anti-CXCR4 labeled liposomes relative to HCC1500. Increased binding correlated with greater cell death relative to IgG labeled liposomes. Quantitative cell characterization may be used to select targeted therapeutics with enhanced efficacy and minimal side effects.
1. Introduction
Approximately 226,870 new cases of breast cancer are reported each year, representing 28% of the total number of new women's cancer cases.[1] The current five-year relative survival rate of metastatic breast cancer (MBC) is 21% compared with 97% for patients with non-metastatic breast cancer.[2, 3] Breast cancer mortality is primarily due to breast cancer metastases to regional lymph nodes, bone marrow, lung, and liver.[4] Therapeutics that effectively target and kill MBC cells may increase patient survival.
Targeting breast cancer cells has proven to be a powerful tool for controlling cancer progression and metastasis.[5] Hormone therapy and targeted therapeutics have been developed for treating estrogen receptor positive (ER+) and human epidermal growth factor receptor 2/neu positive (HER2+) breast cancers, respectively.[6-8] They work by blocking receptor activation, which is required for cancer cells to proliferate and spread. Despite advances in therapeutic development, acquired resistance or lack of response can lead to poor patient prognosis. Receptors that modulate cancer progression may be opportune targets for engineering vehicles that localize in primary and distal breast tumors.
Recent attention has been focused on C-X-C chemokine receptor type 4 (CXCR4, or CD184) for its role in cancer metastasis.[9] CXCR4 is a G protein-coupled receptor (GPCR) that is known for its chemosensory transduction mechanisms responsible for cell migration along chemokine gradients, towards stromal derived factor 1 (SDF1, or CXCL12).[10] Silencing and inhibition of CXCR4 have reduced breast cancer metastasis, confirming its role in cancer progression.[9] Targeting CXCR4 on the breast cancer cell surface may not only enhance liposome binding but also inhibit metastasis.[11]
Doxorubicin hydrochloride (Dox) is a commonly used chemotherapeutic; it binds to DNA and certain enzymes involved in the opening of DNA, which blocks the synthesis of DNA, RNA, and proteins.[12, 13] The total lifetime dose of Dox is limited to 550 mg/m2 to prevent accumulative side effects, such as chronic irreversible cardiotoxicity.[14] Targeted therapeutics may reduce toxicity and enhance antitumor potency. While HER2 targeted therapeutics (Trastuzumab,[15] Lapatinib,[16] and Neratinib [17]) have shown clinical promise in HER2+ breast cancer patients, HER2+ breast cancers represent only 20-25% of all breast cancers.[18] Other receptors (e.g., transferrin receptor and epidermal growth factor receptor) have been investigated for targeting breast tumors;[19, 20] their application is limited by expression on a number of normal tissues.[19, 20] A successful therapeutic target requires differential expression from normal tissues and be broadly identified on a range of breast cancers.
In this report, we engineered liposomes to target CXCR4 expressing breast cancer cells. CXCR4 mRNA and surface expression was quantified on two breast cancer cell lines, MDA-MB-175VII and HCC1500, characterized as having low and high invasiveness, respectively.[21, 22] We hypothesized that breast cancer cell binding to anti-CXCR4 presenting liposomes may be dependent on CXCR4 overexpression, which may ultimately impact cytotoxicity. We measured the ability of CXCR4 targeted, Dox encapsulating liposomes to bind to breast cancer cells relative to MCF10A, a nonneoplastic breast epithelial cell. Dox (Adriamycin) is a common chemotherapeutic used widely in breast cancer therapy because of its hydrophilicity and cytotoxicity. Quantitative parameters that help predict the impact of targeted therapeutics on the antitumor potency of Dox may be useful screening tools for determining tumor response.
2. Materials and Methods
2.1 Materials
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-dodecanoyl (N-dod-PE) and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) were purchased from Avanti Polar Lipids (Alabaster, AL). Mouse anti-human CXCR4 monoclonal antibody (aCXCR4), immunoglobulin G (IgG) isotype control, and NorthernLight® 557 (NL557)-conjugated donkey anti-mouse IgG were purchased from R&D Systems (Minneapolis, MN). Doxorubicin hydrochloride (Dox), 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), Triton X-100, bovine serum albumin (BSA), rhodamine-B isothio-cyanate-conjugated dextran (rhodamine-dextran, 10 kDa MW), ammonium molybdate, ammonium sulfate, ascorbic acid, anhydrous dimethyl sulfoxide (DMSO), Sepharose CL-4B column (fractionation range: 60-20,000 kDa), dialysis tubing cellulose membrane (MWCO 12.4 kDa), and ethanol (EtOH) were purchased from Sigma-Aldrich (St. Louis, MO). Formaldehyde was obtained from EMD Chemicals (Gibbstown, NJ). Phycoerythrin (PE)-conjugated mouse anti-human CXCR4 antibody (PE-aCXCR4) and PE-conjugated mouse IgG isotype (PE-IgG) were purchased from BioLegend (San Diego, CA). Dulbecco's phosphate buffered saline (PBS), 0.25% trypsin/2.6 mM ethylenediaminetetraacetic acid (EDTA) solution, human CXCR4 Taqman gene expression assay (Hs.593413), 4′,6-diamidino-2-phenylindole (DAPI), and CellTracker Green CMFDA (5-chloromethylfluorescein diacetate) were purchased from Invitrogen (Carlsbad, CA). Leibovitz's L-15 Medium and Roswell Park Memorial Institute (RPMI)-1640 Medium were obtained from ATCC (Manassas, VA). Thermo Scientific Lab-Tek II Chamber Slide System was obtained from Thermo Fisher Scientific (Pittsburgh, PA). Nuclepore track-etched membrane (Pore size: 100 nm) was obtained from Whatman (Florham Park, NJ). Slide-A-Lyzer dialysis cassette (MWCO 20 kDa) was obtained from Pierce Biotechnology (Rockford, IL). Quantum Simply Cellular microbeads were purchased from Bangs Laboratory, Inc. (Fishers, IN). Dojindo cell counting kit was purchased from Dojindo Molecular Technologies (Rockville, MD).
2.2 aCXCR4-Dox liposome preparation
The aCXCR4 labeled, doxorubicin encapsulating liposomes (aCXCR4-Dox-LPs) were prepared by the extrusion method.[23-25] Briefly, a mixture of DOPC:N-dod-PE (95:5, mol:mol) was solubilized in chloroform and dried in a rotary evaporator under reduced pressure. The lipid film was dissolved in 1 mL DMSO:EtOH (7:3, v:v). The lipid solution was injected in 9 mL 240 mM (NH4)2SO4 buffer (pH 5.4) while being agitated at 650 rpm with a stir bar to yield a 5 mM lipid solution. Liposomes were extruded via a NorthernLipids Extruder with a 100 nm polycarbonate nanoporous membrane. After extrusion, the liposome solution was dialyzed in phosphate buffered saline (PBS pH 7.4) using a Slide-A-Lyzer dialysis cassette (MWCO 20 kDa) overnight at room temperature (RT). Dox was encapsulated in the liposome via an active transmembrane pH gradient method as described.[26] Liposomes were incubated within a Dox solution (5 mg/mL in PBS) for 6 h to allow Dox loading. Obtained Dox-loaded liposomes were dialyzed in PBS (pH 7.4) using a Slide-A-Lyzer dialysis cassette (MWCO 20 kDa) for 12 h at RT to remove excess Dox. Liposomes were conjugated to aCXCR4 via the N-dod-PE anchor. EDC (2 mg) and NHS (3 mg) were mixed with 1 mmol of lipid (liposomes) in PBS (pH 7.4) and incubated for 6 h at RT. A Slide-A-Lyzer dialysis cassette (MWCO 10 kDa.) was used to remove unreacted EDC and NHS. Then aCXCR4 or the IgG isotype was added to EDC-modified liposomes at a molar ratio of 1:1000 (antibody:phospholipid) and incubated overnight at RT. Unreacted antibodies were removed using a Sepharose CL-4B column (fractionation range: 60-20,000 kDa). In liposome binding experiments, aCXCR4-labeled, rhodamine-dextran encapsulating liposomes (aCXCR4-RD-LPs) were prepared and tested. For aCXCR4-RD-LPs, the preparation process is similar as aCXCR4-Dox-LPs except the 1 mL lipid solution was added to a 9 mL rhodamine-dextran solution (1 mg/mL).
The antibody density of aCXCR4 on liposomes was quantified.[23-25] Liposomes cannot be detected by flow cytometry because of their size. Therefore, 2 μm borosilicate beads were encapsulated within DOPC:N-dod-PE (95:5, mol:mol) liposomes by sonicating small unilamellar liposomes with microbeads in PBS for 6 h. Microbeads were rinsed three times in PBS via suspension-spin cycles to separate free liposomes. Conjugation of PE-aCXCR4 or PE-IgG (nonspecific binding) to microbead encapsulating liposomes was performed using EDC/NHS chemistry. The surface density of aCXCR4 conjugated to each microbead was determined with reference to Quantum Simply Cellular microbeads, which have defined numbers of antibody binding sites per bead. Liposome size and zeta potential were measured by dynamic light scattering on a Zeta-PALS analyzer (Brookhaven Instruments, Holtsville, NY) in PBS (pH 7.4).
2.3 Cell culture
Two metastatic human breast cancer cell lines (HCC1500 and MDA-MB-175VII) and one human nonneoplastic mammary epithelial cell line (MCF10A) were tested. HCC1500, MDA-MB-175VII, and MCF10A were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in RPMI-1640 medium, Leibovitz's L-15 medium, and Gibco®DMEM/F12(1:1) medium with supplements, respectively. HCC1500 and MCF10A cells were maintained at 37°C in a humidified incubator with 5% CO2, and MDA-MB-175VII in an incubator at 37°C in air.
2.4 Quantification of CXCR4 expression
Gene expression level of CXCR4 of breast cancer cell lines was characterized using qRT-PCR. HCC1500, MDA-MB-175VII and MCF10A cells were cultured at 3×105 cells/well in 6-well cell culture plate overnight. Then, HCC1500, MDA-MB-175VII and MCF10A cells were removed from each well by incubating with a trypsin/EDTA solution for 3 min. The cells were washed with PBS 3 times. RNA was extracted, purified using the Qiagen RNeasy minikit (Valencia, CA), and quantified by SpectraMaxPlus 384 UV-Visible Spectrophotometer (Molecular Devices Corp, Sunnyvale, CA). Reverse transcription was conducted using the Applied Biosystems Taqman RT protocol. Detection and quantification of mRNA was performed by the StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA). All PCR samples were referenced to the gene expression of Glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Breast cancer cell CXCR4 surface expression was evaluated by a BD FACSCalibur Flow Cytometer (BD Biosciences, San Jose, CA).[24] Quantification of the CXCR4 density on the cell surface was determined with reference to Quantum Simply Cellular microbeads, using the protocol as provided by the manufacturer. Briefly, 106 cells were collected and rinsed twice through suspension-spin cycles. Cells were blocked by 1% bovine serum albumin (BSA) in PBS for 30 min in an ice bath. After BSA blockage, cells were incubated with PE-aCXCR4 for 1 h at RT. Cells were rinsed with 1% BSA in PBS three times, resuspended in PBS, and evaluated by flow cytometry. Flow cytometry results were obtained on the same machine at the same settings on the same day.
2.5 CXCR4 immunofluorescent staining
HCC1500, MDA-MB-175VII, and MCF10A cells (2×105) were seeded in a Lab-Tek II Chamber Slide System separately with 2 mL medium overnight at 37°C. After medium was removed, cells were rinsed with PBS three times and fixed with 4% formaldehyde in PBS at RT for 10 min, and followed by washing with PBS. Then samples were blocked with 1% BSA in PBS for 30 min in an ice bath. After BSA blocking, samples were stained with aCXCR4 (primary antibody) for 1 h and rinsed with PBS. Samples were then incubated with NorthernLight® 557 conjugated goat anti-mouse secondary antibody (NL557 Abs) for another 1 h followed by washing with PBS. DAPI was used to stain the cell nucleus. Immunofluorescent stained samples were dried overnight in the dark and used for fluorescent microscope imaging. Samples were examined under a Leica TCS SP5 confocal fluorescent microscope (Leica Microsystems, Buffalo Grove, IL, USA). Digital images were captured with AxioVision digital image processing software.
2.6 Liposome binding
Quantitative analysis of liposome binding to breast cancer cells (HCC1500, MDA-MB-175VII, and MCF10A (control)) was studied by flow cytometry as previously described [21-23]. Cells were seeded in 6-well plates (3×105 cells/well) and allowed to adhere overnight. 3×105 cells were placed in each well of a 6-well cell culture plate and incubated for 4 h at 37°C with (1) rhodamine-dextran encapsulated liposomes (RD-LPs); (2) rhodamine-dextran encapsulated nonspecific (IgG) liposomes (IgG-RD-LPs); and (3) aCXCR4-RD-LPs. The concentration used was 1 μmol lipid/106 cells. All liposome treated cells were washed with PBS, harvested using a 0.25% trypsin/2.6 mM EDTA solution, and washed with PBS (pH 7.4) three times. Binding data were acquired using a BD FACSCalibur flow cytometer and analyzed using FlowJo software. The fold-over unconjugated liposome value was calculated by dividing the mean fluorescence intensity for aCXCR4-RD-LP or IgG-RD-LP stained cells by that of the RD-LPs.
2.7 Cell-liposome immunofluorescent staining
Immunofluorescent staining of breast cancer cells was performed as described previously.[23-25] Briefly, 2×105 cells of HCC1500, MDA-MB-175VII and MCF10A cells were seeded separately in Lab-Tek II Chamber Slide System and allowed to adhere overnight. Cells were incubated for 4 h at 37°C with (1) RD-LPs; (2) IgG-RD-LPs; and (3) aCXCR4-RD-LPs, respectively. The concentration used was 1 μmol lipid/106 cells. DAPI and Cell Tracker Green were used to stain the cell nucleus and cytoplasm, respectively. Samples were dried overnight in the dark and examined using a Leica TCS SP5 confocal fluorescent microscope.
2.8 Doxorubicin release
Release of Dox from aCXCR4-Dox-LPs was carried out in PBS at pH 5.5 and 7.4. The aCXCR4-Dox-LP solution (1 mL, 200 μg/mL) was added to a dialysis tube (MWCO -12.4 kDa). The dialysis tube was placed in a beaker with 50 mL PBS (pH 5.5 or 7.4). Then the beaker was sealed with parafilm and incubated at 37°C on a shaker (100 rpm). For each time point, three 100 μL samples were collected from the solution outside of dialysis tube and the fluorescence intensity was measured on a SpectraMaxGEMIN XPS fluorescence spectrophotometer (Molecular Devices Corp, Sunnyvale, CA, USA). The Dox excitation and emission wavelengths were 485 nm and 590 nm, respectively. The release rate of Dox was calculated based on a standard fluorescence concentration calibration curve.
2.9 In vitro cytotoxicity
In vitro cytotoxicity of aCXCR4-Dox-LPs on breast cancer cells were evaluated using a cell viability assay. 104 cells (HCC1500 and MDA-MB-175VII) were seeded in each well of a 96 well plate and incubated for 48 h. Cells were treated with (1) aCXCR4-LP; (2) Dox-LPs; (3) aCXCR4-Dox-LPs and (4) free Dox for 4 h. Cells were rinsed three times with PBS and grown for 48 h. Cell viability was determined by a Dojindo cell counting kit using the protocol from the manufacturer (Rockville, MD).
2.10 Statistical analysis
All of the experimental data were obtained in triplicate unless otherwise mentioned and are presented as mean ± standard deviation. Statistical comparison by analysis of variance was performed at a significance level of p < 0.05 based on a Student's t-test.
3. Results and Discussion
3.1. Characterization of aCXCR4-Dox-LPs
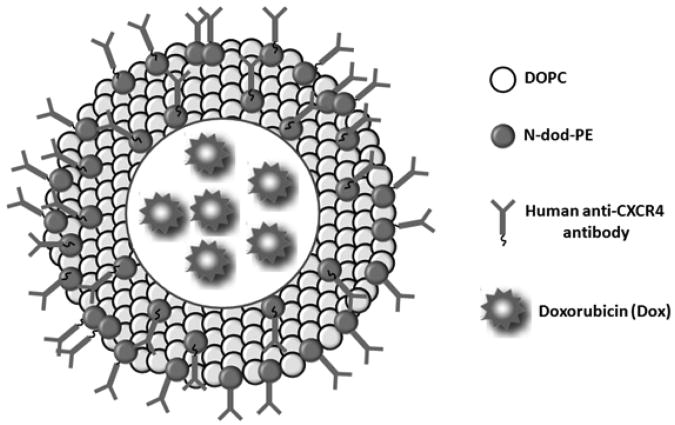
Unilamellar liposomes were prepared by extrusion from a mixture of DOPC:N-dod-PE (95:5, mol:mol). DOPC is an unsaturated lipid with a cis double bond (Δ9) in each C18 chain, which exists in the liquid crystalline phase at RT and 37°C. We have previously shown that liposomes comprised of DOPC have increased cell binding relative to dipalmitoylphosphatidylcholine (DPPC) due to the ability of molecules to rearrange on the membrane surface.[23-25] N-dod-PE was selected as the anchor for aCXCR4 or IgG conjugation. The carboxylic acid on N-dod-PE was used to covalently bond a primary amine group present on aCXCR4 or IgG via EDC/NHS chemistry. The design of aCXCR4-Dox-LPs is illustrated in Figure 1.
Figure 1.
Schematic illustration of the CXCR4-targeted doxorubicin encapsulated liposome (aCXCR4-Dox-LP).
Liposomes were characterized for their physical properties (Table 1). The diameter of aCXCR4-Dox-LPs was 99 ± 1 nm (Table 1). Liposomes with diameters less than 200 nm are desirable for intravenous administration and for passive accumulation within tumors due to the enhanced permeability and retention (EPR) effect. The zeta potential of aCXCR4-Dox-LPs was approximately neutral (−4.0 ± 4.5 mV). The encapsulation efficiency of aCXCR4-Dox-LPs was 94.6 ± 0.7%, which is significantly higher than polymeric nanoparticles (41-72%).[27] A transmembrane pH gradient in aCXCR4-Dox-LPs allows efficient sequestration of Dox within liposomes.[26] Furthermore, the surface density of aCXCR4 was quantified; the density of aCXCR4 was 1720 ± 20 molecules/μm2, which is equivalent to 54 antibodies per liposome.
Table 1. Diameter, size distribution, zeta potential, drug loading, and antibody density of aCXCR4-Dox liposomes.
| Size (nm) | Polydispersity index | Zeta potential (mV) | Encapsulation Ratio (%) | aCXCR4 antibody density (molecule/μm2) |
|---|---|---|---|---|
| 99 ± 1 | 0.06 | −4.0 ± 4.5 | 94,6 ±0.7 | 1,720 ± 20 |
3.2 CXCR4 expression on breast cancer cell surfaces
Numerous breast cancer cell lines are established and used to investigate breast cancer pathobiology and therapeutic strategies.[28] Breast cancer cells are characterized by their ability to invade the surrounding tissue; tumor aggressiveness is described as the primary reason for the poor prognosis for breast cancer patients.[4] MDA-MB-175VII and HCC1500 were selected and characterized for CXCR4 expression because they represent breast cancer cell lines regarded as having low and high invasiveness, respectively.[21, 22] Both HCC1500 and MDA-MB-175VII are ER+ and HER2-. Progesterone receptor (PR) is positively expressed in HCC1500, but not in MDA-MB-175VII.[28]
CXCR4 gene expression was quantified relative to MCF10A, the non-neoplastic mammary epithelial cell line (Figure 2a). HCC1500 and MDA-MB-175VII exhibited 10 and 2.5-fold higher gene expressions than MCF10A, respectively. The highly invasive breast cancer cell line demonstrated higher CXCR4 expression compared to the cell line characterized as low invasive. [9]
Figure 2.
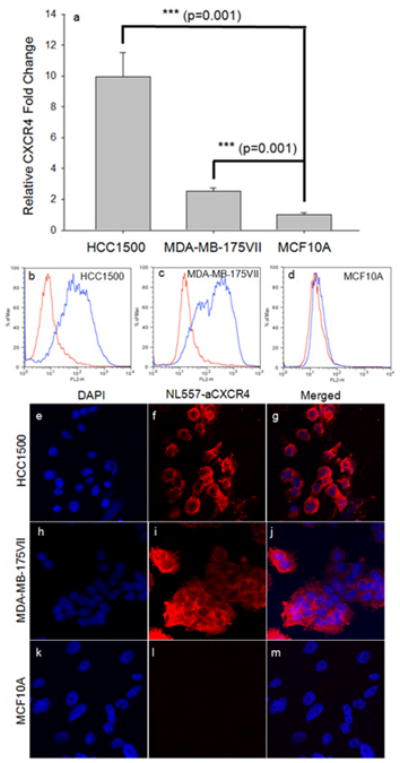
Characterization of CXCR4 gene and surface expression on metastatic breast cancer and normal breast cancer cells. CXCR4 gene expression was quantified by qRT-PCR in Figure 2a. CXCR4 fold change is relative to GAPDH. CXCR4 surface expression was characterized in (b) HCC1500, (c) MDA-MB-175VII and (d) MCF10A cells via a flow cytometry. Blue curve represents the fluorescence of cells stained with PE-conjugated anti-CXCR4 antibody; Red curve represents the fluorescence of cells stained with PE-conjugated-IgG as controls. Figures 2d-l are representative confocal fluorescence microscope images of CXCR4 immunofluorescent staining in HCC1500 (e-g); MDA-MB-175VII (h-j); and MCF10A (k-m). DAPI was used to stained the cell nuclei; Mouse anti-human CXCR4 antibody (primary) and goat anti-mouse NL557 antibody (secondary) were used to stain CXCR4.
CXCR4 surface expression was quantified by flow cytometry. Both breast cancer cell lines had significantly higher CXCR4 surface expression relative to MCF10A (Table 2). CXCR4 expression levels in HCC1500 and MDA-MB-231VII were over 20-fold higher than MCF10A. CXCR4 expression was further confirmed by immunostaining; representative micrographs illustrated high CXCR4 expression on HCC1500 and MDA-MB-175VII and low CXCR4 expression on MCF10A (Figures 2e-m). Overexpression of CXCR4 on both breast cancer cell lines suggested that it may be a possible target for drug delivery.
Table 2. CXCR4 surface density on HCC1500, MDA-MB-175VII, and MCF10A.
| HCC1500 | MDA-MB-175V1I | MCF10A | |
|---|---|---|---|
| CXCR4 (Molecule/Cell) | 104,600 ± 680 | 110,000 ± 1000 | 4,600 ± 100 |
Previous reports have suggested that tumor metastasis may be associated with CXCR4 expression, similar to lymphocyte homing.[29] Despite the differences in gene expression of CXCR4 and invasiveness,[21, 22] HCC1500 and MDA-MB-175VII expressed similar CXCR4 cell surface densities. CXCR4 is expressed on leukocytes, endothelial cells, and hematopoietic stem cells at levels lower than CXCR4 expression on cancer cells.[30-32] While CXCR4 may be useful for targeting drug delivery vehicles, its overexpression was not unique to a highly invasive breast cancer phenotype.
3.3 Breast cancer cells bind liposomes
Cellular binding of rhodamine-dextran (RD) encapsulating liposomes (LPs) was measured by flow cytometry. Flow cytometry measures the fluorescence per cell, which quantitatively assesses the binding and uptake of liposomes. Three different liposome samples: (1) RD-LPs; (2) IgG-RD-LPs; and (3) aCXCR4-RD-LPs were tested in this study. As shown in Figure 3a, breast cancer cells exhibited significantly more binding of CXCR4 targeted liposomes relative to nonspecific, IgG labeled liposomes. This demonstrated that interactions between breast cancer cells and aCXCR4-RD-LPs were CXCR4 dependent. IgG-RD-LPs exhibited higher binding than bare RD-LPs due to electrostatic interactions. Consistent with the higher expression of CXCR4 in breast cancer cells, both HCC1500 and MDA-MB-175VII showed greater binding of aCXCR4-RD-LPs than MCF10As (Figures 3d-l). Given an order of magnitude difference in CXCR4 surface expression, it was surprising that the increase in HCC1500 and MDA-MB-175VII liposome binding was limited to 2 and 3-fold relative to MCF10A, respectively. In comparison, CXCR4 gene expression demonstrated a 10 and 2.5-fold increase for HCC1500 and MDA-MB-175VII, which did not correlate with binding. Breast cancer cells that overexpress CXCR4 had increased aCXCR4-RD-LP binding relative to nonneoplastic cells with low CXCR4 expression.
Figure 3.
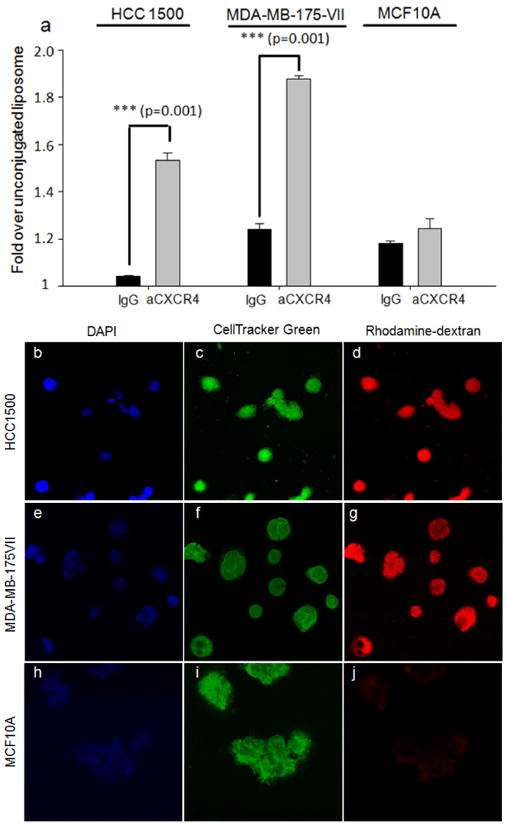
(a) Cellular binding of immunoliposomes in HCC1500, MDA-MB-175VII and MCF10A. Cells were treated with CXCR4-targeted liposome with rhodamine-dextran (aCXCR4) and IgG liposome with rhodamine-dextran (IgG, control), then characterized via flow cytometry. Figures 3(b-j) are representative confocal fluorescent microscope images of immunoliposome cellular binding in HCC1500 (b-d), MDA-MB-175VII (e-f) and MCF10A (g-j). DAPI and cell tracker green were used to stain cell nucleus and cytoplasm. CXCR4-targeted liposome with rhodamine-dextran was tested in this experiment.
3.4 Dox release and toxicity
We measured the release of Dox from aCXCR4-Dox-LPs as a function of time (Figure 4a). Dox is a chemotherapeutic and a robust fluorophore.[33] The release study was carried out at pH 7.4 and 5.5 in order to mimic the extra- and intracellular environments, respectively. Breast cancer cells endocytose liposomes; endosomes are acidic.[34-36] In addition, tumor environments have been reported to have a low pH.[37]
Figure 4.
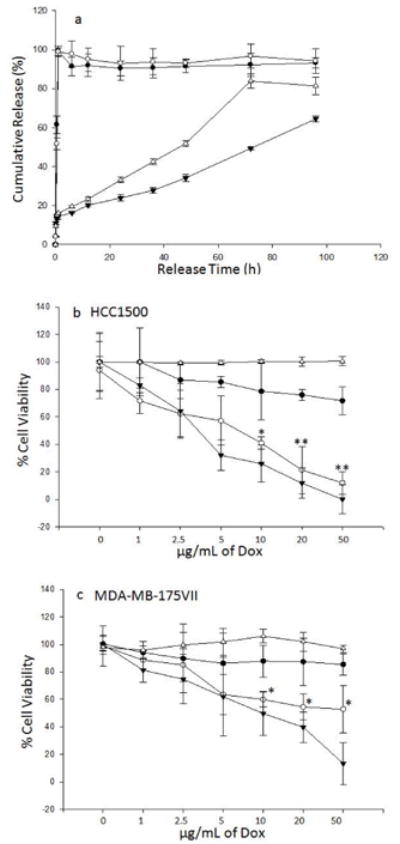
(a) Cumulative Dox release from aCXCR4-Dox-LPs in pH 7.4 (▼) and pH 5.5 (Δ) buffers at 37°C Release of free doxorubicin in pH 7.4 (●) and pH 5.5 (○) buffers; (b and c) In vitro cell cytotoxicity of aCXCR4-Dox-LP on HCC1500 and MDA-MB-175VII. Cells were treated with free Dox (▼), CXCR4 targeted liposomes without Dox (aCXCR4-LPs) (Δ), unconjugated Dox encapsulated liposomes (unconjugated Dox-LPs) (●), or CXCR4 targeted Dox encapsulated liposomes (aCXCR4-Dox-LPs) (○) with varying Dox concentrations. Differences between aCXCR4-Dox-LPs and unconjugated Dox-LPs were analyized by student t-test. * p < 0.05; ** p < 0.01.
Controlled release of Dox was achieved using liposomes. The rate of Dox release was faster at pH 5.5 than pH 7.4 (Figure 4a). Release curves of free Dox at pH 7.4 and pH 5.5 were used as controls. Free Dox is completely released within 1 h at both pH conditions. We hypothesized that increased binding may translate into greater cytotoxicity. The toxicity of Dox-LPs, aCXCR4-Dox-LPs, and free Dox were tested in the two breast cancer cell lines. As seen in Figure 4b, aCXCR4-Dox-LPs demonstrated substantial in vitro cytotoxicity. For HCC1500, the half maximal inhibitory concentration (IC50) of aCXCR4-Dox-LPs was 7.2 μg/mL Dox, which approached that of free Dox (3.6 μg/mL).
This is notably lower than that of Dox-LPs (over 50 μg/mL). At a Dox concentration of 50 μg/mL, only 11% of HCC1500 cells remained viable after treatment with aCXCR4-Dox-LPs. In contrast, cells treated with Dox-LP maintained 72% viability. CXCR4-targeted liposomes without Dox (aCXCR4-LPs) did not show any cytotoxic effect on HCC1500 cells. CXCR4 targeted, Dox encapsulating liposomes demonstrated significantly greater cancer cell toxicity relative to bare, Dox encapsulating liposomes in the invasive cancer cell line.
A similar trend was observed in MDA-MB-175VII cells (Figure 4c). At a Dox concentration of 50 μg/mL, 53% of MDA-MB-175VII cells remained viable after treatment with aCXCR4-Dox-LPs. Although the measured cytotoxicity was significantly greater than Dox-LPs, increased binding of aCXCR4-Dox-LPs failed to result in similar toxicity. Differences in HCC1500 and MDA-MB-175VII response to free Dox were observed. Enhanced binding of targeted therapeutics improved cytotoxicity relative to unlabeled vehicles. Nonetheless, the MDA-MB-175VII cell line was harder to kill than the HCC1500.
Our data suggested that CXCR4 surface overexpression on breast cancer cells represents a novel and useful targeting strategy to enhance the efficacy of Dox independent of tumor aggressiveness. Further investigation is warranted to evaluate the clinical potential of CXCR4 targeted liposomes as a breast cancer therapy.
4. Conclusion
The primary aim of this work was to predict liposome binding and cytotoxicity based on quantitative CXCR4 expression analysis. We engineered a CXCR4 targeted liposomal drug delivery vehicle to improve Dox toxicity. Overexpression of CXCR4 was observed on HCC1500 and MDA-MB-175VII breast cancer cells relative to nonneoplastic MCF10As. Overexpression of CXCR4 on breast cancer cell membranes correlated with increased liposome binding. Increased binding led to improved cytotoxicity. Tumor aggressiveness resulted in differences in cell susceptibility; HCC1500 exhibited greater cell death than MDA-MB-175VII. We have shown that quantitative characterization of CXCR4 surface density predicted enhanced binding and cytotoxicity.
Acknowledgments
JY and MM received support by National Institute of Health Grants R01-CA118764, P01-CA045548, and Breast Cancer Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, Senkus-Konefka E, Fallowfield L, Costa A, Castiglione M. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:15–9. doi: 10.1093/annonc/mdq160. [DOI] [PubMed] [Google Scholar]
- 3.Hickey RC, Gallager HS, Dodd GD, Samuels BI, Paulus DD, Jr, Moore DL. The detection and diagnosis of early, occult and minimal breast cancer. Adv Surg. 1976;10:287–312. [PubMed] [Google Scholar]
- 4.Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 6.Fossati R, Confalonieri C, Torri V, Ghislandi E, Penna A, Pistotti V, et al. Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women. J Clin Oncol. 1998;16:3439–60. doi: 10.1200/JCO.1998.16.10.3439. [DOI] [PubMed] [Google Scholar]
- 7.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. New Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 8.Vogel CL. Efficacy and Safety of Trastuzumab as a Single Agent in First-Line Treatment of HER2-Overexpressing Metastatic Breast Cancer. Journal of Clinical Oncology. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 9.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 10.Wu B, Chien EYT, Mol CD, Fenalti G, Liu W, Katritch V, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–71. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein RJ. Opinion: The CXCL12-CXCR4 chemotactic pathway as a target of adjuvant breast cancer therapies. Nat Rev Cancer. 2004;4:901–9. doi: 10.1038/nrc1473. [DOI] [PubMed] [Google Scholar]
- 12.D'Arpa P, Liu LF. Topoisomerase-targeting antitumor drugs. Biochim Biophys Acta. 1989;989:163–77. doi: 10.1016/0304-419x(89)90041-3. [DOI] [PubMed] [Google Scholar]
- 13.Frederick CA, Williams LD, Ughetto G, Van der Marel GA, Van Boom JH, Rich A, et al. Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry. 1990;29:2538–49. [PubMed] [Google Scholar]
- 14.Tardi PG, Boman NL, Cullis PR. Liposomal doxorubicin. J Drug Targeting. 1996;4:129–40. doi: 10.3109/10611869609015970. [DOI] [PubMed] [Google Scholar]
- 15.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–60. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 16.Wood ER, Truesdale AT, McDonald OB, Yuan D, Hassell A, Dickerson SH, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–9. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 17.Rabindran SK, Discafani CM, Rosfjord EC, Baxter M, Floyd MB, Golas J, et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–65. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 18.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–9. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 19.Qian ZM. Targeted Drug Delivery via the Transferrin Receptor-Mediated Endocytosis Pathway. Pharmacol Rev. 2002;54:561–87. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Z. Targeted cancer therapies based on antibodies directed against epidermal growth factor receptor: status and perspectives. Acta Pharmacol Sin. 2007;28:1476–93. doi: 10.1111/j.1745-7254.2007.00681.x. [DOI] [PubMed] [Google Scholar]
- 21.Prindull G, Zipori D. Environmental guidance of normal and tumor cell plasticity: epithelial mesenchymal transitions as a paradigm. Blood. 2004;103:2892–9. doi: 10.1182/blood-2003-08-2807. [DOI] [PubMed] [Google Scholar]
- 22.Przybylo JA, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition: tumor progression at Snail's pace. Int J Biochem Cell Biol. 2007;39:1082–8. doi: 10.1016/j.biocel.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Gunawan RC, Almeda D, Auguste DT. Complementary targeting of liposomes to IL-1α and TNF-α activated endothelial cells via the transient expression of VCAM1 and E-selectin. Biomaterials. 2011;32:9848–53. doi: 10.1016/j.biomaterials.2011.08.093. [DOI] [PubMed] [Google Scholar]
- 24.Gunawan RC, Auguste DT. Immunoliposomes That Target Endothelium In Vitro Are Dependent on Lipid Raft Formation. Mol Pharmaceutics. 2010 doi: 10.1021/mp9003095. [DOI] [PubMed] [Google Scholar]
- 25.Gunawan RC, Auguste DT. The role of antibody synergy and membrane fluidity in the vascular targeting of immunoliposomes. Biomaterials. 2010;31:900–7. doi: 10.1016/j.biomaterials.2009.09.107. [DOI] [PubMed] [Google Scholar]
- 26.Bolotin EM, Cohen R, Bar LK, Emanuel N, Ninio S, Barenholz Y, et al. Ammonium Sulfate Gradients for Efficient and Stable Remote Loading of Amphipathic Weak Bases into Liposomes and Ligandoliposomes. J Liposome Res. 1994;4:455–79. [Google Scholar]
- 27.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30:592–9. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Kao J, Salari K, Bocanegra M, Choi Y-L, Girard L, Gandhi J, et al. Molecular Profiling of Breast Cancer Cell Lines Defines Relevant Tumor Models and Provides a Resource for Cancer Gene Discovery. Plos One. 2009;4:e6146–e. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MCP. CXCR4 Regulates Growth of Both Primary and Metastatic Breast Cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 30.Gul H, Marquez-Curtis LA, Jahroudi N, Lo J, Turner AR, Janowska-Wieczorek A. Valproic acid increases CXCR4 expression in hematopoietic stem/progenitor cells by chromatin remodeling. Stem Cells and Dev. 2009;18:831–8. doi: 10.1089/scd.2008.0235. [DOI] [PubMed] [Google Scholar]
- 31.Lee B. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci. 1999;96:5215–20. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmud N, Patel H, Hoffman R. Growth factors mobilize CXCR4 low/negative primitive hematopoietic stem/progenitor cells from the bone marrow of nonhuman primates. Biol Blood and Marrow Transplant. 2004;10:681–90. doi: 10.1016/j.bbmt.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Karukstis KK, Thompson EH, Whiles JA, Rosenfeld RJ. Deciphering the fluorescence signature of daunomycin and doxorubicin. Biophys Chem. 1998;73:249–63. doi: 10.1016/s0301-4622(98)00150-1. [DOI] [PubMed] [Google Scholar]
- 34.You J-O, Almeda D, Ye GJC, Auguste DT. Bioresponsive matrices in drug delivery. J Biol Eng. 2010;4:15. doi: 10.1186/1754-1611-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You JO, Auguste DT. Nanocarrier Cross-Linking Density and pH Sensitivity Regulate Intracellular Gene Transfer. Nano Lett. 2009;9:4467–73. doi: 10.1021/nl902789s. [DOI] [PubMed] [Google Scholar]
- 36.You JO, Auguste DT. The effect of swelling and cationic character on gene transfection by pH-sensitive nanocarriers. Biomaterials. 2010;31:6859–66. doi: 10.1016/j.biomaterials.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 37.You JO, Auguste DT. Feedback-regulated paclitaxel delivery based on poly(N,N-dimethylaminoethyl methacrylate-co-2-hydroxyethyl methacrylate) nanoparticles. Biomaterials. 2008;29:1950–7. doi: 10.1016/j.biomaterials.2007.12.041. [DOI] [PubMed] [Google Scholar]