Granulocytic myeloid-derived suppressor cells from tumorbearing mice inhibit the differentiation of iTreg.
Keywords: MDSC, cancer, immunosuppression
Abstract
MDSCs and Tregs play an essential role in the immunosuppressive networks that contribute to tumor-immune evasion. The mechanisms by which tumors promote the expansion and/or function of these suppressive cells and the cross-talk between MDSC and Treg remain incompletely defined. Previous reports have suggested that MDSC may contribute to Treg induction in cancer. Herein, we provide evidence that tumor-induced gr-MDSCs, endowed with the potential of suppressing conventional T Lc, surprisingly impair TGF-β1-mediated generation of CD4+CD25+FoxP3+ iTregs. Furthermore, gr-MDSCs impede the proliferation of nTregs without, however, affecting FoxP3 expression. Suppression of iTreg differentiation from naïve CD4+ cells by gr-MDSC occurs early in the polarization process, requires inhibition of early T cell activation, and depends on ROS and IDO but does not require arginase 1, iNOS, NO, cystine/cysteine depletion, PD-1 and PD-L1 signaling, or COX-2. These findings thus indicate that gr-MDSCs from TB hosts have the unanticipated ability to restrict immunosuppressive Tregs.
Introduction
Evasion of tumor cells from immune elimination represents a major obstacle in cancer immunotherapy. A complex network of interacting mechanisms governs the establishment and maintenance of cancer-induced immunosuppression [1, 2]. The promotion of MDSCs and Tregs by tumors plays a central role in escape from immunosurveillance [3, 4].
MDSCs consist of a heterogeneous population of myeloid cells arrested at early stages of differentiation and endowed with immunosuppressive activity. They include myeloid progenitors and immature myeloid cells [5, 6]. In mice, MDSCs are phenotypically characterized by the expression of the markers CD11b and Gr-1 and by the absence of expression of CD11c, F4/80, and MHC II. Two major subsets of MDSCs have been identified based on their expression of Ly6G and/or Ly6C (two Gr-1 epitopes) and distinct mechanisms of T cell suppression [7]. The CD11b+Ly6G+Ly6Clow subpopulation exhibits a granulocytic phenotype, whereas Mo-MDSCs are characterized by a CD11b+Ly6G−Ly6Chigh phenotype. MDSCs are expanded significantly in cancer patients and in TB animals [8, 9]. A broad panel of tumor-derived factors can trigger MDSC expansion by promoting myelopoiesis and by impairing the differentiation of myeloid progenitors into mature macrophages, DCs, or granulocytes [10, 11]. A cardinal feature of MDSCs is their ability to inhibit T cell proliferation and activation [12, 13] and to suppress innate and adaptive tumor immunity [14]. The mechanisms underlying MDSC immunosuppressive function are multiple, require direct cell–cell contact, and include the production of NO, peroxynitrites, ROS, and the expression of iNOS and/or arginase 1 [9, 10, 15–17].
Regulatory T Lc (Tregs) contribute to the maintenance of self-tolerance and to the prevention of autoimmunity [18, 19]. Multiple populations of immunosuppressive T cells have been described in the past 15 years, including the CD4+CD25+ subset expressing the transcription factor FoxP3 [20, 21]. CD4+CD25+FoxP3+ Tregs can be generated during thymic selection (nTreg) or in the periphery by differentiation from naïve CD4+CD25−FoxP3− T Lc under specific conditions requiring TCR and CD28 stimulation and TGF-β1 [22–24]. Although the exact mechanisms have not been fully elucidated, it has been proposed that Treg expansion observed in cancer patients and in TB animals may result from the conversion of naïve T cells into iTregs and/or from the proliferation of nTregs [25, 26]. The prevalence of one over the other mechanism in cancer patients remains controversial [27, 28]. Tumor-induced Tregs inhibit anti-tumor effector CD4+ and CD8+ T cells and compromise APC function [29–31]. Arguably, targeted strategies that curtail Treg expansion and/or function have been shown to improve cancer immunotherapy [31–33]. However, most of these approaches lack specificity, as they also target activated conventional lymphocytes.
It has been reported that immature DCs, phenotypically and functionally impaired by the tumor-immunosuppressive environment, may be capable of inducing Treg expansion [34]. Similarly, some reports have suggested that tumor-induced MDSCs may contribute to the generation of CD4+CD25+ FoxP3+ Tregs. Most of these studies have, however, focused on the Mo-MDSC subset and/or the ability of MDSCs to induce nTreg proliferation. Whether gr-MDSCs may have a distinct influence on iTreg and/or nTreg expansion and function has not been clearly defined, although gr-MDSCs are the primary subset of MDSCs expanded in cancer patients and TB animals [35–39]. In the current study, we provide evidence that the regulation of Tregs by gr-MDSCs may be more complex than proposed initially. Unexpectedly, gr-MDSCs, which expand in mice bearing established 4T1 breast or LLC lung tumors, are capable of suppressing TGF-β1-induced polarization of naïve T Lc into iTregs. gr-MDSC-mediated inhibition of iTreg generation occurs during the first hours of the differentiation process, requires a direct cell-to-cell contact, and depends on ROS and IDO, but not on arginase 1, cysteine/cystine depletion, PD-1 and PD-L1 interaction, or COX-2. In addition, tumor-expanded gr-MDSCs impede IL-2-, anti-CD3-, and anti-CD28-induced proliferation of nTregs isolated from naïve mice. This idiosyncratic regulation of nTregs and iTregs by gr-MDSCs in cancer provides further insights into the mechanisms underlying tumor-induced expansion and regulation of Tregs.
MATERIALS AND METHODS
Cell lines, mice, and tumor growth in vivo
LLC and 4T1 tumor cell lines were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI 1640 (Thermo Fisher Scientific, Waltham, MA, USA), supplemented with 10% FBS (Thermo Fisher Scientific) antibiotic-antimycotic (Gibco, Carlsbad, CA, USA), MEM (Cellgro, Mediatech, Manassas, VA, USA), and sodium pyruvate (Thermo Fisher Scientific) at 37°C, 5% CO2.
Female BALB/c and C57BL/6 mice were purchased from the National Cancer Institute (Bethesda, MD, USA). FoxP3-EGFP mice, which coexpress the FoxP3 and the GFP under control of the endogenous promoter, were obtained from The Jackson Laboratory (Sacramento, CA, USA; C.Cg-Foxp3tm2Tch/J) and were bred at the University of Arizona Health Sciences Center Animal Care Facility (Tucson, AZ, USA). All cells that express FoxP3 can be identified by flow cytometry analysis though the expression of GFP. Arg+/−, Cox-2−/−, and IDO−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA; 007,741). Animals were used at the age of 6–8 weeks. All experiments were performed in accordance with the University of Arizona Institutional Animal Care and Use Committee guidelines.
For tumor generation, 4T1 or LLC cells were trypsinized and washed three times in PBS (Gibco) and then counted and adjusted to a concentration of 5 × 106 cells/mL. Animals (BALB/c or C57BL/6) were administered with 0.2 mL (1×106 cells) s.c. in both groins. 4T1-bearing mice were killed on Day 14, and animals bearing LLC were killed on Day 21. Tumor volumes were 1000 mm3.
Immunohistochemistry
Mice were injected in both groins (106 4T1 or LLC tumor cells, s.c.) and were killed after 14 days (4T1) or 21 days (LLC). Tumors and spleens were harvested and frozen. Serial sections (5 μm-thick) were performed from each tissue. Frozen slides were first fixed in 100% cold methanol for 10 min. Slides were then washed three times in 1× PBS and transferred to a 3% hydrogen peroxide solution (Sigma-Aldrich, St. Louis, MO, USA) for 15 min. Slides were then blocked for 1 h using the pre-made blocking solution from the HistoMark biotin-streptavidin peroxidase kit, rat primary antibody (KPL, Gaithersburg, MD, USA). Rat anti-mouse CD11b (BD PharMingen, San Diego, CA, USA; clone M1/70) and rat anti-mouse Gr-1 (eBioscience, San Diego, CA, USA; clone RB6-8C5) antibodies were added to the tissue at concentrations of 0.005 mg/mL and 0.02 mg/mL, respectively (overnight, 4°C). The slides were then washed three times with 0.1% TBST, followed by treatment with anti-rat IgG from the KPL kit (30 min, room temperature). Slides were washed again with TBST and incubated for 30 min with streptavidin (KPL kit). A final set of three washes was performed using TBST. The stained slides were then developed using AEC and DAB kits (Vector Laboratories, Burlingame, CA, USA) for Gr-1 and for CD11b, respectively. Images were captured with a Nikon Digital Sight DS-Fi1 camera and NIS-Element software (Nikon Instruments, Melville, NY, USA).
Cell isolation and magnetic cell sorting
Tumors, spleens, and BM were harvested from TB or TF animals. Tumors were minced into thin pieces and were dissociated in collagenase (1.5 mg/mL; Sigma-Aldrich), 0.5% trypsin (Gibco), in RPMI 1640 (Thermo Fisher Scientific) for 1 h at 37°C. Dissociated tumor tissues were then passed though a 100-μm nylon cell strainer to obtain a single-cell suspension. Spleens were mechanically dissociated, and RBCs were lysed in 1× lysis buffer (BD PharMingen). Total BM was flushed out from mouse tibias and femurs, filtered, and RBCs were lysed. Ly6G+ gr-MDSCs were isolated using the mouse MDSC isolation kit, according to the manufacturer's protocol (Miltenyi Biotec, Auburn, CA, USA). CD4+CD62L+ naïve T cells and CD4+CD25+ nTregs were isolated by magnetic cell sorting using a mouse CD4+CD62L+ T cell isolation kit II or a mouse CD4+CD25+ Treg isolation kit, respectively, and an autoMACS separator following the manufacturer's instructions (Miltenyi Biotec).
Generation of iTregs from CD4+CD62L+ T cells in vitro and coculture with gr-MDSCs
CD4+CD62L+ naïve T cells, isolated from FoxP3GFP BALB/c or C57BL/6 mouse splenocytes, were cultured in a 24-well plate for 72 h in complete RPMI medium (106 cells/well) with anti-CD3- and anti-CD28-coated T cell expander beads (Invitrogen, Carlsbad, CA, USA; beads:T cell ratio, 1:1) and with TGF-β1 (5 ng/mL; PeproTech, Rocky Hill, NJ, USA). Some groups were cocultured with Ly6G+ MDSCs isolated from tumors, spleens, or BM of TF, 4T1 TB, or LLC TB mice as indicated. The percentage of CD4+CD25+FoxP3+ and CD4+CD25+FoxP3− cells was determined by flow cytometry analysis.
Generation of CD4+ Th1, Th2, and Th17 lymphocytes
CD4+CD25−CD62L+ naive T cells were isolated from mouse spleens using a CD4+CD62L+ T cell isolation kit (Miltenyi Biotec). The purified cells were then incubated for 3 days in 5% CO2 at 37°C in RPMI-1640 medium (Thermo Fisher Scientific), supplemented with 10% heat-inactivated FBS (Thermo Fisher Scientific) with anti-CD3- and anti-CD28-coated T cell expander beads (1:1 bead:cell ratio; Invitrogen) and with the following specific cytokines and blocking antibodies. For Th1 generation, cells were cultured with IL-2 (20 IU/ml), IL-12 (10 ng/ml), and anti-IL-4-blocking antibody (5 μg/ml). For Th2 differentiation, cells were incubated with IL-2 (20 IU/ml), IL-4 (30 ng/ml), and anti-IFN-γ-blocking antibody (5 μg/ml). Th17 lymphocytes were generated with TGF-β1 (5 ng/ml), IL-6 (50 ng/ml), anti-IFN-γ, and anti-IL-4-blocking antibodies (5 μg/ml each). All cytokines were purchased from PeproTech and blocking antibodies from eBioscience. Some groups were cocultured with Ly6G+ gr-MDSCs isolated from spleens of TB mice as indicated. The expression of the transcription factors T-bet, GATA3, and RORγt was evaluated by flow cytometry.
T cell proliferation and suppression assays
Total T cells were isolated from TF BALB/c or C57BL/6 mouse splenocytes using a nylon wool column (Polysciences, Warrington, PA, USA) [32] and were used as responder T Lc. Cells were plated in sixplicate in a 96-well plate and were incubated (37°C for 72 h) with anti-CD3- and anti-CD28-coated beads, with or without Ly6G+ MDSCs. BrdU (Millipore, Billerica, MA, USA) was added for the last 24 h. The cells were then fixed, and the incorporation of BrdU was detected according to the manufacturer's instructions (Millipore). In other experiments, CD4+CD25+ T cell proliferation was assed using the CellTrace Violet cell-proliferation kit, according to the manufacturer's procedure (Invitrogen). Labeled CD4+CD25+ cells were cultured (37°C for 96 h) with anti-CD3- and anti-CD28-coated beads (cell:bead ratio, 1:1) and 20 IU/mL mouse rIL-2 (PeproTech), with or without Ly6G+ cells, and cell division was analyzed by flow cytomerty, using a BD LSR II (BD PharMingen). Data analysis was performed using FlowJo software (version 5.7.2.).
Antibodies and flow cytometry
Cells (∼106) were washed in PBS and were first incubated with a FcR-blocking antibody (BD PharMingen) for 10 min and then with saturating amounts of the appropriate combination of fluorochrome-conjugated antibodies for 40 min. Cells were then washed and analyzed using a FACSCalibur (BD PharMingen). A minimum of 10,000 events was collected for each sample, and data analysis was performed with CellQuest Pro 6.0 (BD PharMingen) and FlowJo. The following antibodies were purchased from BD PharMingen: anti-mouse CD25-PE (clone PC61), CD25-FITC (clone 7D4), CD4-FITC (clone GK1.5), CD11b-PE (clone M1/70), F4/80 FITC (clone BM8), and IL-4Rα-PE (clone mIL4R-M1). The following antibodies were purchased from eBioscience: CD4-allophycocyanin (clone RM4-5), Gr-1-PE (clone RB6-8C5), Ly6C-allophycocyanin (clone HK1.4), Ly6G-PE RB6-8C5, MHC II-allophycocyanin (clone M5/114.15.2), and B220-PE (clone RA3-6B2). CD11c-allophycocyanin (clone N418) was purchased from Miltenyi Biotec. For detection of transcription factor expression, cells were fixed and permeabilized using the anti-mouse FoxP3 staining kit (eBioscience) and stained using the following antibodies, purchased from eBioscience: anti-mouse FoxP3 (clone FJK-16s), T-bet (clone 4B10), GATA3 (clone TWAJ), and ROR-γt (clone AFKJS-9).
Inhibitors, blocking antibodies, and exogenous proteins
The arginase 1 inhibitor NOHA (Calbiochem, Darmstadt, Germany) was used at a concentration of 50 μM. The iNOS inhibitor L-NMMA was used at a concentration of 1 mM. Anti-mouse PD-1 (clone J43; eBioscience) and anti-mouse PD-L1 (clone MIH5; eBioscience) blocking antibodies were used at 10 μg/mL. Isotype controls consisted in Armenian hamster IgG (eBioscience) or rat IgG2a,γ (eBioscience). Exogenous l-arginine (Sigma-Aldrich) or l-cysteine (Sigma-Aldrich) was used at the concentration of 400 μM or 100 μM, respectively. In other experiments, 50 μM β-ME was added to the culture to prevent l-cysteine oxidation. The antioxidant NAC (Sigma-Aldrich) was used at the indicated concentrations.
Real-time RT-PCR
Real-time RT-PCR was used to evaluate expression of different cytokines and mediators in gr-MDSCs isolated from the spleen of TF or 4T1 TB mice. Total RNA was isolated using TRIzol (Invitrogen), and its integrity was confirmed by denaturing agarose gel electrophoresis and a calculated densitometric 28S:18S ratio. Total RNA (250 ng) was reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Subsequently, 20 μl of the PCR reactions were set up in 96-well plates containing 10 μl 2× IQ Supermix (Bio-Rad), 1 μl TaqMan primer/probe set (ABI, Foster City, CA, USA), 2 μl of the cDNA synthesis reaction (10% of RT reaction), and 7 μl nuclease-free water. Reactions were run and analyzed on a Bio-Rad iCycler iQ real-time PCR detection system. Cycling parameters were determined, and resulting data were analyzed by using the Ct method as a means of relative quantification, normalized to an endogenous reference (TATA box-bonding protein) and relative to a calibrator (normalized Ct value obtained from control mice), and expressed as 2−ΔΔCt (Applied Biosystems User Bulletin #2: Rev B “Relative Quantification of Gene Expression”). For statistical analysis for RT-PCR data, statistical significance was determined by the ANOVA, followed by Fisher protected least significant difference post hoc test with StatView software package v.4.53 (SAS Institute, Cary, NC, USA). Data are expressed as mean ± sem.
Statistics
Unless specified otherwise, all experiments were repeated at least three times. A two-tailed paired Student's t test was performed to determine significant differences.
RESULTS
Granulocytic Ly6G+ MDSC frequency is increased in the spleen, tumor, and BM of LLC- and mammary (4T1) carcinoma-bearing mice
We first evaluated the influence of tumor development on the number of MDSCs in two murine cancer models (4T1, mammary; LLC, lung). A significant increase in the frequency of CD11b+Gr-1+ cells was observed in the spleen and BM of 4T1 TB mice compared with TF animals (Supplemental Figs. 1A and 2A). A similar expansion of CD11b+Gr-1+ cells was detected by immunohistochemistry in the tumor beds of 4T1 TB mice (Supplemental Fig. 2A). Expansion of CD11b+Gr-1+ cells was also observed in the spleen, BM, and tumor beds of LLC TB mice (Supplemental Figs. 1B and 2B). These results thus indicate that consistent with previous studies [15, 39–41], 4T1 and LLC tumors induce the expansion of CD11b+Gr-1+ cells in vivo.
The Gr-1 antigen is a homo- or heterodimer composed of Ly6G and Ly6C. Mo-MDSCs are characterized by a CD11b+Ly6G−Ly6Chigh phenotype, whereas gr-MDSCs are CD11b+Ly6G+Ly6CLow. We determined that the CD11b+Ly6G+Ly6CLow granulocytic subset was increased significantly in the spleen and BM of 4T1 (Supplemental Fig. 1C and D)- or LLC (not shown)-bearing mice. The CD11b+Ly6G−Ly6Chigh monocytic subpopulation was not expanded significantly (Supplemental Fig. 1C). In line with these results, a very low number (∼5.0×105±2.0×105 cells) of Mo-MDSCs could be recovered from mice with 4T1 or LLC tumors. These results are consistent with previous reports indicating that tumors predominantly promote the expansion of the Ly6G+ granulocytic subset of MDSCs [5, 15, 39, 42–46]. We next isolated expanded Ly6G+ cells from the tumor, spleen, or BM of 4T1 TB mice using a magnetic cell-sorting strategy and evaluated their purity and phenotypic characteristics. The data depicted in Supplemental Fig. 3A indicate that the majority of isolated Ly6G+ cells expressed CD11b. Similarly, >93% of isolated Ly6G+ cells were positive for IL-4Rα, a marker expressed by gr-MDSCs, which has been shown to be essential for their suppressive function [47]. Additionally, a high percentage of Ly6G+ cells expressed low or intermediate levels of Ly6C, a characteristic associated with the Ly6G+ granulocytic subset of MDSCs [15]. Expression of MHC II, CD11c, F4/80, and B220 by selected Ly6G+ cells was limited (Supplemental Fig. 3A). These results confirm that 4T1 tumor-expanded CD11b+Ly6G+ cells display a phenotype consistent with that of the granulocytic subset of MDSCs. Similar results were obtained with Ly6G+ cells isolated from the tumor, spleen, and BM of LLC TB mice (data not shown).
To further characterize the functional properties of Ly6G+ cells isolated from the spleen, BM, or tumor of 4T1 or LLC TB mice, their ability to inhibit the proliferation of T Lc induced with anti-CD3- and anti-CD28-coated microbeads was assessed. Ly6G+ cells, isolated from 4T1 TB mice, were capable of significantly hampering responder T Lc proliferation (Supplemental Fig. 3B). The suppression of T cell proliferation depended on the Ly6G+ cell:target T cell ratio (Supplemental Fig. 3C and D). Ly6G+ cells from TB mice were significantly more efficient suppressors compared with Ly6G+ cells from TF animals at ratios of 1:0.5 and 1:1. Likewise, Ly6G+ cells isolated from LLC TB mice significantly suppressed T cell proliferation (data not shown). These results therefore confirm that tumor-induced CD11b+Ly6G+ cells exhibit a phenotype and function consistent with that of gr-MDSCs.
Ly6G+ gr-MDSCs impair TGF-β1-induced differentiation of naïve T cells into FoxP3-expressing iTregs
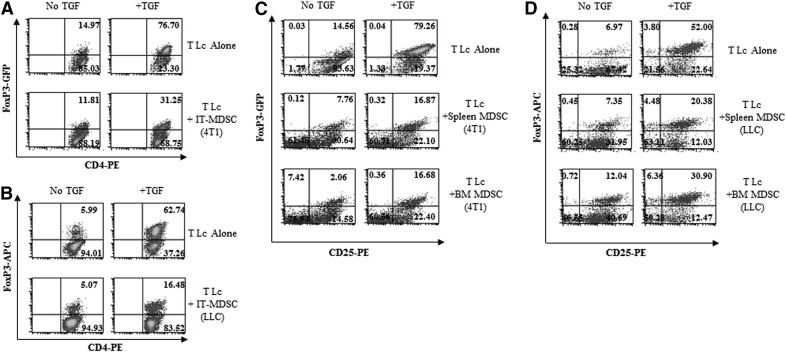
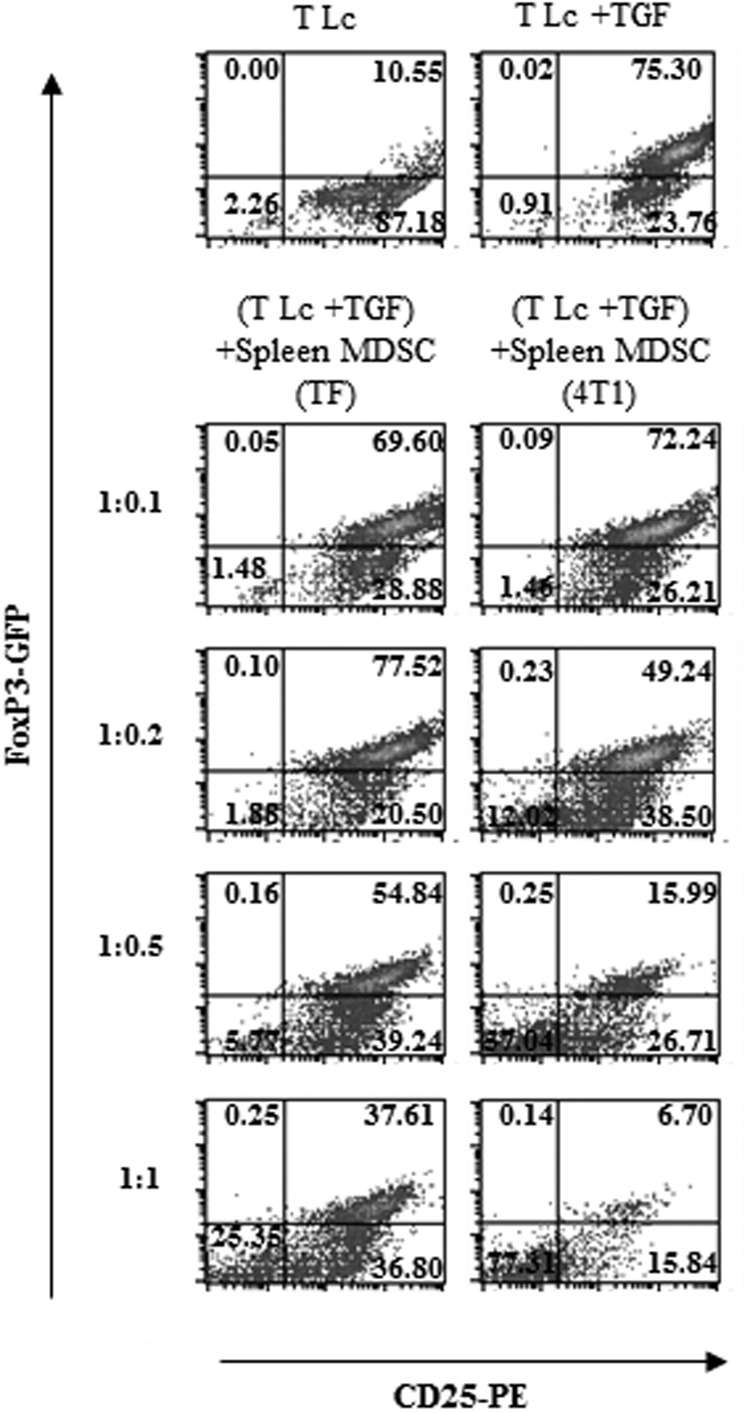
Previous reports have indicated that MDSCs promote Treg proliferation [35–37]. We initially reasoned that these cells may expand Tregs in cancer-bearing hosts by also inducing the differentiation of naïve CD4+ T cells into FoxP3+ iTregs. To address this hypothesis, we evaluated the influence of gr-MDSCs on the generation of FoxP3-expressing iTregs from naïve T cells using FoxP3-EGFP transgenic mice. Tumor-infiltrating gr-MDSCs isolated from 4T1 (Fig. 1A) or LLC (Fig. 1B) tumors not only did not trigger the differentiation of naïve CD4+ T cells into FoxP3-expresssing lymphocytes in the absence of exogenous TGF-β1 (Fig. 1A and B, left panels), but surprisingly, they significantly suppressed TGF-β1-induced conversion of CD4+CD25−FoxP3− T cells into iTregs (Fig. 1A and B, right panels). Likewise, gr-MDSCs, isolated from the spleen or the BM of 4T1 (Fig. 1C) or LLC (Fig. 1D) TB animals, were capable of impairing the generation of FoxP3-expressing lymphocytes. This inhibition depended on the gr-MDSC:T cell ratio, occurred even with low ratios (Fig. 2), and was not related to killing of T cells by MDSCs (data not shown). Interestingly, 4T1 tumor-induced splenic gr-MDSCs were significantly more potent at impairing TGF-β1-induced FoxP3+ iTreg generation compared with gr-MDSCs isolated from TF mice (Fig. 2). Similar results were obtained with gr-MDSCs purified from 4T1 tumors and from the tumor, spleen, or BM of LLC-bearing C57BL6 mice (data not shown). Given these findings, our subsequent studies focused on gr-MDSCs isolated from the spleen of 4T1 TB mice.
Figure 1. Ly6G+ gr-MDSCs suppress TGF-β 1-induced generation of FoxP3+ iTreg.
CD4+CD62L+ naïve T Lc were isolated from FoxP3-EGFP BALB/c (A and C) or C57BL/6 (B and D) mice and cultured for 72 h with activation beads and with or without TGF-β1 (5 ng/mL) in the presence or absence of Ly6G+ gr-MDSCs isolated from 4T1 tumors [intratumoral (IT)-MDSC (4T1); A] or from LLC tumors [intratumoral-MDSC (LLC); B] at a 1:0.5 ratio. The frequency of FoxP3+ cells was determined after 72 h. Representative dot plots are depicted (n=6 mice). The influence of Ly6G+ gr-MDSCs isolated from the spleen and BM of 4T1 (C) or LLC (D) TB mice on FoxP3 expression was examined as described above. APC=Allophycocyanin.
Figure 2. Ly6G+ gr-MDSCs suppress TGF-β1-induced FoxP3+ iTreg generation in a dose-dependent manner.
Ly6G+ gr-MDSCs were isolated from TF or 4T1 TB (4T1) mice and cultured with CD4+ CD25−CD62L+ naïve T Lc isolated from the spleen of FoxP3-EGFP BALB/c mice in the presence of activation beads and TGF-β1 at the indicated T Lc:Ly6G+ gr-MDSC ratios. The frequency of FoxP3+ cells was determined after 72 h. Representative dot plots are depicted (n=6 mice).
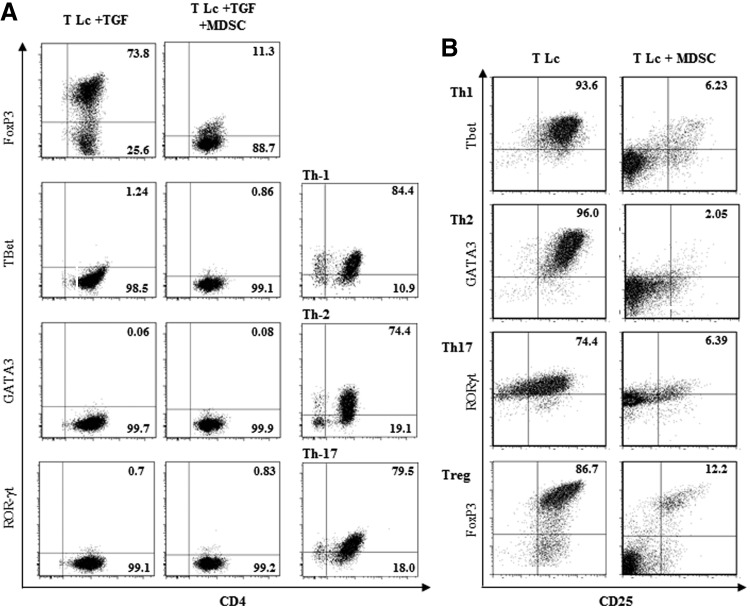
We next sought to address whether gr-MDSCs may redirect TGF-β1-induced Foxp3+ T cell differentiation toward other T Lc lineages. CD4+ T cells obtained after culture with gr-MDSCs expressed low levels of T-bet, GATA3, and ROR-γt (Fig. 3A). These results therefore indicate that gr-MDSCs do not skew TGF-β1-dependent polarization of naïve T cells from FoxP3+ Tregs toward any of the Th1, Th2, or Th17 lineages. Interestingly, the differentiation of naïve CD4+ T Lc into Th1, Th2, or Th17 lymphocyte lineages induced in vitro was also impaired by gr-MDSCs (Fig. 3B).
Figure 3. Ly6G+ gr-MDSCs suppress the differentiation of naïve T cells into Th1, Th2, or Th17 lymphocytes.
(A) CD4+CD25−CD62L+ naïve T Lc, isolated from the spleen of naive mice, were cultured with activation beads and TGF-β1, as indicated in Fig. 2, alone (T Lc+TGF) or in the presence of Ly6G+ gr-MDSCs isolated from TB mice (T Lc+TGF+MDSC; T cells:Ly6G+ gr-MDSC ratio, 1:0.5). After 72 h, the expression of the indicated transcription factors (FoxP3, Treg; T-bet, Th1; GATA-3, Th2; RORγt, Th17) was determined by flow cytometry. Th1, Th2, Th17, and Treg, generated with specific cytokines, as described in Materials and Methods, were used as positive controls. (B) The differentiation of Th1, Th2, Th17, and Treg from naïve T Lc was performed in the conditions described in Material and Methods, in the absence (T Lc) or presence (T Lc+MDSC) of gr-MDSCs isolated from TB mice. The expression of the indicated transcription factors (FoxP3, T-bet, GATA-3, RORγt) was determined after 72 h by flow cytometry.
gr-MDSC-mediated suppression of iTreg generation depends on direct cell-to-cell contact and occurs early in the differentiation process
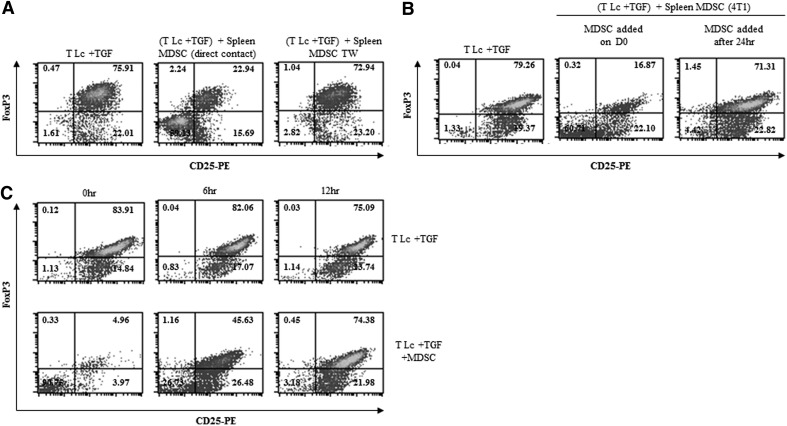
In an effort to delineate the mechanism(s) by which gr-MDSCs suppress TGF-β1-mediated iTreg polarization, gr-MDSCs were separated from T cells by a transwell insert during the differentiation process. The data depicted in Fig. 4A indicate that direct cell-to-cell contact was required for gr-MDSCs to impede FoxP3+ iTreg generation. Interestingly, the addition of gr-MDSCs, 24 h after the initiation of the conversion process, did not influence TGF-β1-induced FoxP3+ iTreg differentiation (Fig. 4B). To further identify the time window of gr-MDSC action, naïve CD4+CD25−FoxP3− T Lc cultures were initiated, with activation beads added at the start of the culture, and gr-MDSCs and TGF-β1 added at 0, 6, and 12 h after the initiation of the culture. Our results indicate that gr-MDSCs were capable of blocking iTreg generation only when added during the first 6 h of the differentiation process (Fig. 4C). As the presence of TGF-β1 was not required for iTreg differentiation during these first hours of culture (Fig. 4C, upper panels), gr-MDSC-mediated inhibition of iTreg generation may not significantly depend on a direct targeting of TGF-β1 signaling. gr-MDSCs may therefore inhibit FoxP3 induction very early in the differentiation of naïve T cells into FoxP3-expressing iTregs, suggesting possible modification of early T cell activation signaling.
Figure 4. Ly6G+ gr-MDSC-mediated suppression of iTreg differentiation depends on direct cell contact and occurs early in the differentiation process.
(A) Ly6G+ gr-MDSCs, isolated from the spleen of 4T1 TB mice, were cultured in direct contact or separated by a transwell insert (TW) with CD4+CD25−CD62L+ naïve T Lc in the presence of activation beads and TGF-β1 (5 ng/mL). After 72 h, the frequency of FoxP3+ cells was determined by flow cytometry. (B) CD4+CD25−CD62L+ naïve T Lc were cultured in the presence of activation beads and TGF-β1 (5 ng/mL), and Ly6G+ gr-MDSCs, isolated from the spleen of 4T1 TB mice, were added from the beginning [Ly6G+ gr-MDSCs added on Day 0 (D0)] or 24 h after (Ly6G+ gr-MDSCs added after 24 h) the initiation of the T cell culture. The frequency of FoxP3+ cells was determined as described above. (C) CD4+CD25−CD62L+ naïve T Lc were incubated with activation beads at the time the culture was initiated. TGF-β1 (5 ng/mL) and Ly6G+ gr-MDSCs (T Lc:Ly6G+ gr-MDSC, 1:0.5) were added to the T Lc at 0, 6, or 12 h after the initiation of the culture. The frequency of FoxP3+ cells was determined by flow cytometry analysis as described above.
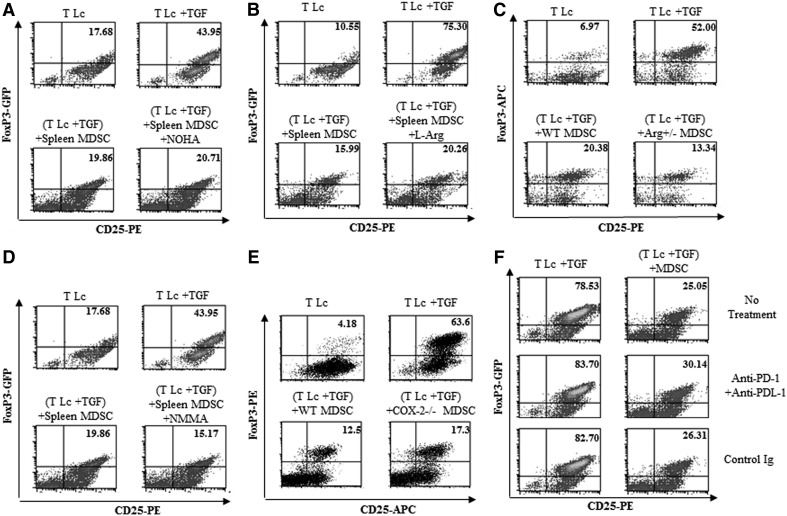
Inhibition of FoxP3+ iTreg generation by gr-MDSCs does not depend on arginase 1, cystine or cysteine, NO, COX-2, or PD-1/PD-L1
The expression of arginase 1 by MDSCs has been identified as one of the key mechanisms underlying the immunosuppressive function of these cells [48]. We therefore examined whether arginase 1 may play a role in gr-MDSC-mediated inhibition of iTreg generation. As expected, arginase 1 mRNA was significantly higher in gr-MDSCs isolated from the spleen of 4T1 TB mice compared with gr-MDSCs isolated from the spleen of TF animals (Supplemental Fig. 4A). gr-MDSCs were then treated with NOHA, a specific inhibitor of arginase 1, and cultured with naïve T cells in the presence of TGF-β1. NOHA did not prevent gr-MDSC-mediated suppression of FoxP3+ iTreg differentiation (Fig. 5A). Moreover, the addition of exogenous l-arginine to overcome any possible arginase 1-mediated depletion of this amino acid had no effect on the inhibition of iTreg generation by gr-MDSCs (Fig. 5B). Consistent with these data, the ability of splenic gr-MDSCs, isolated from arginase 1-deficient (Arg1+/−) TB animals, which expressed a significantly reduced level of arginase 1 (data not shown), to impede TGF-β1-mediated differentiation of naïve T cells into iTreg, was also not impaired (Fig. 5C). Taken together, these results rule out a significant role for arginase 1 in gr-MDSC-mediated inhibition of iTreg generation.
Figure 5. Ly6G+ gr-MDSC-mediated inhibition of iTreg generation does not depend on arginase 1, iNOS and NO, COX-2, or PD-1 and PD-L1.
CD4+CD25−CD62L+ naïve T Lc (T Lc) were cultured with activation beads, TGF-β1 (5 ng/mL), Ly6G+ gr-MDSCs, and (A) NOHA, (B) l-arginine (l-Arg), or (D) l-NMMA (NMMA). The frequency of FoxP3+ cells was determined after 72 h. Ly6G+ gr-MDSCs were isolated from LLC tumor-bearing wild type mice (WT MDSC) or from (C) LLC TB arginase 1 +/− (+Arg+/−MDSC) mice or (E) LLC TB COX-2−/− (+COX−/− MDSC) mice and cultured for 72 h with CD4+CD25−CD62L+ naïve T Lc isolated from C57BL/6 mice in the presence of activation beads and TGF-β1 (5 ng/mL). The frequency of FoxP3+ cells was determined as described above. (F) CD4+CD25−CD62L+ naïve T Lc were first pretreated with anti-PD-1- and anti-PD-L1-blocking antibodies or isotype control antibodies. T Lc were then cultured for 72 h with activation beads, TGF-β1 (5 ng/mL), and Ly6G+ gr-MDSCs. The frequency of FoxP3+ cells was determined by flow cytometry analysis.
It has been reported that MDSCs may inhibit T cell function through the consumption of cystine. MDSCs can take up cystine, but unlike APCs, they do not export cysteine, an amino acid essential for T cell activation [49]. MDSCs have also been shown to oxidize exogenous cysteine. Neither the addition of exogenous cysteine nor the addition of β-ME, a chemical capable of inhibiting cysteine oxidation, significantly modified the ability of gr-MDSCs to hinder iTreg differentiation (Supplemental Fig. 4B).
MDSCs may also exert their function through the release of NO produced by iNOS [9, 17]. l-NMMA, an iNOS inhibitor, did not affect gr-MDSC-mediated inhibition of FoxP3+ iTreg generation (Fig. 5D). Consistent with this result, nitrites, the main metabolites of NO, were not detectable in gr-MDSCs and differentiating T cell culture supernatants (Supplemental Fig. 4C).
COX-2 has been reported to play a role in the suppression of adaptive T cell responses against cancer and may contribute to MDSC expansion and enhanced suppressive function [50]. We did not detect any significant expression of COX-2 by gr-MDSCs using real-time PCR, and more importantly, MDSCs from COX-2 knockout mice demonstrated a similar potential to impair iTreg generation than MDSCs from control WT mice (Fig. 5E), indicating that this enzyme does not play a major role in the modulation of iTreg generation by gr-MDSCs.
PD-1 is a membrane protein from the same family of T cell regulators as CD28 and CTLA4. PD-1 is expressed by activated T and B cells and binds to PD-L1, expressed on MDSCs, tumor cells, activated macrophages, some subsets of DCs, and resting and activated T Lc. The engagement of PD-1 with PD-L1 results in the inhibition of T cell proliferation and activation [51]. In addition, the binding of CD80 expressed by APCs with PD-L1, expressed by resting or activated T cells, may also lead to T Lc suppression. We sought to determine whether the PD-1/PD-L1 axis may play a role in gr-MDSC-mediated suppression of iTreg differentiation. The results shown in Fig. 5F indicate that anti-PD-1- and anti-PD-L1-blocking antibodies did not significantly prevent the inhibition of iTreg generation by gr-MDSCs.
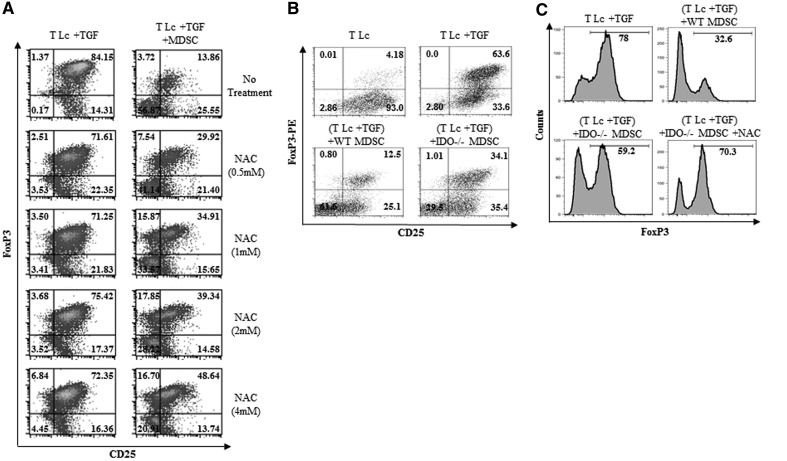
The inhibition of iTreg generation by Ly6G+ gr-MDSCs depends on ROS and IDO
MDSCs have been reported to suppress T cell activation through the production of ROS [5, 52]. Although the mechanism(s) of ROS-mediated MDSC suppression remain to be fully elucidated, in patients treated with ROS inhibitors, the ability of MDSCs to suppress T cells was inhibited significantly [5]. We therefore sought to evaluate whether ROS may play a role in gr-MDSC-mediated suppression of iTreg generation using the ROS inhibitor, NAC. Our results indicate that NAC reverted, although not completely, the inhibition of TGF-β-mediated generation of iTreg by gr-MDSCs (Fig. 6A). NAC did not significantly modify the ability of Ly6G+ gr-MDSCs to suppress the proliferation of conventional T Lc induced with anti-CD3/anti-CD28 (not shown). As NAC only partially restored iTreg polarization, we next sought to examine the possible contribution of IDO, an immunomodulatory enzyme responsible for the catabolism of the essential amino acid tryptophan and involved in T cell suppression, in this phenomenon. gr-MDSCs, isolated from TB IDO−/− mice, were less potent at impairing iTreg generation than their counterparts isolated from WT animals (Fig. 6B). In addition, NAC further inhibited the capability of gr-MDSCs from IDO−/− mice to suppress iTreg differentiation (Fig. 6C), indicating that IDO and ROS are both required for MDSC-mediated inhibition of iTreg generation.
Figure 6. The suppression of iTreg differentiation by Ly6G+ gr-MDSCs partially depends on ROS and IDO.
(A) Ly6G+ gr-MDSCs were isolated from the spleen of 4T1 TB mice, pretreated for 1 h with NAC at the indicated concentrations and cultured with CD4+CD25−CD62L+ naïve T Lc (T Lc:Ly6G+ MDSC ratio, 1:0.5) for 72 h in the conditions indicated in Fig. 5 and in the presence of NAC at the same concentrations. The frequency of FoxP3+ cells was then determined by flow cytometry. (B) Ly6G+ gr-MDSCs were isolated from TB WT MDSCs or IDO−/− (+IDO−/− MDSC) mice and cultured with naïve CD4+CD25−CD62L+ T cells as described in A. The frequency of FoxP3+ cells was then determined by flow cytometry after 72 h. (C) The same experiment as described in B was performed, and NAC (4 mM) was added to the coculture. The percentage of FoxP3+ cells was determined after gating on the CD4+ T Lc population.
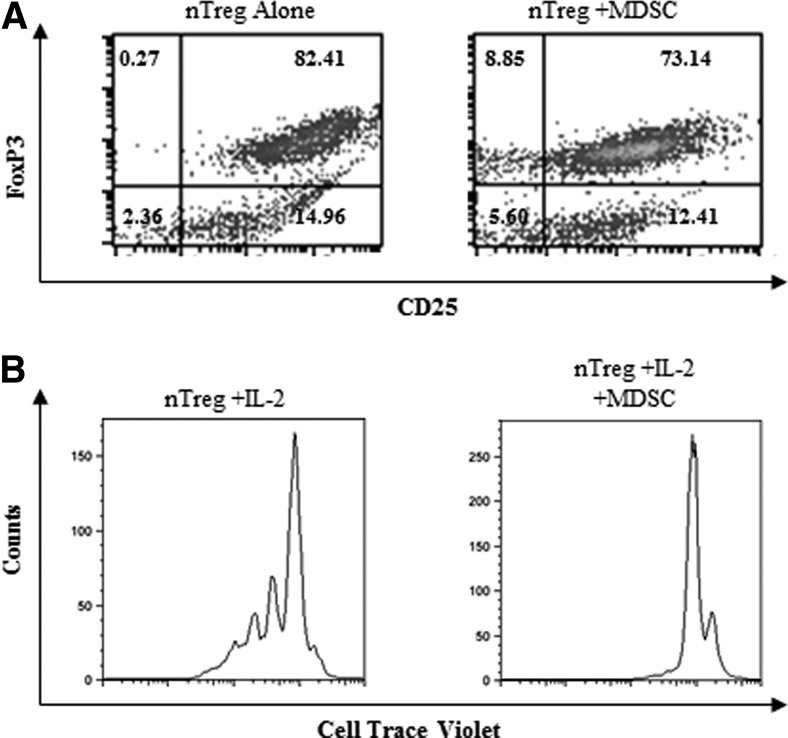
gr-MDSCs do not modify FoxP3 expression but impair the proliferation of Tregs isolated from naïve mice
It has been reported that Mo-MDSC [38] or gr-MDSC [35] may enhance the proliferation of nTregs. To clarify the influence of gr-MDSCs expanded in 4T1 TB mice on already differentiated Tregs, FoxP3+ T Lc were isolated from naïve FoxP3-EGFP mice and were cultured for 48 h with gr-MDSCs in the presence of activation beads and IL-2. Sixty-eight percent of these FoxP3+EGFP+ cells expressed the Ikaros transcription factor Helios, primarily expressed by nTregs. Our results indicated that gr-MDSCs did not affect FoxP3 expression by already differentiated Tregs (Fig. 7A) but impaired their proliferation (Fig. 7B).
Figure 7. Ly6G+ gr-MDSCs do not modulate FoxP3 expression of already-differentiated CD4+ CD25+ cells but impair their proliferation.
(A) CD4+CD25+FoxP3+ (nTreg) cells were isolated from the spleen of TF BALB/c mice by magnetic cell sorting. Sixty-eight percent of the cells express Helios. Cells were cultured with Ly6G+ gr-MDSCs isolated from the spleen of 4T1 TB mice (nTreg:Ly6G+ gr-MDSC ratio, 1:0.5) in the presence of activation beads and IL-2 (20 IU/mL). After 48 h, the expression of FoxP3 was detected by flow cytometry. (B) CD4+CD25+FoxP3+, isolated as explained in A, were stained with CellTrace Violet and were cultured with activation beads and IL-2 (20 IU/mL), with or without Ly6G+ gr-MDSCs (nTreg:Ly6G+ gr-MDSC ratio, 1:0.5). Proliferation of gated FoxP3-EGFP cells was assessed by measuring CellTrace Violet dilution by flow cytometry.
DISCUSSION
CD4+CD25+FoxP3+ Tregs and MDSCs represent two immunosuppressive cell populations that play a crucial role in the establishment and maintenance of tumor-immune tolerance. They thereby constitute a major obstacle for successful cancer immunotherapy. However, the cross-talk between tumor-induced MDSCs and tumor-induced Tregs remains incompletely defined. Whether MDSCs may promote the expansion and function of Tregs, which in turn, may induce MDSCs by a positive-feedback amplification loop remains unclear. Previous reports have proposed that MDSCs may contribute to the observed increase in Treg frequency in animal cancer models, in part, by fostering nTreg expansion [35–37]. We reasoned that gr-MDSCs might promote the generation of iTreg from naïve T cells, as one of the mechanisms accounting for Treg expansion in cancer. Surprisingly, our results indicate that gr-MDSCs were not only incapable of promoting iTreg differentiation, but they also hampered TGF-β1-induced generation of these cells with a more pronounced effect with gr-MDSCs isolated from TB animals. These observations, seemingly in opposition with the concept that these two immunosuppressive cell populations may feed on each other, are in fact not unexpected, as blockade of T Lc activation represents a primary mode of action of MDSCs and as the differentiation of iTreg critically depends on activation signals leading to CD25 expression. Inhibition of naïve T cell activation by MDSCs may thus prevent their further differentiation into FoxP3-expressing Tregs. Multiple groups, including ours, have reported that MDSCs and Tregs are expanded in TB hosts. It is possible that tumors may concomitantly promote MDSCs and Tregs (which share the common ability to suppress conventional effector T Lc and NK cells) as two overlapping mechanisms to impair anticancer immunity. Although differentiating Tregs may be under the pressure of negative signals conveyed by tumor-induced MDSCs, the complex immunosuppressive tumor environment may also provide positive signals fostering Treg expansion and function. The integration of these signals may overall lead to the promotion of these cells in the context of growing tumors.
In an effort to identify the mechanism(s) underlying gr-MDSC-mediated suppression of iTreg generation, we extensively investigated the possible role for the main suppressive factors by which MDSCs mediate their immunoinhibitory function. Arginase 1 has been described as a key enzyme involved in MDSC-mediated suppression of T cells. Arginase 1 activity leads to the depletion of arginine, which results in the inhibition of the re-expression of the CD3-ζ chain in activated T cells [48]. Our results indicate that arginase 1 does not play a major role in the inhibition of iTreg generation by gr-MDSCs. Similarly iNOS expression and NO production may contribute to MDSC activity [9, 17]. We demonstrate that iNOS is not responsible for gr-MDSC-mediated suppression of iTreg differentiation. COX-2 has also been shown to play a critical role in the induction of MDSCs and the maintenance of cancer tolerance [50]. Our data, using COX-2 knockout mice, indicate that this enzyme was, however, not involved in the inhibition of iTreg generation by gr-MDSCs. The engagement of PD-1 expressed on T cells with PD-L1 expressed by tumor cells leads to the inhibition of IL-2 production by and proliferation of T Lc [53]. Interestingly, it has been documented that CD4+CD25+FoxP3+ Tregs can be negatively regulated following PD-1/PD-L1 interaction by a mechanism involving the down-regulation of STAT-5 phosphorylation [54]. However, we determined that the PD-1/PD-L1 axis does not significantly contribute to gr-MDSC modulation of iTregs.
Previous reports have indicated that ROS produced by MDSCs play an important role in the suppressive activity of these cells [5]. For instance, it has been reported that ROS, in conjunction with peroxynitrites, may interfere with the recognition of antigens by the TCR, resulting in CD8+ T cell inhibition [55]. IDO is an immunomodulatory enzyme responsible for the catabolism of the essential amino acid tryptophan. IDO-mediated depletion of tryptophan and the subsequent production of immunosuppressive products such as kynurenine may also lead to T cell suppression through down-regulation of the TCR-CD3-ζ chain [56]. Treg differentiation depends on activation signals relayed by the TCR and CD28 converging toward the regulation of specific gene expression such as FoxP3 [57]. Our data indicate that gr-MDSCs may inhibit FoxP3 induction very early in the differentiation of naïve T cells into FoxP3-expressing iTregs and that a negative targeting of the TGF-β1 signaling is unlikely. This observation points toward a negative modulation of early T cell activation signaling cascades by tumor-derived gr-MDSCs. Consistent with these considerations, we demonstrated that the ROS inhibitor NAC partially reversed the effects of gr-MDSCs on iTreg differentiation and that gr-MDSCs from IDO−/− mice were less efficient than their WT counterpart at impairing iTreg generation. Furthermore, in the presence of NAC, gr-MDSCs from IDO−/− mice were further impaired in their ability to block the conversion of naïve T cells into iTregs, suggesting that ROS and IDO may act in concert to suppress the activation signaling cascades in differentiating T cells.
Our results therefore suggest that the interplay between these two immunosuppressive cell populations in cancer may be more complex than envisioned initially and advocate for a new concept that gr-MDSCs may have the peculiar ability to suppress iTreg generation and to impair already-differentiated nTreg proliferation. Some reports have indicated that MDSCs may foster the proliferation of Tregs [35, 38]. However, these studies primarily focused on the Mo-MDSC subset, which is not expanded predominantly in cancer [15] or used tumor models associated with a modest induction of MDSCs [35]. Our current data further highlight gr-MDSCs as central determining factors in the development of Tregs during tumor progression and provide more insights into understanding the complex interactions between these two immunosuppressive cell populations.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the U.S. National Institutes of Health grant R01 CA104926 (NL and EK), Cancer Biology Training grant T32 CA009213 (SMC, CJL, DA), AZ Cancer Center grant CA023074, Cancer Center Support grant CA 023074, Tee Up for Tots, and PANDA Funds (NL and EK). We thank Paula Campbell (AZCC/ARL, Division of Biotechnology, Cytometry Core Facility, Arizona Cancer Center, University of Arizona, Tucson, AZ, USA) and Alexis Bucknam for technical assistance.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- BM
- bone marrow
- CD62L
- CD62 ligand
- Ct
- comparative threshold
- FoxP3
- forkhead box P3
- GATA3
- guanine adenine thymine adenine sequence-binding protein 3
- gr-MDSC
- granulocytic myeloid-derived suppressor cell
- iTreg
- inducible regulatory T cell
- l-NMMA
- NG-monomethyl-l-arginine
- LLC
- Lewis lung carcinoma
- MDSC
- myeloid-derived suppressor cell
- Mo-MDSC
- monocytic myeloid-derived suppressor cell
- NAC
- N-acetyl cysteine
- NOHA
- Nω-hydroxy-nor-l-arginine
- nTreg
- naturally occurring regualtory T cell
- PD-1
- programmed death 1
- PD-L1
- programmed death ligand 1
- RBC
- red blood cell
- RORγt
- Retinoic acid-related orphan receptor γt
- T-bet
- Th1-specific T box transcription factor
- T Lc
- T lymphocyte
- TB
- tumor-bearing
- TF
- tumor-free
- Treg
- regulatory T cell
- −/−
- homozygote mice for targeted deletion of the gene of interest
- +/−
- heterozygote for targeted deletion of the gene of interest
AUTHORSHIP
S.M.C. and M.T. performed research. C.B.L., C.J.L., N.T.H., J.K., and D.A. provided technical assistance. C.B.L., C.J.L., D.A., N.J., and B.B. discussed data. N.J. and B.B. read and commented on the manuscript. S.M.C., M.T., and N.L. analyzed data. S.M.C., M.T., N.L., and E.K. wrote the paper. N.L. and E.K. designed and directed research.
REFERENCES
- 1. Drake C. G., Jaffee E., Pardoll D. M., James P., Allison G. D., Frederick W. A. (2006) Mechanisms of immune evasion by tumors. Adv. Immunol. 90, 51–81 [DOI] [PubMed] [Google Scholar]
- 2. Nicolini A., Carpi A. (2009) Immune manipulation of advanced breast cancer: an interpretative model of the relationship between immune system and tumor cell biology. Med. Res. Rev. 29, 436–471 [DOI] [PubMed] [Google Scholar]
- 3. Zou W. (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 6, 295–307 [DOI] [PubMed] [Google Scholar]
- 4. Marigo I., Dolcetti L., Serafini P., Zanovello P., Bronte V. (2008) Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol. Rev. 222, 162–179 [DOI] [PubMed] [Google Scholar]
- 5. Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brandau S., Trellakis S., Bruderek K., Schmaltz D., Steller G., Elian M., Suttmann H., Schenck M., Welling J., Zabel P., Lang S. (2011) Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J. Leukoc. Biol. 89, 311–317 [DOI] [PubMed] [Google Scholar]
- 7. Movahedi K., Guilliams M., Van den Bossche J., Van den Bergh R., Gysemans C., Beschin A., De Baetselier P., Van Ginderachter J. A. (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111, 4233–4244 [DOI] [PubMed] [Google Scholar]
- 8. Melani C., Chiodoni C., Forni G., Colombo M. P. (2003) Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 102, 2138–2145 [DOI] [PubMed] [Google Scholar]
- 9. Liu C. Y., Wang Y. M., Wang C. L., Feng P. H., Ko H. W., Liu Y. H., Wu Y. C., Chu Y., Chung F. T., Kuo C. H., Lee K. Y., Lin S. M., Lin H. C., Wang C. H., Yu C. T., Kuo H. P. (2010) Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14−CD15+CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 136, 35–45 [DOI] [PubMed] [Google Scholar]
- 10. Kusmartsev S., Gabrilovich D. (2006) Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol. Immunother. 55, 237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kusmartsev S., Gabrilovich D. (2002) Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol. Immunother. 51, 293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serafini P., De Santo C., Marigo I., Cingarlini S., Dolcetti L., Gallina G., Zanovello P., Bronte V. (2004) Derangement of immune responses by myeloid suppressor cells. Cancer Immunol. Immunother. 53, 64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ostrand-Rosenberg S. (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 59, 1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ostrand-Rosenberg S., Sinha P. (2009) Myeloid-derived suppressor cells: linking inflammation and cancer J. Immunol. 182, 4499–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Youn J. I., Nagaraj S., Collazo M., Gabrilovich D. I. (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ochoa A. C., Zea A. H., Hernandez C., Rodriguez P. C. (2007) Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin. Cancer Res. 13, 721s–726s [DOI] [PubMed] [Google Scholar]
- 17. Angulo I., de las Heras F. G., Garcia-Bustos J. F., Gargallo D., Munoz-Fernandez M. A., Fresno M. (2000) Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood 95, 212–220 [PubMed] [Google Scholar]
- 18. Kronenberg M., Rudensky A. (2005) Regulation of immunity by self-reactive T cells. Nature 435, 598–604 [DOI] [PubMed] [Google Scholar]
- 19. Sakaguchi S. (2000) Regulatory T cells: key controllers of immunologic self-tolerance. Cell 101, 455–458 [DOI] [PubMed] [Google Scholar]
- 20. Xu L., Xu W., Qiu S., Xiong S. (2010) Enrichment of CCR6+Foxp3+ regulatory T cells in the tumor mass correlates with impaired CD8+ T cell function and poor prognosis of breast cancer. Clin. Immunol. 135, 466–475 [DOI] [PubMed] [Google Scholar]
- 21. Camisaschi C., Casati C., Rini F., Perego M., De Filippo A., Triebel F., Parmiani G., Belli F., Rivoltini L., Castelli C. (2010) LAG-3 expression defines a subset of CD4+CD25highFoxp3+ regulatory T cells that are expanded at tumor sites. J. Immunol. 184, 6545–6551 [DOI] [PubMed] [Google Scholar]
- 22. Curotto de Lafaille M. A., Lafaille J. J. (2009) Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30, 626–635 [DOI] [PubMed] [Google Scholar]
- 23. Khattar M., Chen W., Stepkowski S. (2009) Expanding and converting regulatory T cells: a horizon for immunotherapy. Arch. Immunol. Ther. Exp. (Warsz) 57, 199–204 [DOI] [PubMed] [Google Scholar]
- 24. Piccirillo C. A. (2008) Regulatory T cells in health and disease. Cytokine 43, 395–401 [DOI] [PubMed] [Google Scholar]
- 25. Liyanage U. K., Moore T. T., Joo H-G., Tanaka Y., Herrmann V., Doherty G., Drebin J. A., Strasberg S. M., Eberlein T. J., Goedegebuure P. S., Linehan D. C. (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 169, 2756–2761 [DOI] [PubMed] [Google Scholar]
- 26. Viguier M., Lemaître F., Verola O., Cho M-S., Gorochov G., Dubertret L., Bachelez H., Kourilsky P., Ferradini L. (2004) Foxp3 expressing CD4+CD25high regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 173, 1444–1453 [DOI] [PubMed] [Google Scholar]
- 27. Sakaguchi S., Miyara M., Costantino C. M., Hafler D. A. (2010) FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 10, 490–500 [DOI] [PubMed] [Google Scholar]
- 28. Valzasina B., Piconese S., Guiducci C., Colombo M. P. (2006) Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25− lymphocytes is thymus and proliferation independent. Cancer Res. 66, 4488–4495 [DOI] [PubMed] [Google Scholar]
- 29. Chen X., Zhou B., Li M., Deng Q., Wu X., Le X., Wu C., Larmonier N., Zhang W., Zhang H., Wang H., Katsanis E. (2007) CD4+CD25+FoxP3+ regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin. Immunol. 123, 50–59 [DOI] [PubMed] [Google Scholar]
- 30. Larmonier N., Marron M., Zeng Y., Cantrell J., Romanoski A., Sepassi M., Thompson S., Chen X., Andreansky S., Katsanis E. (2007) Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-β and IL-10. Cancer Immunol. Immunother. 56, 48–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Larmonier N., Janikashvili N., LaCasse C. J., Larmonier C. B., Cantrell J., Situ E., Lundeen T., Bonnotte B., Katsanis E. (2008) Imatinib mesylate inhibits CD4+CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL− tumors. J. Immunol. 181, 6955–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Janikashvili N., Lacasse C. J., Larmonier C., Trad M., Herrell A., Bustamante S., Bonnotte B., Har-Noy M., Larmonier N., Katsanis E. (2011) Allogeneic effector/memory Th-1 cells impair FoxP3+ regulatory T lymphocytes and synergize with chaperone-rich cell lysate vaccine to treat leukemia. Blood 117, 1555–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghiringhelli F., Larmonier N., Schmitt E., Parcellier A., Cathelin D., Garrido C., Chauffert B., Solary E., Bonnotte B., Martin F. (2004) CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 34, 336–344 [DOI] [PubMed] [Google Scholar]
- 34. Chung D. J., Rossi M., Romano E., Ghith J., Yuan J., Munn D. H., Young J. W. (2009) Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 114, 555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Serafini P., Mgebroff S., Noonan K., Borrello I. (2008) Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 68, 5439–5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang B., Pan P. Y., Li Q., Sato A. I., Levy D. E., Bromberg J., Divino C. M., Chen S. H. (2006) Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 66, 1123–1131 [DOI] [PubMed] [Google Scholar]
- 37. Yang R., Cai Z., Zhang Y., Yutzy W. H., IV, Roby K. F., Roden R.B. (2006) CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 66, 6807–6815 [DOI] [PubMed] [Google Scholar]
- 38. Pan P-Y., Ma G., Weber K. J., Ozao-Choy J., Wang G., Yin B., Divino C. M., Chen S-H. (2010) Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 70, 99–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kodumudi K. N., Woan K., Gilvary D. L., Sahakian E., Wei S., Djeu J. Y. A. (2010) Novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. 16, 4583–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sinha P., Clements V. K., Fulton A. M., Ostrand-Rosenberg S. (2007) Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 67, 4507–4513 [DOI] [PubMed] [Google Scholar]
- 41. Sinha P., Clements V. K., Bunt S. K., Albelda S. M., Ostrand-Rosenberg S. (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 179, 977–983 [DOI] [PubMed] [Google Scholar]
- 42. Youn J-I., Collazo M., Shalova I. N., Biswas S. K., Gabrilovich D. I. (2012) Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J. Leukoc. Biol. 91, 167–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sawanobori Y., Ueha S., Kurachi M., Shimaoka T., Talmadge J. E., Abe J., Shono Y., Kitabatake M., Kakimi K., Mukaida N., Matsushima K. (2008) Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111, 5457–5466 [DOI] [PubMed] [Google Scholar]
- 44. Kodumudi K. N., Woan K., Gilvary D. L., Sahakian E., Wei S., Djeu J. Y. (2010) A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin. Cancer Res. 16, 4583–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ko J. S., Zea A. H., Rini B. I., Ireland J. L., Elson P., Cohen P., Golshayan A., Rayman P. A., Wood L., Garcia J., Dreicer R., Bukowski R., Finke J. H. (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 [DOI] [PubMed] [Google Scholar]
- 46. Rodriguez P. C., Ernstoff M. S., Hernandez C., Atkins M., Zabaleta J., Sierra R., Ochoa A. C. (2009) Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 69, 1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Minton K. (2006) The dual personality of myeloid suppressor cells. Nat. Rev. Immunol. 6, 792–792 [Google Scholar]
- 48. Rodriguez P. C., Quiceno D. G., Zabaleta J., Ortiz B., Zea A. H., Piazuelo M. B., Delgado A., Correa P., Brayer J., Sotomayor E. M., Antonia S., Ochoa J. B., Ochoa A. C. (2004) Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 64, 5839–5849 [DOI] [PubMed] [Google Scholar]
- 49. Srivastava M. K., Sinha P., Clements V. K., Rodriguez P., Ostrand-Rosenberg S. (2010) Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 70, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Veltman J., Lambers M., van Nimwegen M., Hendriks R., Hoogsteden H., Aerts J., Hegmans J. (2010) COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer 10, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu Y., Zeng B., Zhang Z., Zhang Y., Yang R. (2008) B7-H1 on myeloid-derived suppressor cells in immune suppression by a mouse model of ovarian cancer. Clin. Immunol. 129, 471–481 [DOI] [PubMed] [Google Scholar]
- 52. Kusmartsev S., Nefedova Y., Yoder D., Gabrilovich D. I. (2004) Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172, 989–999 [DOI] [PubMed] [Google Scholar]
- 53. Iwai Y., Ishida M., Tanaka Y., Okazaki T., Honjo T., Minato N. (2002) Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 99, 12293–12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Franceschini D., Paroli M., Francavilla V., Videtta M., Morrone S., Labbadia G., Cerino A., Mondelli M. U., Barnaba V. (2009) PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phosphorylation in patients chronically infected with HCV. J. Clin. Invest. 119, 551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nagaraj S., Gupta K., Pisarev V., Kinarsky L., Sherman S., Kang L., Herber D. L., Schneck J., Gabrilovich D. I. (2007) Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med. 13, 828–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Munn D. H., Mellor A. L. (2007) Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 117, 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Von Boehmer H., Nolting J. (2008) What turns on Foxp3? Nat. Immunol. 9, 121–122 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.