Abstract
Hexose export from chloroplasts at night has been inferred in previous studies of mutant and transgenic plants. We have tested whether hexose export is the normal route of carbon export from chloroplasts at night. We used nuclear magnetic resonance to distinguish glucose (Glc) made from hexose export and Glc made from triose export. Glc synthesized in vitro from fructose-6-phosphate in the presence of deuterium-labeled water had deuterium incorporated at C-2, whereas synthesis from triose phosphates caused C-2 through C-5 to become deuterated. In both tomato (Lycopersicon esculentum L.) and bean (Phaseolus vulgaris L.), Glc from sucrose made at night in the presence of deuterium-enriched water was deuterated only in the C-2 position, indicating that >75% of carbon is exported as hexoses at night. In darkness the phosphate in the cytosol was 28 mm, whereas that in the chloroplasts was 5 mm, but hexose phosphates were 10-fold higher in the cytosol than in the chloroplasts. Therefore, hexose phosphates would not move out of chloroplasts without the input of energy. We conclude that most carbon leaves chloroplasts at night as Glc, maltose, or higher maltodextrins under normal conditions.
Chloroplasts provide all of the reduced carbon in higher plants: from photosynthesis during the day and from starch degradation at night. During the day reduced carbon is exported as triose phosphate, especially dihydroxyacetone phosphate (Walker and Herold, 1977), which is used to make Suc and similar transport sugars. A membrane-bound antiporter that exchanges triose phosphate for phosphate has been isolated and sequenced, and the gene has been cloned (Flügge and Heldt, 1991). The triose-phosphate translocator is unregulated; the flow of carbon is determined by the metabolism in the chloroplast and cytosol. During the day, production of triose phosphates inside the chloroplast and production of phosphate outside result in concentration gradients that drive triose phosphate export and phosphate import.
Starch breakdown can be amylolytic, resulting in maltodextrins, maltose, and Glc, or phosphorolytic, resulting in hexose and triose phosphates (Levi and Gibbs, 1976; Preiss, 1982). The phosphorolytic pathway has a high capacity when phosphate levels are high (Heldt et al., 1977) and preserves energy contained in the Glc-Glc bond in starch. Phosphorolytic starch breakdown is presumed to lead to the production of triose phosphates, which can be exported on the phosphate translocator. Amylolytic starch breakdown leads to maltose and Glc, both of which can be transported, presumably on the Glc transporter (Schäfer et al., 1977; Herold et al., 1981). A number of studies have concluded that the phosphorolytic pathway is the primary pathway for starch breakdown (Heldt et al., 1977; Levi and Preiss, 1978; Stitt and Heldt, 1981a).
Recent work with mutant and transgenic plants has indicated that when triose phosphate export or metabolism in the cytosol is blocked, carbon export during the day declines and export at night increases. For example, (a) a mutant of Flaveria linearis deficient in cytosolic FBPase activity but with no obvious phenotypic changes was found to export little carbon during the day (Sharkey et al., 1992; Zrenner et al., 1996); (b) antisense triose-phosphate- translocator plants were found to have reduced carbon export during the day and enhanced export during the night (Riesmeier et al., 1993; Heineke et al., 1994a; Häusler et al., 1998); and (c) a mutant of Arabidopsis that accumulates starch was found to lack a hexose transporter (Trethewey and ap Rees, 1994).
These observations indicate that at night there is a path for carbon export from chloroplasts that does not function during the day and that does not involve either the triose-phosphate translocator or cytosolic FBPase. The nighttime pathway could work by export of Glc, with or without esterified phosphate. However, known hexose-phosphate transporters in plastids have low activity (Batz et al., 1992) and are generally considered important primarily in starch accumulation (Heldt et al., 1991; Overlach et al., 1993; Schott et al., 1995; Häusler et al., 1998). The Glc/maltose transporter described by Schäfer et al. (1977) and Herold et al. (1981) could explain the nighttime export.
We have developed a method to test whether plants normally export hexoses or trioses from chloroplasts during starch breakdown, and have made measurements to distinguish between phosphorylated and unphosphorylated compounds. During ketose phosphate/aldose phosphate isomerization by sugar isomerases, such as triose phosphate isomerase and phosphoglucoisomerase, one hydrogen of C-1 of a ketose and C-2 of the corresponding aldose can exchange with hydrogen in the medium (Hanson and Rose, 1975). If sugar leaves the chloroplast as hexose and is then converted to Suc following isomerization by phosphoglucoisomerase, i.e. if hexose units stay intact from starch breakdown to Suc synthesis, the Glc moiety of Suc should exchange only the hydrogen on C-2 with the medium. However, if triose phosphates were involved, the hydrogen on C-2 would exchange as before and triose phosphate isomerase would allow exchange of hydrogens on what will become C-3 and C-5 of Glc. In addition, aldolase would allow for the exchange of hydrogen at C-4. Therefore, the Glc moiety of Suc made in the dark in 2H-labeled medium would be enriched in 2H only at C-2 if carbon export were only by hexoses, but at carbons 2 through 5 if triose phosphate were involved.
We tested whether chloroplasts normally export hexoses or trioses at night by allowing starch degradation in the presence of water labeled with 2H and using NMR to determine the intramolecular distribution of 2H on Glc liberated from starch. We also measured the concentration of Glc-6-P and Fru-6-P in chloroplasts and the cytosol in leaves harvested in darkness to determine whether there is a sufficient gradient in these phosphorolytic breakdown products to cause substantial export.
MATERIALS AND METHODS
Plant Material
Bean (Phaseolus vulgaris L. cv Linden) and tomato (Lycopersicon esculentum L. cv UC82B) plants were grown in growth chambers with a 16-h day. The day temperature was 22°C and the night temperature was 18°C. Light was provided by a mixture of high-pressure sodium and metal halide lamps and was 500 μmol m−2 s−1. The RH was 60%. Plants were grown in 3-L pots in Metro-Mix 360 (Scotts- Sierra Horticultural Products, Marysville, OH).
Experimental Protocol
Two types of labeling experiments were carried out. In the first type, leaves were surrounded with a plastic bag containing deuterated water. By diffusion, the water in the leaf became labeled. Starch breakdown was allowed to proceed normally in darkness for 2.5 or 6 h. The leaf was then cut off the plant, frozen, and stored at −80°C until analyzed. In the second type of experiment, leaves were detached and the petiole was put into 2H-enriched water containing EDTA to enhance phloem export from the cut tip. The leaf was allowed to export Suc for 24 h. The irrigation water containing the leaf exudate was then frozen and stored for later analysis.
Derivatization for NMR
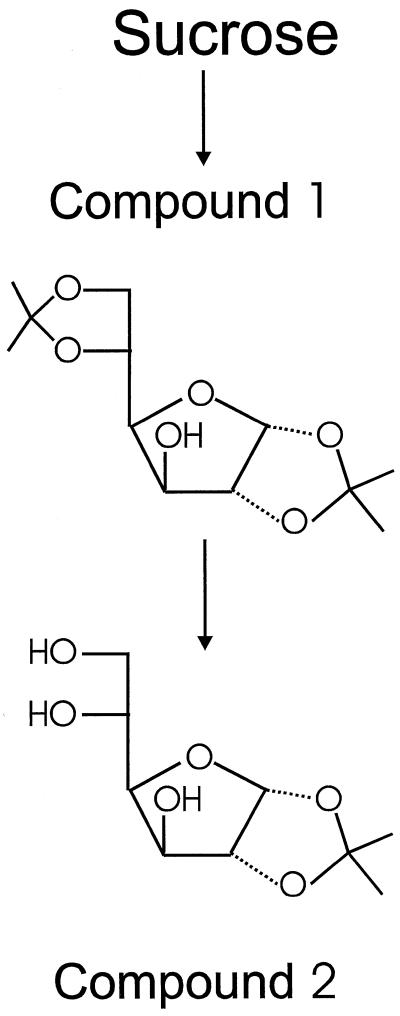
Per gram of crude sugar, 5 g of anhydrous ZnCl2, 0.5 mL of 85% H3PO4, and 200 mL of anhydrous acetone were added and the reaction mixture was stirred for 2 d at room temperature under exclusion of moisture (Glen et al., 1951). After the 1st d, any solid material was loosened from the wall of the flask with a spatula. This procedure resulted in the formation of an equilibrium mixture of approximately 7% 1,2-O-isopropyliden-α-d-glucofuranose (compound 2, Fig. 1), 86% 1,2–5,6-di-O-isopropylidene-α-d-glucofuranose (compound 1, Fig. 1), and 7% Glc (measured by integration of the signals of the anomeric protons by proton NMR). The reaction mixture was poured into 1.2 volumes of ice water and the pH was adjusted to 2.1 with 6 mol L−1 HCl. This solution was stirred at room temperature to hydrolyze compound 1 to compound 2. After 30 h, the reaction mixture contained approximately 88% compound 2, 5% compound 1, and 7% Glc. The diisopropylidene derivative of Fru that was formed from Suc was stable under these conditions. The solution was neutralized by pouring it into a solution of Na2CO3 (160 g L−1).
Figure 1.
Isolation of Glc derivatives for 2H-NMR spectroscopy. Reaction conditions and yields are given in Methods. Compound 1, 1,2–5,6-di-O-isopropyliden-α-d-glucofuranose; compound 2, 1,2-O-isopropylidene-α-d-glucofuranose.
The precipitate of zinc compounds was removed by filtration, the solution was evaporated to dryness under reduced pressure, and the residue (sodium salts and compound 2) was dried in a vacuum at 60°C for 30 min. The powdered residue was extracted four times with boiling ethyl acetate (30 mL g−1 sugar educt), and the hot solution was filtered through a glass funnel into a flask containing a small amount of solid NaHCO3. The ethyl acetate extract was concentrated and compound 2 was allowed to crystallize from the hot solution. Compound 2 was isolated by filtration, washed with cold diethyl ether, and dried. An additional crystallization from ethyl acetate was often required to remove the diisopropylidene derivative of Fru.
NMR Methodology
For the NMR measurements, compound 2 was dissolved at 50°C in 2.4 mL of acetonitrile (HPLC grade) in a 10-mm NMR tube (catalog no. Z18, 398–9, Aldrich) containing a few crystals of NaHCO3, 2H-depleted water (50–250 μL for amounts of compound 2 from 100–300 mg), and 20 μL of a 2% (v/v) solution of hexadeuterobenzene in acetonitrile. This sample composition and the measurement temperature of 50°C reduced the viscosity of the solution and therefore the 2H signal width. The addition of water increased the solubility of compound 2 and ensured that a single signal outside of the range of the carbon-bound hydrogens was observed for 2HHO and the hydroxy protons of compound 2. Longitudinal relaxation times of the 2H signals were determined using an inversion-recovery pulse sequence (Vold et al., 1968). The longest longitudinal relaxation times were 300 to 400 ms. 2H-NMR spectra were acquired at 50°C on DMX-400 and DMX-500 spectrometers (Bruker Instruments, Billerica, MA) in an unlocked mode using a 90° excitation pulse. A repetition time (and acquisition time) of 1 s (approximately 2 longitudinal relaxation times of the slowest relaxing signal) was used. Proton decoupling using the globally optimized alternating-phase rectangular pulses (Shaka et al., 1985) sequence was applied during the acquisition of the 2H spectra to obtain singlet Lorentzian signals. It has been reported (Martin and Martin, 1990) and was confirmed here (not shown) that this did not cause measurable intensity changes as a result of nuclear Overhauser enhancements. The free induction decays (raw data) were zero-filled and Fourier-transformed with a line broadening of 1 Hz using the program Felix (Molecular Simulations, San Diego, CA). The signals of the solvent and the added hexadeuterobenzene were used for phasing. The sample sizes given in Results refer to independently prepared samples from separate plants. The assignment of the compound 2 signals were taken from Mackie and Perlin (1965) and were confirmed by analysis of the proton NMR spectrum. The stereospecific assignment of the C-6 methylene hydrogens of compound 2 was obtained from a 2H-NMR spectrum of a stereospecifically deuterated sample (see below).
Control Experiments
Analysis for hydrogen exchange with the solvent was accomplished by carrying out the isolation sequence in the presence of 2% D2O at each step. 2H-NMR spectra of compound 2 formed in the presence of D2O and in H2O were compared. To identify possible isotope effects of the derivatization reactions, control experiments were performed in which some reaction steps were carried out with low turnover so as to maximize the 2H discrimination caused by isotope effects. Partial turnover of Suc was achieved by reacting Suc with acetone and sulfuric acid in the presence of water. Partial turnover of compound 1 to 2 was achieved by stopping the hydrolysis at low turnover. 2H-NMR spectra of the control samples were compared with spectra obtained from normal preparations (yields ≥ 88%). Epimerization of the C-6 methylene hydrogens was ruled out by synthesis of stereospecifically deuterated Glc. (6S)-1,6-Anhydro-2,3,4-tri-O-benzoyl-β-d-glucopyranose-6-2H was synthesized according to the method of Ohrui et al. (1983) and was hydrolyzed to (6S)-1,6-anhydro-β-d-glucopyranose-6-2H. This was reacted directly to yield (6S)-1,2-O-isopropyliden-α-d-glucofuranose-6-2H.Com-parison of the 2H-NMR spectra of this compound and of the precursor (6S)-1,6-anhydro-2,3,4-tri-O-benzoyl-β-d-glucopyranose-6-2H showed that no epimerization at C-6 had occurred.
We confirmed the predicted intramolecular labeling for hexose phosphate isomerase and triose phosphate isomerase by derivatizing Glc made from Fru-6-P or from triose phosphates in the presence of 2H-labeled water. The intramolecular labeling was determined by NMR analysis.
Subcellular Metabolite Levels
Subcellular metabolite levels were determined by nonaqueous fractionation of bean leaves using the method of Gerhardt and Heldt (1984), as modified by Sharkey and Vanderveer (1989). Freeze-dried leaves were sonicated in cold heptane and then layered on a heptane/tetrachloroethylene density gradient. After centrifugation, the gradient was separated into six fractions. Each fraction was assayed for NADP-glyceraldehyde-3-P dehydrogenase (chloroplast marker), PEP carboxylase (cytosol marker), and α-mannosidase (vacuole marker) plus each metabolite of interest. No indication of hexose phosphate in the vacuole was seen and therefore the data were evaluated assuming only two compartments, the chloroplast and the cytosol, for hexose phosphates. Glc and Fru were not resolved well on the gradient, probably because a large fraction of each was located in the vacuole (Heineke et al., 1994b); therefore, only whole-leaf levels of these compounds are reported. The phosphate nutrition of the bean plants was restricted to lower the phosphate level in the vacuole (Sharkey and Vanderveer, 1989) but there was still a substantial amount present. To determine the amounts of phosphate in the chloroplast, cytosol, and vacuole, an empirical three-compartment analysis was used (Riens et al., 1991). In this analysis each possible distribution of phosphate among the three compartments was considered and the predicted distribution in the gradient was compared with the observed distribution. The assumed subcellular distribution that predicted a distribution through the density gradient closest to the observed one was taken as the best estimate of the subcellular distribution.
RESULTS
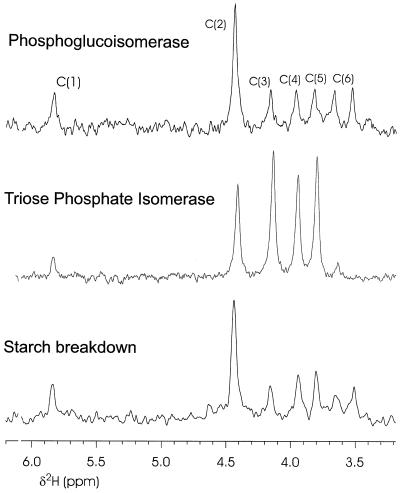
The labeling pattern resulting from the action of phosphoglucoisomerase in vitro is shown in the first trace of Figure 2. In this spectrum, C-2 is heavily deuterated, whereas the other hydrogens have natural abundance levels of 2H. Whereas the labeling is only about 4-fold over natural abundance, the exclusive labeling of C-2 is obvious. Enzyme isotope effects can only deplete 2H in the C-2 position (J. Schleucher, unpublished data), so accumulation of 2H at C-2 must have resulted from incorporation from the medium. The second trace shows that there was substantial deuteration of C-2 through C-5 in Glc made from triose phosphate in vitro when 2H-labeled water was present in the medium.
Figure 2.
2H-NMR spectra of 2H-labeled samples of the Glc derivative 1,2-O-isopropylidene-d-glucofuranose. Top trace, Glc was synthesized from Fru-6-P via phosphoglucoisomerase and Glc-6-phosphatase in 2H-labeled water. Middle trace, Glc was synthesized in 2H-labeled water from dihydroxyacetone phosphate via triose phosphate isomerase, aldolase, Fru bisphosphatase, phosphoglucoisomerase, and Glc 6-phosphatase. Bottom trace, Intact bean leaves were placed at the beginning of darkness in plastic bags containing paper soaked with 2H-labeled water, the leaves were harvested after 3 h and treated with invertase, and the Glc liberated from Suc was derivatized.
We tested whether starch conversion to Suc at night involved triose phosphates by putting a bag with 2H-labeled water around a bean leaf at night after the lights of the growth chamber had been turned off. The 2H-labeled water was allowed to diffuse into the leaf and we assumed that substantial 2H was present throughout the leaf sap. Leaves were left with bags around them for 2.5 h, during which time the amount of starch broken down was about the same as the Suc content in the leaf at the end of the day. About 63% of the Suc in the leaf after the incubation should have originated from starch breakdown. Preexisting Suc made in the light would have had no enrichment in 2H, since 2H was present only after the lights were off. The third trace in Figure 2 shows that the labeling pattern of the Glc moiety of Suc was essentially identical to the labeling pattern caused by phosphoglucoisomerase. In other experiments with bean and tomato plants, leaves were harvested at other times during the night and similar results were found (Table I).
Table I.
Ratio of 2H signal at C-2 to C-4 for four experiments and two controls
To determine whether sufficient gradients exist to cause export of hexose phosphates at night, we measured the concentrations of hexose phosphates and phosphate in the chloroplast and cytosol. Bean leaves were quickly frozen in the dark and then subjected to nonaqueous fractionation. The gradient for hexose phosphates was in the wrong direction for simple diffusion of these compounds (Table II). The gradient for phosphate was large and in the proper direction to cause exchange of phosphate from the cytosol for hexose phosphate from the stroma. Assuming 50 μL liquid volume mg−1 chlorophyll for the stromal volume and 25 μL mg−1 chlorophyll for the cytosolic volume (average of values reported for barley and spinach [Winter et al., 1993, 1994]), we could calculate that phosphate was 28 mm in the cytosol and 5 mm in the stroma. The hexose phosphates were 1 mm in the cytosol and not in isomerase equilibrium. In the stroma, Glc-6-P was 0.1 mm and Fru was undetectable.
Table II.
Metabolite concentrations in the chloroplast and cytosol
| Compound | Chloroplast | Cytosol | Vacuole |
|---|---|---|---|
| nmol mg−1 chlorophyll | |||
| Glc-6-P | 5.4 ± 1.9 | 29.0 ± 4.2 | |
| Fru-6-P | −2.1 ± 1.7 | 23.1 ± 5.6 | |
| Pi | 253 ± 113 | 710 ± 83 | 394 ± 90 |
Bean leaves were analyzed by nonaqueous fractionation. Leaves were quickly frozen in the dark.
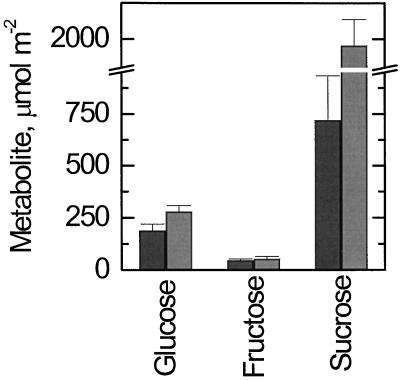
Fru levels were low and constant day and night, whereas Glc levels were higher during the day than at night (Fig. 3). Suc was very high during the day and lower but still substantial during the night. The Glc level varied between day and night but much less than the Suc level. Because most of the hexoses are located in the vacuole, it was not possible to resolve the intracellular location of the free hexoses.
Figure 3.
Levels of Glc, Fru, and Suc in bean leaves during the day and at night. Dark bars, Data determined using leaf samples taken at midnight; lighter bars, data from samples taken at noon.
DISCUSSION
The NMR data clearly indicate that most carbon leaves chloroplasts at night as hexoses, not as trioses. Previously, 13C-NMR was used to show that amyloplasts take up hexoses and not trioses (Keeling et al., 1988). The hexose exported from chloroplasts at night cannot be phosphorylated, since the concentration gradient for both Glc-6-P and Fru-6-P is in the wrong direction. A large gradient in phosphate could theoretically drive hexose phosphate export against its concentration gradient. However, the phosphate concentration was 6-fold higher in the cytosol (Table II) but needs to be 10-fold higher to force hexose phosphate export from the chloroplast. The level of Glc could be sufficient to cause hexose export; however, accurate measurements of the distribution between the chloroplast and cytosol are difficult because of the amount of Glc in the vacuole. Some plastids can transport hexose phosphates but the gene for the Glc-6-P/P antiporter of plastids is not expressed in leaves and, therefore, this transporter is not present in chloroplasts (Kammerer et al., 1998). Thus, all available evidence indicates that free hexoses, possibly including maltose and higher maltodextrins, are the primary transport compounds during Suc synthesis from starch.
Hexose export has been postulated to explain the results from mutant and transgenic plants (Sharkey et al., 1992; Heineke et al., 1994a; Zrenner et al., 1996); we now show that hexose export is the normal route of carbon export from chloroplasts at night in untransformed, nonmutant plants. From the data in Figure 1 and Table I, we calculate that at least 75%, and possibly 100%, of the carbon is exported from chloroplasts at night as hexose, not triose. The finding that plants lacking the Glc transporter accumulate starch (Trethewey and ap Rees, 1994) indicates that the phosphorolytic pathway of starch breakdown and export cannot replace the amylolytic pathway.
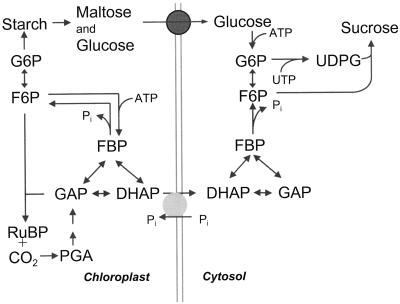
Cytosolic FBPase and Suc-phosphate synthase are important regulatory points in the synthesis of Suc (Stitt et al., 1987). Suc phosphate synthase activity can decline at night but often does not (Huber et al., 1989). However, cytosolic FBPase is nearly always regulated to have a very low activity in darkness. This enzyme is strongly inhibited by Fru 2,6-bisP (Stitt, 1990), which is high at night in most plants studied (Sicher et al., 1986; Sicher and Bunce, 1987). Thus, cytosolic FBPase activity is reduced much more at night than is Suc phosphate synthase. If hexose is exported at night, then Suc phosphate synthase, not cytosolic FBPase, activity is needed. Therefore, the regulation of the two important regulatory steps in Suc synthesis is consistent with hexose export as the primary path for carbon export from chloroplasts at night. These pathways are shown in Figure 4. Given the low activity of cytosolic FBPase restricting triose phosphate supply for Suc synthesis and the unfavorable concentration gradient and lack of carrier for hexose phosphates, it is easy to see why the phosphorolytic pathway cannot compensate for the loss of the Glc transporter in the Arabidopsis mutant TC265 (Trethewey and ap Rees, 1994).
Figure 4.
Pathways of carbon metabolism described in Discussion. A triose phosphate/phosphate antiporter is the source of carbon for Suc synthesis during the day, whereas the Glc/maltose uniporter is the source of carbon for Suc synthesis during the night. DHAP, Dihydroxyacetone-3-P; F6P, Fru-6-P; FBP, Fru-1,6-bisP; GAP, glyceraldehyde-3-P; G6P, Glc-6-P; PGA, phosphoglyceric acid; RuBP, ribulose 1,5-bisP; and UDPG, UDP-Glc.
The pathway of starch degradation is not yet certain. Most studies have found that the chloroplastic α-amylase (endoamylase) is the only enzyme to attack intact starch granules. The maltodextrins released by α-amylase are debranched by the chloroplastic R-enzyme and broken down by β-amylase to maltose and higher maltodextrins (Okita and Preiss, 1980). Whereas most starch-degrading enzymes occur both inside and outside of the chloroplast, maltase activity occurs only outside of the chloroplast (Okita et al., 1979; Kakefuda et al., 1986). Thus, maltose could be an important component of the carbon export from chloroplasts at night.
The energetics of the phosphorolytic versus amylolytic pathways have been discussed by Beck (1985). The phosphorolytic pathway leading to triose phosphate export would require three A(U)TPs per Suc molecule (two ATPs at phosphofructokinase inside the chloroplast plus one UTP at UDP-Glc pyrophosphylase; Fig. 4). The amylolytic pathway with Glc exported would require the same amount of energy (two ATP at hexokinase in the cytosol plus one UTP). If maltose were exported and broken down by phosphorolysis in the cytosol, the total energy required could be reduced by up to one-third. Thus, the amylolytic pathway is potentially more energy efficient than the phosphorolytic pathway of starch breakdown. Phosphate speeds starch breakdown, but the Glc-1-P formed may be used to synthesize maltose for export (Kruger and ap Rees, 1983).
Starch breakdown has been reported in illuminated leaves. If, as seems likely, this indicates amylolytic breakdown followed by export of carbon from the chloroplast, then this carbon would bypass the cytosolic FBPase. The coordinate regulation of the cytosolic and chloroplastic FBPases is crucial in determining what proportion of carbon is exported from chloroplasts during the day (Stitt et al., 1987). Daytime starch degradation and export of amylolytic products must ordinarily be severely restricted, since mutant and transgenic plants lacking parts of the triose phosphate export pathway export most or all of their carbon at night. One signal that influences starch breakdown is the concentration of phosphate. In the stroma the concentration of phosphate is higher at night than during the day (Santarius and Heber, 1965; Sharkey and Vanderveer, 1989). The lower phosphate level during the day could inhibit starch breakdown (Stitt and Heldt, 1981b); however, this would affect phosphorolytic starch breakdown, whereas data reported here indicate that most starch breakdown is amylolytic.
The amylolytic breakdown of starch could be restricted during the day by the high pH of the chloroplast, since the amylases have relatively low pH optima. Pongratz and Beck (1978) reported that amylases in chloroplasts exhibit a diurnal oscillation, being more active at night. This does not seem to be the result of thiol reduction processes, since amylase activity is not affected by DTT (Okita and Preiss, 1980). Extending this finding, Kakefuda and Preiss (1997) reported evidence of an endoamylase that changes activity by more than 5-fold between day and night (i.e. is more active at night). The regulation of this enzyme could explain how plants avoid bypassing the regulation by cytosolic FBPase during the day.
In conclusion, the results presented here indicate that carbon from starch breakdown at night is normally exported from chloroplasts as hexose (Fig. 4). The amylolytic pathway is the dominant pathway for starch breakdown and export despite the high levels of phosphate in chloroplasts at night because phosphorylated compounds are not exported. Amylolytic breakdown of starch is regulated so that very little occurs during the day under normal conditions, forcing carbon through the cytosolic FBPase.
ACKNOWLEDGMENT
We would like to thank Prof. John Markley for the use of the National Magnetic Resonance Facility at Madison, which is supported by the National Institutes of Health, the University of Wisconsin, the National Science Foundation, and the U.S. Department of Agriculture.
Abbreviation:
- FBPase
Fru-1,6-bisphosphatase
Footnotes
This research was supported by the U.S. Department of Energy (grant no. DE-FG-02-87ER13785). J.S. was supported in part by a grant from the Deutsche Forschungsgemeinschaft.
LITERATURE CITED
- Batz O, Scheibe R, Neuhaus HE. Transport processes and corresponding changes in metabolite levels in relation to starch synthesis in barley (Hordeum vulgare L.) etioplasts. Plant Physiol. 1992;100:184–190. doi: 10.1104/pp.100.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck E (1985) The degradation of transitory starch granules in chloroplasts. In RL Heath, J Preiss, eds, Regulation of Carbon Partitioning in Photosynthetic Tissue. American Society of Plant Physiologists, Rockville, MD, pp 27–44
- Flügge U-I, Heldt HW. Metabolite translocators of the chloroplast envelope. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:129–144. [Google Scholar]
- Gerhardt R, Heldt HW. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 1984;75:542–547. doi: 10.1104/pp.75.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glen WL, Myers GS, Grant GA (1951) Monoalkyl hexoses: improved procedures for the preparation of 1- and 3-methyl ethers of fructose, and of 3-alkyl ethers of glucose. J Chem Soc 2568–2572
- Hanson KR, Rose IA. Interpretations of enzyme reaction stereospecificity. Acc Chem Res. 1975;8:1–10. [Google Scholar]
- Häusler RE, Schlieben NH, Schulz B, Flügge UI. Compensation of decreased triose phosphate translocator activity by accelerated starch turnover and glucose transport in transgenic tobacco. Planta. 1998;204:366–376. doi: 10.1007/s004250050268. [DOI] [PubMed] [Google Scholar]
- Heineke D, Kruse A, Flügge U-I, Frommer WB, Riesmeier JW, Willmitzer L, Heldt HW. Effect of antisense repression of the chloroplast triose-phosphate translocator on photosynthetic metabolism in transgenic potato plants. Planta. 1994a;193:174–180. [Google Scholar]
- Heineke D, Wildenberger K, Sonnewald U, Willmitzer L, Heldt HW. Accumulation of hexoses in leaf vacuoles: studies with transgenic tobacco plants expressing yeast-derived invertase in the cytosol, vacuole or apoplasm. Planta. 1994b;194:29–33. [Google Scholar]
- Heldt HW, Chon CJ, Maronde D, Herold A, Stankovic ZS, Walker DA, Kraminer A, Kirk MR, Heber U. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977;59:1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt HW, Flügge U-I, Borchert S. Diversity of specificity and function of phosphate translocators in various plastids. Plant Physiol. 1991;95:341–343. doi: 10.1104/pp.95.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold A, Leegood RC, McNeil PH, Robinson SP. Accumulation of maltose during photosynthesis in protoplasts isolated from spinach leaves treated with mannose. Plant Physiol. 1981;67:85–88. doi: 10.1104/pp.67.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SC, Nielson TH, Huber JLA, Pharr DM. Variation among species in light activation of sucrose-phosphate synthase. Plant Cell Physiol. 1989;30:277–285. [Google Scholar]
- Kakefuda G, Duke SH, Hostak MS. Chloroplast and extrachloroplastic starch-degrading enzymes in Pisum sativum L. Planta. 1986;168:175–182. doi: 10.1007/BF00402961. [DOI] [PubMed] [Google Scholar]
- Kakefuda G, Preiss J. Partial purification and characterization of a diurnally fluctuating novel endoamylase from Arabidopsis thaliana leaves. Plant Physiol Biochem. 1997;35:907–913. [Google Scholar]
- Kammerer B, Fischer K, Hilpert B, Schubert S, Gutensohn M, Weber A, Flügge UI. Molecular characterization of a carbon transporter in plastids from heterotrophic tissues: the glucose 6-phosphate phosphate antiporter. Plant Cell. 1998;10:105–117. doi: 10.1105/tpc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling PL, Wood JR, Tyson RH, Bridges IG. Starch biosynthesis in the developing wheat grain. Evidence against the direct involvement of triose phosphate in the metabolic pathway. Plant Physiol. 1988;87:311–319. doi: 10.1104/pp.87.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger NJAP, Rees T. Maltose metabolism by pea chloroplasts. Planta. 1983;158:179–184. doi: 10.1007/BF00397712. [DOI] [PubMed] [Google Scholar]
- Levi C, Gibbs M. Starch degradation in isolated chloroplasts. Plant Physiol. 1976;57:933–935. doi: 10.1104/pp.57.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi C, Preiss J. Amylopectin degradation in pea chloroplast extracts. Plant Physiol. 1978;61:218–220. doi: 10.1104/pp.61.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie W, Perlin AS. 1,2-O-Isopropylidene-α-d-glucofuranose-5-d and -5,6,6′-d3. Can J Chem. 1965;43:2921–2924. [Google Scholar]
- Martin ML, Martin GJ. Deuterium NMR in the study of site-specific natural isotope fractionation (SNIF-NMR) In: Diehl P, Fluck E, Günther H, Kosfield R, Seelig J, editors. NMR Basic Principles and Progress, Vol 23. Berlin: Springer-Verlag; 1990. pp. 1–61. [Google Scholar]
- Ohrui H, Horiki H, Megruno H. Synthesis of (6R)- and (6S)-d-glucose-6-2H through stereospecific photo-bromination of 1,6-anhydro-β-d-glucopyranose derivative. Agric Biol Chem. 1983;47:1101–1106. [Google Scholar]
- Okita TW, Greenberg E, Kuhn DN, Preiss J. Subcellular localization of the starch degradative and biosynthetic enzymes of spinach leaves. Plant Physiol. 1979;64:187–192. doi: 10.1104/pp.64.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita TW, Preiss J. Starch degradation in spinach leaves. Isolation and characterization of the amylases and R-enzyme of spinach leaves. Plant Physiol. 1980;66:870–876. doi: 10.1104/pp.66.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overlach S, Diekmann W, Raschke K. Phosphate translocator of isolated guard-cell chloroplasts from Pisum sativum L. transports glucose-6-phosphate. Plant Physiol. 1993;101:1201–1207. doi: 10.1104/pp.101.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz P, Beck E. Diurnal oscillation of amylolytic activity in spinach chloroplasts. Plant Physiol. 1978;62:687–689. doi: 10.1104/pp.62.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Regulation of the biosynthesis and degradation of starch. Annu Rev Plant Physiol. 1982;33:431–454. [Google Scholar]
- Riens B, Lohaus G, Heineke D, Heldt HW. Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol. 1991;97:227–233. doi: 10.1104/pp.97.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier JW, Flügge U-I, Schulz B, Heineke D, Heldt H-W, Willmitzer L, Frommer WB. Antisense repression of the chloroplast triose phosphate translocator affects carbon partitioning in transgenic potato plants. Proc Natl Acad Sci USA. 1993;90:6160–6164. doi: 10.1073/pnas.90.13.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius KA, Heber U. Changes in the intracellular levels of ATP, ADP, AMP and Pi and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965;102:39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Schäfer G, Heber U, Heldt HW. Glucose transport into spinach chloroplasts. Plant Physiol. 1977;60:286–289. doi: 10.1104/pp.60.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott K, Borchert S, Müller-Röber B, Heldt HW. Transport of inorganic phosphate and C3- and C6-sugar phosphates across the envelope membranes of potato tuber amyloplasts. Planta. 1995;196:647–652. [Google Scholar]
- Shaka AJ, Barker PB, Freeman R. Computer-optimized decoupling scheme for wideband applications and low-level operation. J Magn Reson. 1985;64:547–552. [Google Scholar]
- Sharkey TD, Savitch LV, Vanderveer PJ, Micallef BJ. Carbon partitioning in a Flaveria linearis mutant with reduced cytosolic fructose bisphosphatase. Plant Physiol. 1992;100:210–215. doi: 10.1104/pp.100.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Vanderveer PJ. Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol. 1989;91:679–684. doi: 10.1104/pp.91.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicher RC, Bunce JA. Effects of light and CO2 on fructose 2,6-bisphosphate levels in barley primary leaves. Plant Physiol Biochem. 1987;25:525–530. [Google Scholar]
- Sicher RC, Kremer DF, Harris WG. Control of photosynthetic sucrose synthesis in barley primary leaves. Role of fructose 2,6-bisphosphate. Plant Physiol. 1986;82:15–18. doi: 10.1104/pp.82.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:153–185. [Google Scholar]
- Stitt M, Heldt HW. Physiological rates of starch breakdown in isolated intact spinach chloroplasts. Plant Physiol. 1981a;68:755–761. doi: 10.1104/pp.68.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Heldt HW. Simultaneous synthesis and degradation of starch in spinach chloroplasts in the light. Biochim Biophys Acta. 1981b;638:1–11. [Google Scholar]
- Stitt M, Huber S, Kerr P (1987) Control of photosynthetic sucrose formation. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants. Academic Press, New York, pp 327–409
- Trethewey RN, ap Rees T. The role of the hexose transporter in the chloroplasts of Arabidopsis thaliana L. Planta. 1994;195:168–174. [Google Scholar]
- Vold RL, Waugh JS, Klein MP, Phelps DE. Measurements of spin relaxation in complex systems. J Chem Phys. 1968;48:3831–3832. [Google Scholar]
- Walker DA, Herold A (1977) Can the chloroplast support photosynthesis unaided? Plant Cell Physiol 51: 295–310
- Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in barley leaves. Planta. 1993;191:180–190. [Google Scholar]
- Winter H, Robinson DG, Heldt HW. Subcellular volumes and metabolite concentrations in spinach leaves. Planta. 1994;193:530–535. [Google Scholar]
- Zrenner R, Krause KP, Apel P, Sonnewald U. Reduction of the cytosolic fructose-1,6-bisphosphatase in transgenic potato plants limits photosynthetic sucrose biosynthesis with no impact on plant growth and tuber yield. Plant J. 1996;9:671–681. doi: 10.1046/j.1365-313x.1996.9050671.x. [DOI] [PubMed] [Google Scholar]