Cftr is directly involved in myeloid cell function, contributing to the pathophysiological phenotype of the CF lung.
Keywords: immunity, Pseudomonas aeruginosa, murine knockout model, potential difference, lysozyme-M
Abstract
The absence or reduction of CFTR function causes CF and results in a pulmonary milieu characterized by bacterial colonization and unresolved inflammation. The ineffectiveness at controlling infection by species such as Pseudomonas aeruginosa suggests defects in innate immunity. Macrophages, neutrophils, and DCs have all been shown to express CFTR mRNA but at low levels, raising the question of whether CFTR has a functional role in these cells. Bone marrow transplants between CF and non-CF mice suggest that these cells are inherently different; we confirm this observation using conditional inactivation of Cftr in myeloid-derived cells. Mice lacking Cftr in myeloid cells overtly appear indistinguishable from non-CF mice until challenged with bacteria instilled into the lungs and airways, at which point, they display survival and inflammatory profiles intermediate in severity as compared with CF mice. These studies demonstrate that Cftr is involved directly in myeloid cell function and imply that these cells contribute to the pathophysiological phenotype of the CF lung.
Introduction
Inflammation and infection as well as viscous secretions and impaired mucociliary clearance characterize the lungs and airways of CF patients and contribute significantly to the morbidity and mortality associated with the disease. These processes involve the functions of and interactions among multiple cell types, including ciliated epithelial cells, goblet cells, and immune cells. The epithelial cells and goblet cells line the airway and are responsible for barrier function, secretions, and mucociliary clearance. The immune cells assist in host response to environmental perturbations by neutralizing pathogens as well as returning the host to homeostasis. Although earlier models proposed most of the pulmonary complications of CF to be direct consequences of epithelial dysfunction caused by the absence of CFTR, more recent data have indicated that other cell types, such as lymphocytes [1], neutrophils [2], macrophages [3–5], and even DCs [6], are affected directly by the absence of CFTR function, impairing their ability to resolve infections and inflammation.
Whereas in vitro cell culture studies have demonstrated the expression of CFTR in specific cell types, they do not empirically test the physiological role of CFTR or the consequences of its absence. Recent bone marrow transplant studies between CF and non-CF mice have demonstrated that responses to bacterial LPS are influenced by bone marrow-derived cells [5], further suggesting that these cell types have an important role in the pathophysiology of CF lung disease.
To explore the role of CFTR in immune cells in vivo, we applied a pulmonary infection model to study inflammatory pathways and bacterial killing in CF mice using a conditional expression model of murine Cftr [7–10]. We have used the conditional model to assess the role of Cftr in other cell types [1, 11] and applied it here using a myeloid-targeted Cre-recombinase LysMCre [12], which is expressed in cells such as neutrophils, macrophages, and DCs. The effects of conditional Cftr inactivation in response to bacterial infection indicate that the CF immune system is impaired, requiring a much longer time course to resolve inflammation, and is less effective at infection resolution. This is in the context of having no detectable effect on epithelial ion transport or other CF-associated phenotypes, such as growth reduction or intestinal obstruction, as observed in the total absence of functional Cftr. The data presented in this manuscript suggest that the pathophysiology of the CF lung is not simply the result of defective epithelial cell function but a combined effect of airway epithelial cell dysfunction and inefficient immune cell management of the inflammatory response to infection.
MATERIALS AND METHODS
Mice
All procedures involving mice were reviewed and approved by the CWRU Institutional Animal Care and Use Committee. C57BL/6J mice were obtained originally from The Jackson Laboratory (Bar Harbor, ME, USA), and CF mice (B6.129P-2 Cftrtm1Unc) carry a null allele of Cftr, Cftrtm1Unc. Creation and genotyping of the exon 10-floxed allele of Cftr, Cftrtm1Cwr, referred to here as Cftrfl10, as well as the exon 10-deleted allele, Cftrtm1.1Cwr, referred to here as CftrΔ10, are described elsewhere [6]. Cftrfl10 homozygous mice carrying the LysMCre transgene [2] were generated to produce myeloid-specific Cftr inactivation (Cftrfl10+LysMCre). All mice, including those carrying the floxed Cftr alleles and the LysMCre transgene, were congenic (>10 generations) on C57BL/6J. All animal groups are housed in the same facility with quarterly sentinel surveillance by the Animal Resource Center, and additional quality-control assays were carried out by the CWRU CF Mouse Models Core. To evaluate the presence of infection, whole lung homogenates and BAL were collected from untreated littermates of experimental animals and cultured for growth under a variety of conditions. BALF from some of the animal studies was evaluated for mycoplasm contamination (PCR Mycoplasma Test Kit I/RT, PromoKine, Heidelberg, Germany).
BAL cells
Mice were injected s.c. with ketamine (80 mg/kg) and xylazine (10 mg/kg) as described previously [3]. The thoracic cavity was opened and lungs exposed, followed by inserting a cannula through the trachea into the bronchi and infusing 1 × 1 ml aliquot of warm PBS containing 0.2% lidocaine to do the BAL. The BALF samples were recovered by aspirating the liquid with a syringe. The BAL was evaluated for total cell count, cellular differential, and the presence of proinflammatory cytokines.
Pertioneal neutrophils
Peritoneal-derived neutrophils were obtained by lavaging the peritoneal cavity 3 days after treatment with thioglycollate as described previously [4].
Splenic T cells
Spleens were removed from mice and minced, followed by negative selection using the T cell separation protocol (Stem Cell Separation, San Diego, CA, USA). Flow cytometry was used to evaluate the purity of the preparation, looking at CD3 versus F4/80 (macrophage marker) and Ly6G (neutrophil marker), as detailed elsewhere [5].
Bone marrow-derived macrophages
The generation of bone marrow-derived macrophages was done as described previously [3]. The femur and tibia were removed and wiped with alcohol, followed by immersion in RPMI cell culture medium containing antibiotics. With the use of sterile, 1-cc syringes, fresh RPMI was pushed through the bone marrow cavity. The bone marrow cells were counted for viability (trypan blue exclusion), followed by culture for 7–10 days with L929 support medium [13].
Infection model
P. aeruginosa PAM57-15 (mucoid clinical isolate) was used at a sublethal dose of 105 viable cfu/ml (calculated 0.2 bacteria/bead), embedded in agarose beads, and instilled into the right mainstem bronchus [6, 7]. Total bacterial dose was defined by viability of bacteria postbead preparation. Lung bacterial load was determined by culturing BALF and whole lung homogenates at sacrifice. Infection was allowed to persist for 3 or 10 days.
Clinical and lung pathology scores
Mice were assessed clinically once daily for coat quality, posture, ability to right themselves after being placed in lateral recumbence, ambulation, and body weight [6]. At Day 3 or 10, animals were euthanized, and lungs were isolated and assessed for gross lung pathology in addition to quantitative bacteriological assessment. A description of the clinical and gross lung pathology scoring categories are indicated in Table 1.
Table 1. Key to Clinical and Gross Lung Pathology Scores.
| Score | Clinical scorea | Gross lung pathologya |
|---|---|---|
| 0 | healthy appearance and activity | within normal limits |
| 1 | scruffy appearance | darker red |
| 2 | scruffy and dehydrated | few nodules |
| 3 | scruffy, dehydrated, decreased activity | several nodules, <25% consolidation |
| 4 | scruffy, dehydrated, minimal activity | numerous nodules 25–50% consolidation |
| 5 | moribund or dead | numerous nodules >50% consolidation |
Bioelectric measurements
NPD
The PD across the nasal epithelium was measured as described previously [8]. Briefly, mice were anesthetized with 8.5 mg/ml ketamine, 1.7 mg/ml xylazine, and 0.3 mg/ml acepromazine in sterile saline (i.p., 0.012 ml/g body weight). A PE-10 tube stretched to ∼0.3 mm outer diameter was inserted 2 mm into the mouse nostril and placed against the septum to serve as a bridge for the lumenal electrode. The lumenal surface of the septum was superfused with a series of solutions, each connected to calomel electrodes via Ringers/4% agar bridges. The HEPES-buffered Ringers superfusion solutions contained the following reagents (in mM): 128 NaCl, 5 KCl, 2.5 Na2HPO4, 1.8 CaCl, 1.0 MgSO4, and 10 HEPES-NaOH, pH 7.4. Low Cl− solution was prepared by isomolar replacement of NaCl with Na-gluconate. Amiloride (100 μM) was present in all solutions to block epithelial sodium channels, and forskolin (100 μM) was present in the low Cl− solution to activate Cftr channels. The superfusion rate was 5 μl/min with a 15-s “dead-space” distal to the solution-switching valve. A needle filled with Ringers/4% agar was inserted s.c. in the mouse's back and connected to a calomel electrode to serve as a reference electrode for the subepithelial compartment. The transepithelial PD was measured with a voltmeter (ISO-DAM-D, World Precision Instruments, Sarasota, FL, USA), and the output was recorded on a chart recorder. The nasal septum was superfused with amiloride-containing Ringer's solution for ∼5 min and then switched to low Cl− solution containing amiloride and forskolin. The PD immediately after the switch to low Cl− solution (marked by the nearly instantaneous bionic junction potential) and the value 4 min later were recorded; the difference served as a measure of the CFTR activity.
Gastrointestinal short-circuit current
Mice were rendered unconscious by CO2 asphyxiation and euthanized by exsanguination. The small intestine was removed and placed in ice-cold HEPES-buffered Ringers solution. The intestine was cut longitudinally and mounted in an Ussing chamber with an aperture of 0.125 cm2. Tissues were bathed on both sides by 5 ml mammalian Krebs-Ringer bicarbonate solution, which was warmed to 37°C and circulated with 95% O2–5% CO2. Tissues were maintained under short-circuit conditions to record basal current and then treated with forskolin/IBMX (10/100 μM) to increase intracellular cAMP and stimulate CFTR-dependent short-circuit current.
In vitro studies
BAL cells were harvested and after 1 h adherence, were cultured in the presence and absence of Escherichia coli LPS (Sigma Chemical Co., St. Louis, MO, USA; 0.5 μg/ml) for 24 h. Cellular mRNA was extracted and processed for cDNA. Each sample was assayed using RT-PCR (Applied Biosystems, Life Technologies, Carlsbad, CA, USA; Taqman 7500), using Applied Biosystems-validated primers for TNF-α, IL-1β, KC, MIP-1α, and IL-6. All samples were concurrently evaluated for the expression of GAPDH. Ct values for the cytokines were normalized to the Ct values for GAPDH and then compared with the unstimulated control to determine the ΔΔCt values and relative gene expression and responsiveness to LPS or infection with P. aeruginosa [9].
Statistics
Statistical analysis was performed using SAS v 9.2 (Cary, NC, USA) or Prism Software (San Jose, CA, USA). Data in the manuscript are shown as the mean ± sem, unless indicated otherwise. Pairwise comparisons of survival curves of the WT versus Cftrfl10 + LysMCre or Cftrtm1Unc were made using a stratified Cox proportional hazards regression model, stratifying on experiment with the assistance of the CWRU Cystic Fibrosis Center Biostatistics Core. Statistics for the direct comparison between groups and sampling time comparisons used repeated measures ANOVA and t-tests. For nonparametric tests, Wilcoxon-Mann Whitney tests were used, often including the Bonferroni method to adjust for multiple testing. All statistical applications are described within the figure legends of the manuscript. Graphical representations show data within different groups as frequency histograms with quantitative values as means and sem for the number of values in the group (mean±sem) All tests were two-sided, and P values ≤0.05 were considered statistically significant.
RESULTS
With the use of murine lines congenic on an inbred C57BL/6J background (backcrossed >10 generations), mice lacking Cftr in myeloid-lineage cells were generated by crossing a LysM promoter-driven Cre recombinase transgene [12, 14] into a “floxed” Cftr line carrying a Cftr allele with LoxP sites flanking exon 10 [10]. The floxed mice, with and without the Cre transgene, were compared with mice globally lacking Cftr exon 10 [11] for specificity and extent of recombination. As Fig. 1A (Cftrfl10+LysMCre) and B (Cftrfl10) shows, only the tissues from mice carrying the LysMCre transgene have the recombined (deleted) floxed Cftr allele.
Figure 1. Molecular characterization of conditional, myeloid expression.
Cre-mediated recombination was first assessed in a panel of tissues from mice homozygous for the Cftrfl10 allele with LysMCre transgene (A), with or without the LysMCre transgene (B). Tissues surveyed include thymus (T), spleen (S), lung (Lu), intestine (I), heart (H), and liver (Li). Recombination, as evidenced by the 148-bp band, was only found in Cre-containing mice. To examine the extent of recombination, DNA from bone marrow-derived macrophages was examined by PCR for the exon 10 deletion (C). Bone marrow-derived macrophages from Cftrfl10 homozygous mice lacking Cre (CftrΔfl10) show only the 408-bp nonrecombined form of the allele, whereas macrophages carrying the LysMCre transgene (Cftrfl10+LysMCre) show only the 148-bp recombined product, identical to mice with systemic absence of exon 10 (Cftr10). To examine cell-type specificity of recombination, purified populations of various myeloid and nonmyeloid immune cells were surveyed (D). Controls, in the absence of Cre (−) show only the 408-bp product, whereas systemic absence of exon 10 (+) shows only the 148-bp product. Alveolar macrophages (AM), peritoneal neutrophils (PN), splenic T cells (ST), and bone marrow-derived macrophages (BM) were compared from mice carrying Cre (LysMCre+) and mice lacking Cre (Cre−). Only myeloid-derived cells showed recombination, as T cells showed no sign of recombination.
The data demonstrate that recombination only occurs in the presence of Cre, but they do not address cell specificity of recombination or the extent of recombination in a given cell type, as these tissues represent a complex mixture of cell types. To examine the extent of recombination in cell types of interest, bone marrow-derived macrophages were isolated and their genomic DNA analyzed by PCR for the presence or absence of exon 10. Figure 1C shows that in the absence of LysMCre, no recombination is detected, but when LysMCre is present, loss of exon 10 approaches 100%, as nonrecombined Cftr is not detectable. Cell-type specificity was examined by isolating various cell types from the adaptive and innate immune systems, including alveolar macrophages, peritoneal neutrophils, splenic T-lymphocytes, and bone marrow-derived macrophages from the same Cftrfl10 + LysMCre animal assayed for the presence or absence of exon 10. As Fig. 1D illustrates, T lymphocytes from the same Cftrfl10 + LysMCre mouse were unaffected by the presence of LysMCre transgene, but alveolar and bone marrow-derived macrophages, as well as peritoneal neutrophils, all show complete loss of exon 10 and thus, carry no active Cftr. These studies were repeated on three different animals, which yielded similar results.
To determine if this manipulation affected CF-related processes, such as epithelial ion transport, transepithelial short-circuit measurements were made across duodenal sections of C57BL/6J mice: mice homozygous for a null mutation in Cftr, Cftrtm1Unc(Cftr-null) [15], and mice homozygous for Cftrfl10, with and without the LysMCre transgene. Figure 2A shows that the duodenum of C57BL/6J mice respond with a robust response, with an increase in short-circuit current to Cftr stimulation by agonists that raise cAMP levels. Mice carrying the floxed allele (Cftrfl10) had a reduced response (Fig. 2B) compared with C57BL/6J mice (Fig. 2A) but still greater than Cftrtm1Unc mice (having no response at all; Fig. 2C). The magnitude of the cAMP-induced short-circuit current response was not affected by the presence of the LysMCre transgene (Fig. 2D). The results from several animals are summarized in Fig. 2E.
Figure 2. Gastrointestinal epithelial Cftr function is unaffected by myeloid-specific deletion of Cftr exon 10.
(A–D) Representative traces of short-circuit current across sections of excised duodenum from each of the mouse lines. At the time indicated by the arrow, forskolin (10 μM) and IBMX (100 μM) were added to the basolateral bathing solution to stimulate CFTR-mediated short-circuit current. (E) Summary data for forskolin/IBMX-stimulated short-circuit current in each of the four mouse lines (mean±sem; n=4–7 animals in each group; *P<0.05 using the standard paired t-test comparing C57BL/6J mice with Cftrtm1Unc or comparing Cftrfl10 mice with Cftrfl10+LysMCre). One-way ANOVA analysis was used for the comparison between C57BL/6J and Cftrfl10 and Cftrfl10 + LysMCre.
As a correlate to the short-circuit current, we also examined intestinal obstruction, a well-characterized phenotype of CF mice [11, 15, 16]. Mice homozygous for the Cftrfl10 allele, with and without the LysMCre transgene, were compared with C57BL/6J and Cftrtm1Unc mice from birth up to 3 weeks of age. Whereas mortality from intestinal obstruction is high in Cftrtm1Unc mice (53.3%; see Table 2) and occurs predominantly around weaning, C57BL/6J and Cftrfl10 homozygous mice, with and without LysMCre, all showed similar survival outcomes, with a mortality rate of only 2–3% for all groups. These data show that the Cftrfl10 + LysMCre genotype has no detectable adverse physiological effect on intestinal function.
Table 2. Mortality in First 3 Weeks of Life.
| Mice | (#) Genotyped | (#) Died | (%) Mortality |
|---|---|---|---|
| C57BL/6J | 360 | 10 | 2.78 |
| Cftrfl10 | 100 | 2 | 2.00 |
| Cftrfl10 + LysMCre | 112 | 3 | 2.68 |
| Cftrtm1Unc | 227 | 121 | 53.30 |
CF mice, similar to human CF, tend to exhibit signs of endocrine disruption, phenotypically exhibited as reduced and delayed growth. We therefore assessed animals from these colonies for signs of growth differences, comparing them again with non-CF and CF mice using mice of similar ages (Fig. 3A). Cftrfl10 homozygous mice, with and without the LysMCre transgene of similar ages, were indistinguishable in weight from the C57BL/6J controls (Fig. 3B), and all three of these groups were significantly larger than the Cftrtm1Unc mice (Fig. 3B; P≤0.05).
Figure 3. Age and weight of animals.
C57BL/6J (n=51), Cftrfl10 (n=47), Cftrfl10 + LysMCre (n=80), and Cftrtm1Unc (n=31) were evaluated at a comparable age range (A) for weight differences (B). (A) Animals of each genotype group were of comparable age. (B) Animals homozygous for the floxed allele, with or without the LysMCre transgene, were indistinguishable by weight from C57BL/6J mice. Cftrtm1Unc were considerably smaller than the other groups (*P≤0.05 using one-way ANOVA analysis).
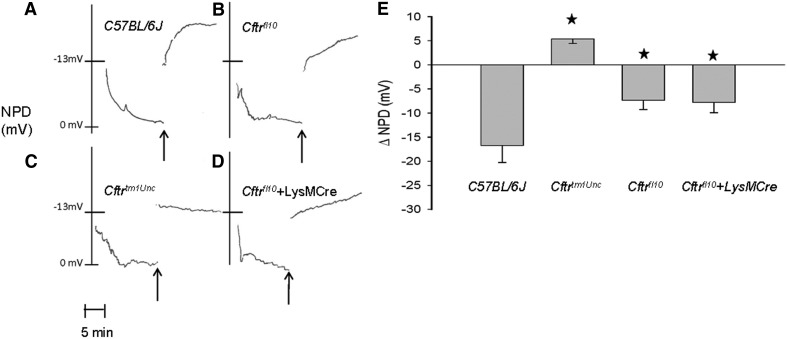
As a final characterization in preparation for airway infection studies, we investigated the potential effect of the conditional expression system on airway epithelial function by measuring NPD in response to cAMP-elevating maneuvers, as described previously [17–19]. As Fig. 4 demonstrates, the responses are analogous to the intestinal characterization. Figure 4A shows that the nasal epithelium of C57BL/6J mice had a robust response, with a hyperpolarization of NPD in response to low Cl− plus forskolin. Mice carrying the floxed allele (Cftrfl10) had less response (Fig. 4B) than C57BL/6J mice (Fig. 4A) but much greater than Cftrtm1Unc mice, having no response at all (Fig. 4C). The cAMP response was not affected by the presence of the LysMCre transgene (Fig. 4D). These results from several animals are summarized in Fig. 4E.
Figure 4. NPD is not affected by absence of Cftr from myeloid cells.
Change in NPD (ΔNPD) was measured as a response to low Cl− plus forskolin. C57BL/6J mice have the greatest hyprpolarization of NPD (A), and Cftrtm1Unc mice (C) have no detectable response, but Cftrfl10 mice (B) and Cftrfl10 carrying the LysMCre transgene (D) showed significant responses to stimulation and are comparable with each other. (E) Mean responses are shown (mean±sem; n=4–7 animals/group). *P ≤ 0.05 using the standard paired t-test comparing C57BL/6J mice with Cftrtm1Unc or comparing Cftrfl10 mice with Cftrfl10 + LysMCre. One-way ANOVA analysis was used for the comparison between C57BL/6J and Cftrfl10 and Cftrfl10 + LysMCre.
These initial characterizations demonstrate that the presence of LysMCre does not confer CF-like phenotype in Cftrfl10 homozygous mice; however, Cftrfl10 homozygous can be distinguished from C57BL/6J mice by electrophysiologic methods. The electrophysiologic differences may reflect the 129Sv origin of the floxed allele, as we have found differential expression of Cftr depending on strain background [20]. Regardless of the etiology of the differences, the results above indicate that the appropriate controls for evaluating the effects of LysMCre must include Cftrfl10 homozygous mice without LysMCre and not just C57BL/6J animals.
Previous reports suggest that the absence of Cftr from myeloid cells impairs bacterial killing or resolution of infection [1–6]. We tested this concept by applying an infection model, instilling P. aeruginosa-laden agarose beads (105 cfu/animal) [7–9] into the right mainstem bronchus of mice homozygous for the Cftrfl10 allele, with and without the LysMCre transgene, as well as Cftrtm1Unc mice. To see if survival differed systematically across experiments, the survival curves of mice from different experiments were compared among mice of the same type. Log rank tests were used to test whether survival differed across experiments, among mice of the same type: Cftrfl10 + LysMCre, Cftrtm1Unc, Cftrfl10, C57BL/6J. Among Cftrfl10 + LysMCre mice, the experiments did not differ in survival (P=0.98); however, survival of the Cftrtm1Unc mice did; post hoc Bonferroni-corrected tests showed that experiments differed significantly (P=0.0004). There was also significant differences between experiments for the WT mice showing a Bonferroni-adjusted P value of (P=0.039); however, there was not a difference between the C57BL/6 and Cftrfl10 control groups. These results reinforce the importance of adjusting for experiment when comparing different mouse types but did allow the control groups to be combined. Because of the significant effects of the experiment on survival, pair-wise comparisons of the three types of mice—controls (WT), Cftrfl10 + LysMCre, and Cftrtm1Unc—were made using a stratified Cox proportional hazards regression analysis, including only experiments where Cftrfl10 + LysMCre, Cftrtm1Unc, Cftrfl10, and C57BL/6J (WT) mice were included (Fig. 5). The experiment was the stratification factor. Results showed that the WT group had better survival than the Cftrfl10 + LysMCre (P=0.04) and Cftrtm1Unc (P<0.0001) groups. The survival curves of the Cftrtm1Unc and Cftrfl10 + LysMCre did not differ significantly (P=0.09). Even with the variability seen with the in vivo infection experiments, the difference between the controls and the Cftrfl10 + LysMCre and the Cftrtm1Unc were still significant, further strengthening the observed importance of myeloid cell Cftr in host response to pathogen exposure.
Figure 5. Survival analysis of mice infected with P. aeruginosa.
Mice homozygous for the Cftrfl10 and C57BL/6J WT controls were combined (n=114; individual curves were almost identical) and showed better survival following infection than Cftrfl10 mice carrying the LysMCre transgene (n=53) or Cftrtm1Unc mice (n=47; P=0.04 and P<0.0001, respectively, using Cox regression analysis stratifying on experiment). The survival curves of Cftrtm1Unc and Cftrfl10 mice carrying the LysMCre transgene did not differ significantly (P=0.09; see text for detailed description of statistical applications).
The survival profile of each of the animals was consistent with the clinical scores obtained at Day 3 versus Day 10 (Fig. 6), based on the criteria outlined in Table 1. Figure 6 shows that there was no difference in clinical scores at Day 3, but by Day 10, Cftrfl10 + LysMCre and Cftrtm1Unc mice had significantly higher clinical scores relative to the Cftrfl10 controls (P≤0.05), which is consistent with the decreased survival in Fig. 5.
Figure 6. Clinical scores postinfection with P. aeruginosa.
Cftrfl10 + LysMCre (n=42), Cftrtm1Unc (n=49), and controls (n=32) were infected with P. aeruginosa-laden agarose beads and euthanized at Day 3 or Day 10. For the duration of the study, animals were evaluated for clinical scores based on the criteria outlined in Table 2. Clinical scores at Day 3 were not different among the three animal groups. At Day 10, Cftrfl10 + LysMCre and Cftrtm1Unc (*P≤0.05) were significantly higher than the Cftrfl10 controls. Nonparametric Mann-Whitney tests were done to determine significance of Cftrfl10 + LysMCre and controls and Cftrtm1Unc and controls.
As the survival data demonstrate that the absence of Cftr from myeloid cells has an effect on host response to infection in vivo, we next examined how it influences various cell types prior to and during an infection. To examine innate differences—those that are apparent prior to infection—mice were euthanized and lungs lavaged to determine cell counts and differentials. To minimize the potential for infection obscuring the lung milieu, sentinels from each colony were evaluated for infectious status and mycoplasm using quantitative PCR (Quanti-iT-HS PCR Mycoplasma Test Kit, Molecular Probes, Life Technologies, Eugene, OR, USA). Figure 7A shows that total cell counts were elevated in Cftrtm1Unc and mice lacking Cftr in myeloid cells relative to Cftrfl10 mice (P≤0.05). Examining the differentials (Figs. 7B–D), the elevation of total cell numbers appears to be a result of macrophages and neutrophils in the mice lacking Cftr in only the myeloid compartment (Cftrfl10+LysMCre), whereas the neutrophils, but not macrophages, are elevated in Cftrtm1Unc animals. In contrast to the myeloid cells, lymphocyte numbers were similar between the two different Cftr knockout strains and the Cftrfl10 control. This profile suggests that total cell recruitment and the differential phenotype of the cell recruited are dependent on the cells that lack the expression of Cftr.
Figure 7. Immune cell composition in the lung in the absence of infection.
BALF was obtained from Cftrfl10 (n=12), Cftrfl10 + LysMCre (n=14), and Cftrtm1Unc (n=16) mice and evaluated for total cell count (A) and relative composition of neutrophils (B), macrophages (C), and lymphocytes (D). Total cell counts were elevated in Cftrfl10 + LysMCre and Cftrtm1Unc mice at baseline relative to controls (*P≤0.05). The shift in differential was toward neutrophils in the Cftrtm1Unc mice (*P≤0.05) with elevated neutrophils and macrophages in the BAL of Cftrfl10 + LysMCre (*P≤0.05). Nonparametric Mann-Whitney tests were done to determine significance.
To examine the effects during infection, mice were inoculated with P. aeruginosa-laden agarose beads, as described for Fig. 5, and euthanized at Day 3 or Day 10 postinfection, at which time, BAL was performed to measure total cell count and differentials. Although all three genotypes had comparable total cell counts at Day 3, suggesting efficient, acute response to pathogen exposure, the similarities in total cell counts changed in the resolution phase at Day 10. Both the Cftrfl10 + LysMCre (P=0.07) and Cftrtm1Unc (P≤0.05) continued to have elevated total cell counts, predominantly as a result of neutrophils compared with the controls. As the differentials show in Fig. 8, the neutrophils were elevated compared with preinfection, regardless of genotype at Days 3 and 10, with levels higher at Day 3 than Day 10 for each group. These observations suggest that all three models showed signs of resolving airway inflammation postinfection. However, neutrophil proportions were still higher in the Cftrfl10 + LysMCre and Cftrtm1Unc mice than Cftrfl10 mice at Day 10, suggesting that the absence of Cftr from myeloid cells prolongs the resolution of airway inflammation. There were no detectable differences in alveolar macrophages or lymphocytes at Day 3 or Day 10, suggesting that recruitment and/or resolution of these cell types, unlike neutrophils, are not influenced by the Cftr status in any cell type.
Figure 8. Immune response to infection with P. aeruginosa.
Cftrfl10 + LysMCre (n=42), Cftrtm1Unc (n=49), and controls (n=32) were infected with P. aeruginosa-laden agarose beads and euthanized at Day 3 or Day 10. BALF was obtained for total cell count (A) and the relative composition of neutrophils (B), macrophages (C), and lymphocytes (D). There was no difference in total white cell recruitment at Day 3 between the groups. Cftrfl10 + LysMCre (·P=0.07) and Cftrtm1Unc (*P≤0.05) had elevated white blood cells compared with controls at Day 10. The composition of the elevated cell counts was associated with elevated neutrophils (Fig. 7B; *P≤0.05) but not alveolar macrophages or lymphocytes. Nonparametric Mann-Whitney tests were done to determine the significance between Cftrfl10 + LysMCre and controls and Cftrtm1Unc and controls.
Histopathology was also performed to assess the effects of infection on lung gross architecture. For this, the lungs of all three infected murine models at Day 10 were evaluated for gross lung pathology using the criteria outlined in Table 1. As Fig. 9 demonstrates, the mice carrying the LysMCre transgene had higher gross lung pathology scores than mice without LysMCre (P≤0.05).
Figure 9. Gross lung pathology at Day 10.
At Day 10, animals were euthanized, and lungs were evaluated for gross lung pathology as outlined in Table 2. Cftrfl10 + LysMCre (n=14) and Cftrtm1Unc (n=11) mice had elevated gross lung pathology scores compared with controls (n=15 from three different experiments; *P≤0.05). Nonparametric Mann-Whitney tests were done to determine significance comparing Cftrfl10 + LysMCre and controls and Cftrtm1Unc and controls.
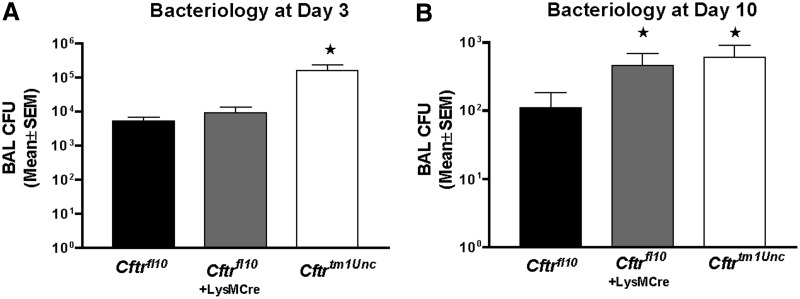
As a final assessment of immune function, we compared BAL cytokine levels and bacterial load at Days 3 and 10 postinoculation. As Fig. 10 shows, bacterial load was elevated significantly in Cftrtm1Unc mice at Day 3 (P≤0.05) but not the Cftrfl10 mice, regardless of the presence of the LysMCre transgene. By Day 10, however, myeloid Cftr-deficient mice and Cftrtm1Unc mice had bacterial loads five times greater than that of the Cftrfl10, non-CF controls (P≤0.05). These data suggest that the development of chronic infection is complex from the perspective of the immune system, in that acute or early-stage response to infection is impaired in CF mice but not as a consequence of myeloid Cftr deficiency. In contrast, impaired bacterial killing, apparent in a more chronic model of infection, is largely a result of lack of Cftr in myeloid cells.
Figure 10. Bacteriology at Days 3 and 10 postinfection.
BALF and whole lung homogenate were obtained from Cftrfl10, Cftrfl10 + LysMCre, and Cftrtm1Unc mice and controls and cultured for P. aeruginosa growth from three different experiments. All values of P ≤ 0.05 are indicated with a star. At Day 3, Cftrtm1Unc mice had a higher bacterial load than Cftrfl10 (P=0.03; n=10 mice), but Cftrfl10 and Cftrfl10 + LysMCre were not different (P>0.05). At Day 10, Cftrtm1Unc (P=0.001; n=11) and Cftrfl10 + LysMCre (P=0.07; n=15) mice had elevated P. aeruginosa growth, relative to Cftrfl10 (n=15). Nonparametric Mann-Whitney tests were done to determine the significance Cftrfl10 + LysMCre and controls and Cftrtm1Unc and controls.
In terms of the cytokine response to acute and chronic infection with P. aeruginosa, Cftrfl10 + LysMCre and Cftrtm1Unc animals had elevated BAL levels of IL-1β, KC, IL-6, IL-17, and MCP-1 at Day 3 (Table 3A) and elevated IL-1β, IL-6, IFN-γ, and MCP-1 levels at Day 10 (Table 3B). The cytokine levels that were associated exclusively with the Cftrfl10 + LysMCre response to infection included GM-CSF, IFN-γ, and IL-10 at Day 3 and GM-CSF and TNF-α at Day 10. These cytokines are active in promoting phenotypic changes in macrophage activation and the ensuing interaction with other cells of the adaptive immune system [10, 11]. The BAL cytokine levels that were associated exclusively with the Cftrtm1Unc response to infection included MIP-2 at Day 3 and KC, MIP-2, and IL-17 at Day 10. These chemokines are produced in the context of a global deficiency of Cftr, most likely as an aberrant epithelial response to P. aeruginosa infection consistent with other in vivo modeling studies [12, 14]. IL-10 levels were decreased significantly in the Cftrtm1Unc BALF at Day 10, similar to other studies [15].
Table 3. Cytokines and Inflammation Resolution.
| Animal strain | Cytokine concentration (mean pg/ml±sem) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GM-CSF | IL-1β | KC | TNF-α | MIP-2 | IL-6 | IFN-γ | IL-17 | IL-10 | MCP-1/JE | |
| A: BAL cytokines at Day 3 | ||||||||||
| Cftrfl10+ LysMCre | 44 ± 10a | 137 ± 21a | 144 ± 27a | 113 ± 16 | 709 ± 92 | 3717 ± 577a | 18 ± 2a | 150 ± 83b | 7 ± 1a | 284 ± 69a |
| Cftrtm1Unc | 14 ± 8 | 147 ± 24a | 171 ± 30a | 91 ± 20 | 1018 ± 219b | 3489 ± 765a | 67 ± 25 | 99 ± 20a | 2 ± 0.3 | 194 ± 96b |
| WT | 15 ± 3 | 82 ± 20 | 76 ± 24 | 117 ± 18 | 546 ± 70 | 2282 ± 852 | 42 ± 8 | 48 ± 14 | 3 ± 1 | 55 ± 13 |
| B: BAL cytokines at Day 10 | ||||||||||
| Cftrfl10 + LysMCre | 1 ± 0.4a | 11 ± 5b | 56 ± 22 | 6 ± 2b | 132 ± 73 | 44 ± 17a | 9 ± 2b | 8 ± 4 | 0.6 ± 0.1 | 52 ± 14 |
| Cftrtm1Unc | 0.5 ± 0.2 | 24 ± 10a | 128 ± 23a | 5 ± 3 | 87 ± 24b | 41 ± 16a | 12 ± 1a | 1 ± 0.5b | 0.4 ± 0.1a | 40 ± 2 |
| WT | 0.2 ± 0.1 | 3 ± 2 | 48 ± 20 | 2 ± 1 | 39 ± 14 | 15 ± 8 | 5 ± 2 | 7 ± 4 | 1 ± 0.1 | 27 ± 7 |
Three different survival experiments were done with n = 2–4 BAL samples evaluated at Days 3 and 10 from each group for each study. A minimum of eight BAL samples was evaluated for each analytes.
P ≤ 0.05;
P ≤ 0.07.
To begin to investigate the contributor to the overall cytokine response to P. aeruginosa infection, we evaluated adherent BAL cells obtained from the Cftrfl10 + LysMCre myeloid-specific knockout and the Cftrfl10 control for cytokine gene expression in response to endotoxin stimulation. Adherent BAL cells were obtained from the Cftrfl10 and Cftrfl10 + LysMCre mice and cultured in vitro, with or without LPS stimulation (5×105 adherent cells, 0.5 μg/ml, for 24 h), to determine how the BAL cells might contribute directly to the cytokines generated by the total lung highlighted in Table 3. Table 4 shows the relative change in cytokine gene expression of the Cftrfl10 mice versus Cftrfl10 + LysMCre when comparing a subset of the cytokines highlighted in Table 3. Cftrfl10 + LysMCre-adherent BAL cells expressed higher levels of TNF-α, IL-1β, IL-6, KC (P≤0.05), and MIP-2 (P=0.07) than the Cftrfl10 control. This is consistent with previous studies with LPS-stimulated Cftrtm1Unc bone marrow-derived macrophages and BAL macrophages [16]. Future studies will investigate the mechanistic difference that contributes to this difference in myeloid cell cytokine gene expression.
Table 4. Deficient Myleoid Cftr Results in Elevated Cytokine mRNA Production.
| Cytokine | BAL cell response to LPSa |
|
|---|---|---|
| Cftrfl10 + LysMCre | Cftrfl10 | |
| TNF-α | 3.0 ± 1.0b | 0.7 ± 0.5 |
| IL-1β | 7.3 ± 1.4b | 1.8 ± 0.6 |
| IL-6 | 6.8 ± 1.6b | 1.2 ± 0.1 |
| MIP-2 | 2.8 ± 1.4 | 0.6 ± 0.4 |
| KC | 3.8 ± 0.4b | 1.0 ± 0.2 |
Data are derived from RT-PCR of mRNA derived from basal BAL. Cells were cultured in the presence and absence of 0.5 μg/ml LPS for 24 h (n=4). ΔCt is normalized to GAPDH for each sample and then compared with the unstimulated control. Data are presented as mean ΔΔCt±sem; n=4–5.
P ≤ 0.05 using nonparametric Wilcoxon-Mann Whitney paired t tests.
DISCUSSION
Defective CFTR results in a variety of changes in the pulmonary milieu, which contributes to the inefficient resolution of lung infection with P. aeruginosa in CF. Studies have focused predominantly on the contributory role of defective airway clearance and airway epithelial production of proinflammation proteins. Traditionally, it has been suggested that individuals with CF do not have defective immune systems even in the context of the inability to efficiently resolve chronic lung infections. In this manuscript, we use in vivo mouse models, in which we mechanically impair airway clearance to model the human airway, but also modulate CFTR expression in myeloid cells directly, assessing the specific role of CFTR on myeloid cell phenotype prior to infection and after response to chronic P. aeruginosa infection. We demonstrate in this manuscript that with the use of the Cre-Lox system, we effectively and specifically knocked out Cftr in the myeloid compartment of C57BL/6J mice. At baseline, in the absence of airway obstruction or inflammation, there was no overt pulmonary phenotype. Upon bacterial instillation, however, the survival of the Cftrfl10 + LysMCre knockout mice was statistically less than the WT and floxed control mice, trending toward the severe phenotype observed in the Cftrtm1Unc mice. We showed further that defective myeloid Cftr contributed to inefficient resolution of infection and inflammation even in the absence of the defective epithelial cell Cftr. In the context of deficient myeloid CFTR but sufficient epithelial cell CFTR, the animals had poor survival, increased inflammation with recruitment of neutrophils, elevated cytokine production, and inability to resolve infection. The studies highlighted in this manuscript also showed that adherent BAL cells, obtained from only mice Cftr-deficient in myeloid cells and therefore, not differentiated in the context of Cftr-based epithelial cell defects, resulted in enhanced cytokine gene expression when stimulated with endotoxin in vitro. These observations suggest that the CF lung phenotype is not simply a consequence of Cftr-dependent airway epithelial cell defects and that the immune system is an important contributor to the overall pathophysiology of CF lung disease.
CF is a lethal, inherited disorder that results from mutations in the gene encoding CFTR, a chloride channel [17]. Airway inflammation and recurrent pulmonary infections play a central role in the progression of CF lung disease [18]. CF has been characterized by a perpetuating cycle of airway obstruction as a result of deficient CFTR, chronic bacterial infection, and a robust inflammatory response [18, 19]. Studies have suggested that the inflammatory process may begin before infection, potentially as a result of the altered lung environment because of deficient airway epithelial CFTR [20]. The development of the Cftr-deficient pig has suggested that the overt inflammatory response is secondary to the bacterial colonization, along with a greater propensity for infection in the first place [21]. In any regard, it is unclear whether the propensity for excessive inflammation is a result of deficient CFTR function in inflammatory cells or as a result of insufficient resolution of inflammation associated with a prior suboptimal-transient infection [18]. In addition, CFTR correctors and potentiators [13, 22] offer great promise in improving CF lung disease; however, the beneficial impact of these new pharmaceuticals may be augmented by enhancing the activity of other cells, including neutrophils, macrophages, and lymphocytes.
In the studies highlighted in this manuscript, the efficient and specific deletion of Cftr in only cells of the myeloid lineage provided a means to compare the “contributory” role of the immune system in regulating the pulmonary response with bacterial colonization in CF. Although deficient myeloid cell Cftr did not alter NPD, gastrointestinal electrophysiology, growth, or intestinal obstruction, it contributed to an altered pulmonary milieu, shifting the baseline differential in the direction of increased neutrophils and macrophages. This was in the absence of detectable infection by BAL, PCR, or whole lung homogenate culture. Inoculation of Cftrfl10 + LysMCre animals with agarose beads impregnated with P. aeruginosa resulted in increased mortality, elevated neutrophil numbers, and bacterial load compared with controls, which was not as severe as Cftrtm1Unc mice infected at the same time but was more severe than the control mice. The intermediate phenotype of the Cftrfl10 + LysMCre mice was more apparent at the resolution phase of the infection model. When the C57BL/6J or Cftrfl10 mice began to resolve the response to pathogen exposure and attenuate the inflammatory response, the Cftrfl10 + LysMCre and Cftrtm1Unc mice still had statistically elevated white cell counts consisting of predominately neutrophils. Further, even with the elevated levels of inflammatory cells, resolution of the infection was incomplete, as there were still statistically elevated numbers of bacteria relative to the controls. Although these studies focus on a single pathogen, P. aeruginosa, as well as a single dose (105 cfu), future studies will evaluate the inflammatory and infection resolution response to different dosages of P. aeruginosa compared with other pathogens such as Streptococcus pneumonia and Staphylococcus aureus. These studies will define the overall sensitivity of the Cftrfl10 + LysMCre to pathogen dose versus bacteria compared with the Cftrtm1Unc null mice, similar to previous studies focusing on defining the murine model of CF lung infection and inflammation [23].
In terms of the inflammatory cytokine responses, IL-1β, IL-6, and MCP-1 cytokines were elevated at Days 3 and 10 in the Cftrfl10 + LysMCre and Cftrtm1Unc mice. KC appears to be an early cytokine, whereas MCP-1 appears to be a distinguishing marker for late responses to infection, implying phenotype specificity at different phases of the infection and inflammatory response. The myeloid cytokines GM-CSF, IFN-γ, IL-10, and TNF-α were dominant in the Cftrfl10 + LysMCre and are important in defining adaptive and innate responses [10, 11, 24]. The BAL from the Cftrtm1Unc model had elevated levels of chemokines MIP-2, KC, and IL-17, whereas IL-10 levels became deficient at Day 10. MIP-2, KC [17, 20, 25, 26], IL-17 [27, 28], GM-CSF [29, 30], and IL-10 [15, 31] have all been implicated in the pathophysiology of CF, including the recruitment of T cells and neutrophils. The observation that the Cftrtm1Unc IL-10 BAL levels were deficient at Day 10 but not Day 3 suggests that the impact of low levels of IL-10 is dominant in the chronic phase of infection and inflammation resolution. Changes in the levels of GM-CSF may be important in myeloid cell activation and differentiation, as this CSF is essential for alveolar macrophage function [29, 32, 33]. The contributors to the overall cytokine production are many, including the airway epithelium, interstitial cells, and mesenchymal cells. To determine if myeloid cells deficient in Cftr could ultimately contribute to the overall cytokine dysregulation in the Cftr-deficient lung, we showed that lung myeloid cells (predominately macrophages) are more sensitive to endotoxin stimulation than WT cells. This gets at the overall phenotype of the Cftr macrophages and implies that these cells may or may not have a phenotype that lies within the description of the traditional groups of classical versus alternative macrophages [10, 34, 35]. Future studies will delve into the phenotype of the Cftrfl10 + LysMCre versus Cftrtm1Unc macrophage and whether it is species- or tissue-specific by evaluating alveolar macrophages, bone marrow-derived macrophages, peritoneal macrophages, peripheral blood monocytes, as well as neutrophils.
Neutrophils, DCs, and monocyte/macrophages are professional phagocytic cells that are able to phagocytose and destroy infectious agents, making them key anti-infectious participants in host defense [36]. However, in the process of responding to pathogen exposure, the products of macrophages/neutrophils can result in tissue damage [37]. Understanding the function and activity of these cells in the context of chronic infection and inflammation resolution is important in defining the balance between the beneficial and detrimental contributions of these cells in CF. The complexity of immune cell function includes phagocytosis, pathogen recognition, and active communication among neutrophils, monocyte/macrophages, and DCs[36, 38]. Neutrophil products stimulate bacterial phagocytosis and intracellular killing in monocytes and macrophages, regulating antimicrobial activities [36, 39]. Apoptotic neutrophil recognition and the subsequent engulfment by macrophages are essential components associated with inflammation resolution [40], both of which have been implicated in contributing to high molecular-weight DNA and increased mucous in CF lungs [41]. Alveolar macrophages, DCs, and neutrophils regulate the inflammatory response to infection through pathogen recognition with cell surface receptors, such as TLRs [42], which results in cytokine production and cell recruitment. Significant efforts have been made to dissect the molecular mechanisms governing phagocytosis and pathogen recognition and killing by macrophage/neutrophil TLRs and the ensuing immune response [43]. Previous studies have shown that the overall process of phagocytosis is not different between CF and WT cells using ex vivo BAL macrophages from humans [44] or mice [45]. However, defects have been described in total phagocytic ability in the context of the milieu of the Cftr-deficient lung, which may be related to soluble factors [46–48]. Further, in vivo differences may be a result of changes in acidification, although significant controversy still exists around this effect of Cftr on myeloid cells [45, 49–51]. In the context of our studies, pathogen and TLR interactions were likely mechanisms associated with the BAL cytokines detected in the mouse models post-P. aeruginosa infection. TLRs, such as TLR-4, are efficient ligands for gram-negative bacteria recognition and activation of the host's innate immune response [52]. In CF, it has been suggested that monocyte/macrophages have an altered expression of triggering receptor expressed on myeloid cell-1 and impaired antigen presentation, whereas still having potent and efficient phagocytic activity [53, 54]. Further, TLR dysregulation has been documented in CFTR-deficient epithelial cells [55] and macrophages [56].
Dysregulated electrolyte transport and mucus clearance are important features of the human CF lung [57]. Although the murine model of CF appears to have the capacity to maintain mucus clearance, the data in this manuscript suggest a model for CF lung disease, in which there are at least two key components. One is clearance, dictated by mucosal hydration through CFTR in the epithelium and the other innate defense, at least partially as a result of the absence of CFTR in immune cells. Therefore, the murine model of sufficient mucus clearance provides the unique opportunity to investigate differences in immune cell function in the absence of defective clearance but enhanced proinflammatory airway epithelium phenotype. With the use of our animal models of “cell-specific” Cftr deficiency [6], we show that myeloid cells also have a direct, contributory role in the dysfunctional inflammatory response to infection and are not just recruited, “innocent victims” of the pathophysiology of CF. The change in CF myeloid cells contributes to the overt inflammatory response to lung infection in vivo. The data presented in this manuscript support a direct contribution of Cftr deficiency on myeloid cell phenotype and function in CF. Although the mechanisms by which deficient Cftr expression alters myeloid cell function are unknown, it appears that the absence of Cftr results in a phenotype of enhanced sensitivity to the surrounding environment, such as bacterial exposure. Further, the data suggest that there is active communication between the epithelium and the myeloid compartment in the response to infection. In all, it seems that defective Cftr impacts not just the epithelium but also the mechanisms of communication between innate and adaptive immune response. The data presented here imply that therapeutic intervention for CF at the myeloid level may have significant impact on immunity by itself but may also provide a strategy to augment current epithelial-targeted therapies.
ACKNOWLEDGMENTS
This work was funded by the gracious support of the Cystic Fibrosis Foundation (Pilot and Feasibility grant) and U.S. National Institutes of Health grants R01 GM 088,823, P30 DK 027,651, R21 HL104322, and R24 RR-032425. We thank the Animal Core, Inflammatory Mediator Core, and Biostatistics Core of CWRU Cystic Fibrosis Center for their assistance in the animal model, analyte analysis, and the survival analysis of the studies, respectively.
Footnotes
- BALF
- BAL fluid
- CF
- cystic fibrosis
- CFTR/Cftr
- cystic fibrosis transmembrane regulator
- Ct
- comparative threshold
- CWRU
- Case Western Reserve University
- KC
- keratinocyte-derived chemokine
- LysM
- lysozyme-M
- NPD
- nasal potential difference
- PD
- potential difference
AUTHORSHIP
T.L.B. planned all experiments, did all tissue procurement, performed infection studies, and wrote the manuscript. C.A.H. generated the myeloid-specific knockout, did all Cftr PCR reactions, added perspective on PCR results, and participated in writing related components of the manuscript. C.U.C. did both NPD and gastrointestinal electrophysiology, added perspective on electrophysiology results, and participated in writing related components of the manuscript. M.L.D. actively participated in the development and critical evaluation of the myeloid-specific knockout, critically reviewed the manuscript, and actively participated in the presentation and interpretation of all of the data.
REFERENCES
- 1. Mueller C., Braag S. A., Keeler A., Hodges C., Drumm M., Flotte T. R. (2011) Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am. J. Respir. Cell Mol. Biol. 44, 922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babaev V. R., Yancey P. G., Ryzhov S. V., Kon V., Breyer M. D., Magnuson M. A., Fazio S., Linton M. F. (2005) Conditional knockout of macrophage PPARγ increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 25, 1647–1653 [DOI] [PubMed] [Google Scholar]
- 3. Bonfield T. L., Thomassen M. J., Farver C. F., Abraham S., Koloze M. T., Zhang X., Mosser D. M., Culver D. A. (2008) Peroxisome proliferator-activated receptor-γ regulates the expression of alveolar macrophage macrophage colony-stimulating factor. J. Immunol. 181, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomassen M. J., Barna B. P., Malur A. G., Bonfield T. L., Farver C. F., Malur A., Dalrymple H., Kavuru M. S., Febbraio M. (2007) ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J. Lipid Res. 48, 2762–2768 [DOI] [PubMed] [Google Scholar]
- 5. Bonfield T., Raychaudhuri L., Malur B., Abraham A., Trapnell S. B. C., Kavuru M. S., Thomassen M. J. (2003) PU. 1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L1132–L1136 [DOI] [PubMed] [Google Scholar]
- 6. Van Heeckeren A. M., Schluchter M. D. (2002) Murine models of chronic Pseudomonas aeruginosa lung infection. Lab. Anim. 36, 291–312 [DOI] [PubMed] [Google Scholar]
- 7. Van Heeckeren A. M., Tscheikuna J. R. W., Walenga M. W., Konstan P., Davis B., Erokwu B., Haxhiu M. A., Ferkol T. W. (2000) Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am. J. Respir. Crit. Care Med. 161, 271–279 [DOI] [PubMed] [Google Scholar]
- 8. Hodges C. A., Cotton C. U., Palmert M. R., Drumm M. L. (2008) Generation of a conditional null allele for Cftr in mice. Genesis 46, 546–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Δ Δ C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 10. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 11. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siveke J. T., Hamann A. (1998) Cutting edge: T helper 1 and T helper 2 cells respond differentially to chemokines. J. Immunol. 160, 550–554 [PubMed] [Google Scholar]
- 13. Ramsey B. W., Davies J., McElvaney N. G., Tullis E., Bell S. C., Drevinek P., Griese M. E. F., McKone C. E., Wainwright M., Konstan W., Moss R., Ratjen F., Sermet-Gaudelus I., Rowe S. M., Dong Q., Rodriguez S., Yen K., Ordonez C., Elborn J. S. (2011) A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 365, 1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Heeckeren A. M., Davis P. B. (2004) Examining the contribution of resident and migratory cells in mediating the exaggerated inflammatory response in cystic fibrosis lung infections with mucoid Pseudomonas aeruginosa. Pediatric Pulmonol. 27, 274 [Google Scholar]
- 15. Chmiel J. F., Konstan M. W., Knesebeck J. E., Hilliard J. B., Bonfield T. L., Dawson D. V., Berger M. (1999) IL-10 attenuates excessive inflammation in chronic pseudomonas infection in mice. Am. J. Respir. Crit. Care Med. 160, 2040–2047 [DOI] [PubMed] [Google Scholar]
- 16. Bruscia E. M., Zhang P. X., Ferreira E., Caputo C., Emerson J. W., Tuck D., Krause D. S., Egan M. E. (2009) Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator−/− mice. Am. J. Respir. Cell Mol. Biol. 40, 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonfield T. L., Panuska J. R., Hilliard K. A., Hilliard J. B., Ghnaim H., Berger M. (1995) Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 152, 2111–2118 [DOI] [PubMed] [Google Scholar]
- 18. Chmiel J. F., Konstan M. W. (2007) Inflammation and anti-inflammatory therapies for cystic fibrosis. Clin. Chest Med. 28, 331–346 [DOI] [PubMed] [Google Scholar]
- 19. Vandivier R. W., Fadok V. A., Ogden C. A., Hoffmann P. R., Brain J. D., Accurso F. J., Fisher J. H., Greene K. E., Henson P. M. (2002) Impaired clearance of apoptotic cells from cystic fibrosis airways. Chest 121, 89S. [DOI] [PubMed] [Google Scholar]
- 20. Bonfield T. L., Konstan M. W., Berger M. (1999) Altered respiratory epithelial cell cytokine production in cystic fibrosis. J. Allergy Clin. Immunol. 104, 72–78 [DOI] [PubMed] [Google Scholar]
- 21. Rogers C. S., Stoltz D. A., Meyerholz D. K., Ostedgaard L. S., Rokhlina T., Taft P. J., Rogan M. P., Pezzulo A. A., Karp P. H., Itani O. A., Kabel A. C., Wohlford-Lenane C. L., Davis G. J., Hanfland R. A., Smith T. L., Samuel M., Wax D., Murphy C. N., Rieke A., Whitworth K., Uc A., Starner T. D., Brogden K. A., Shilyansky J., McCray P. B., Jr., Zabner J., Prather R. S., Welsh M. J. (2008) Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 321, 1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Erlinger S. (2011) Molecular repair of a defective CFTR protein in cystic fibrosis. Clin. Res. Hepatol. Gastroenterol. 35, 254–256 [DOI] [PubMed] [Google Scholar]
- 23. Van Heeckeren A. M., Schluchter M. D., Xue W., Davis P. B. (2006) Response to acute lung infection with mucoid Pseudomonas aeruginosa in cystic fibrosis mice. Am. J. Respir. Crit. Care Med. 173, 288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edwards J. P., Zhang X., Frauwirth K. A., Mosser D. M. (2006) Biochemical and functional characterization of three activated macrophage populations. J. Leukoc. Biol. 80, 1298–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moser C., Van Gennip M., Bjarnsholt T., Jensen P. O., Lee B., Hougen H. P., Calum H., Ciofu O., Givskov M., Molin S., Hoiby N. (2009) Novel experimental Pseudomonas aeruginosa lung infection model mimicking long-term host-pathogen interactions in cystic fibrosis. APMIS 117, 95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nichols D., Chmiel J., Berger M. (2008) Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin. Rev. Allergy Immunol. 34, 146–162 [DOI] [PubMed] [Google Scholar]
- 27. Dubin P. J., Kolls J. K. (2011) IL-17 in cystic fibrosis: more than just Th17 cells. Am. J. Respir. Crit. Care Med. 184, 155–157 [DOI] [PubMed] [Google Scholar]
- 28. Tan H. L., Regamey N., Brown S., Bush A., Lloyd C. M., Davies J. C. (2011) The Th17 pathway in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 184, 252–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ballinger M. N., Paine R., III, Serezani C. H., Aronoff D. M., Choi E. S., Standiford T. J., Toews G. B., Moore B. B. (2006) Role of granulocyte macrophage colony-stimulating factor during gram-negative lung infection with Pseudomonas aeruginosa. Am. J. Respir. Cell Mol. Biol. 34, 766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bonfield T. L., Konstan M. W., Burfeind P., Panuska J. R., Hilliard J. B., Berger M. (1995) Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 13, 257–261 [DOI] [PubMed] [Google Scholar]
- 31. Buff S. M., Yu H., McCall J. N., Caldwell S. M., Ferkol T. W., Flotte T. R., Virella-Lowell I. L. (2010) IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia. Gene Ther. 17, 567–576 [DOI] [PubMed] [Google Scholar]
- 32. Trapnell B. C., Zsengeller Z. Z., Chroneos C., Whitsett J. A., Berclaz Y. P. (2000) Alveolar macrophages from granulocyte-monocyte colony stimulating factor (GM-CSF) deficient mice are arrested at an early differentiation stage. Am. J. Respir. Crit. Care Med. 161, A667 [Google Scholar]
- 33. Shibata Y., Berclaz Y. P., Chroneos Z. C., Yoshida M., Whitsett J. A., Trapnell B. C. (2001) GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU. 1. Immunity 15, 557–567 [DOI] [PubMed] [Google Scholar]
- 34. Porcheray F., Viaud S., Rimaniol A. C., Leone C., Samah B., Dereuddre-Bosquet N., Dormont D., Gras G. (2005) Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 142, 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kantari C., Pederzoli-Ribeil M., Witko-Sarsat V. (2008) The role of neutrophils and monocytes in innate immunity. Contrib. Microbiol. 15, 118–146 [DOI] [PubMed] [Google Scholar]
- 37. Witko-Sarsat V., Descamps-Latscha B. (1994) Neutrophil-derived oxidants and proteinases as immunomodulatory mediators in inflammation. Mediators Inflamm. 3, 257–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Choi J. C., Jung J. W., Kwak H. W., Song J. H., Jeon E. J., Shin J. W., Park I. W., Choi B. W., Kim J. Y. (2008) Granulocyte macrophage-colony stimulating factor (GM-CSF) augments acute lung injury via its neutrophil priming effects. J. Korean Med. Sci. 23, 288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Beg A. A. (2002) Endogenous ligands of Toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 23, 509–512 [DOI] [PubMed] [Google Scholar]
- 40. Hickling T. P., Clark H., Malhotra R., Sim R. B. (2004) Collectins and their role in lung immunity. J. Leukoc. Biol. 75, 27–33 [DOI] [PubMed] [Google Scholar]
- 41. McKeon D. J., Condliffe A. M., Cowburn A. S., Cadwallader K. C., Farahi N., Bilton D., Chilvers E. R. (2008) Prolonged survival of neutrophils from patients with Δ F508 CFTR mutations. Thorax 63, 660–661 [DOI] [PubMed] [Google Scholar]
- 42. Aderem A., Ulevitch R. J. (2000) Toll-like receptors in the induction of the innate immune response. Nature 406, 782–787 [DOI] [PubMed] [Google Scholar]
- 43. Beutler B., Hoebe K., Du X., Ulevitch R. J. (2003) How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74, 479–485 [DOI] [PubMed] [Google Scholar]
- 44. Del P. P., Cifani N., Guarnieri S., Di Domenico E. G., Mariggio M. A., Spadaro F., Guglietta S., Anile M., Venuta F., Quattrucci S., Ascenzioni F. (2011) Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One 6, e19970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Di A., Brown M. E., Deriy L. V., Li C., Szeto F. L., Chen Y., Huang P., Tong J., Naren A. P., Bindokas V., Palfrey H. C., Nelson D. J. (2006) CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat. Cell Biol. 8, 933–944 [DOI] [PubMed] [Google Scholar]
- 46. Entezari M., Weiss D. J., Sitapara R., Whittaker L., Wargo M. J., Li J., Wang H., Yang H., Sharma L., Phan B. D., Javdan M., Chavan S. S., Miller E. J., Tracey K. J., Mantell L. L. (2012) Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol. Med. 18, 477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomassen M. J., Demko C. A., Wood R. E., Sherman J. M. (1982) Phagocytosis of Pseudomonas aeruginosa by polymorphonuclear leukocytes and monocytes: effect of cystic fibrosis serum. Infect. Immun. 38, 802–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thomassen M. J., Demko C. A. (1981) Serum bactericidal effect on Pseudomonas aeruginosa isolates from cystic fibrosis patients. Infect. Immun. 33, 512–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haggie P. M., Verkman A. S. (2007) Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J. Biol. Chem. 282, 31422–31428 [DOI] [PubMed] [Google Scholar]
- 50. Painter R. G., Valentine V. G., Lanson N. A., Jr., Leidal K., Zhang Q., Lombard G., Thompson C., Viswanathan A., Nauseef W. M., Wang G., Wang G. (2006) CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 45, 10260–10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Painter R. G., Bonvillain R. W., Valentine V. G., Lombard G. A., LaPlace S. G., Nauseef W. M., Wang G. (2008) The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J. Leukoc. Biol. 83, 1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prince L. R., Whyte M. K., Sabroe I., Parker L. C. (2011) The role of TLRs in neutrophil activation. Curr. Opin. Pharmacol. 11, 397–403 [DOI] [PubMed] [Google Scholar]
- 53. Del Fresno C., Gómez-Piña V., Lores V., Soares-Schanoski A., Fernández-Ruiz I., Rojo B., Alvarez-Sala R., Caballero-Garrido E., García F., Veliz T., Arnalich F., Fuentes-Prior P., García-Río F., López-Collazo E. (2008) Monocytes from cystic fibrosis patients are locked in an LPS tolerance state: down-regulation of TREM-1 as putative underlying mechanism. PLoS One 3, e2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Del Fresno C., García-Río F., Gómez-Piña V., Soares-Schanoski A., Fernandez-Ruiz I., Jurado T., Kajiji T., Shu C., Marin E., Gutierrez del Prados A. A., Arnalich C., Fuentes-Prior F., Biswas P. S. K., López-Collazo E. (2009) Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J. Immunol. 182, 6494–6507 [DOI] [PubMed] [Google Scholar]
- 55. Muir A., Soong G., Sokol S., Reddy B., Gomez M. I., van Heeckeren A., Prince A. (2004) Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 30, 777–783 [DOI] [PubMed] [Google Scholar]
- 56. Bruscia E. M., Zhang P. X., Satoh A., Caputo C., Medzhitov R., Shenoy A., Egan M. E., Krause D. S. (2011) Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J. Immunol. 186, 6990–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sabroe I., Whyte M. K. (2007) Incapacitating the immune system in cystic fibrosis. Nat. Med. 13, 1417–1418 [DOI] [PubMed] [Google Scholar]