Cannabinoids differentially modulate T cell function with concomitant dysregulation of the NFAT-calcium signaling cascade.
Keywords: immunomodulation, cytotoxic T lymphocytes, antigen specificity, cytokines, NFAT-calcium signaling
Abstract
Approximately 25% of immunocompromised HIV patients smoke marijuana for its putative therapeutic benefit. The goal of these studies was to test the hypothesis that marijuana-derived cannabinoids have immunomodulatory effects on HIV antigen-specific T cell effector function. A surrogate mouse model to induce polyclonal T cell responses against HIVgp120 was established. THC, a marijuana-derived cannabinoid, suppressed or enhanced mouse CD8+ T cell proliferation and the gp120-specific CTL response depending on the magnitude of the IFN-γ response. To determine the molecular mechanisms by which cannabinoids differentially modulate T cell responses, P/I or anti-CD3/CD28 antibodies were used for stimulation, and another marijuana-derived cannabinoid, CBD, was also investigated. THC or CBD suppressed or enhanced IFN-γ and IL-2 production by mouse splenocytes under optimal or suboptimal stimulation, respectively. Similar differential effects of cannabinoids on cytokine production were also observed on nuclear translocation of NFAT and with human PBMCs in response to P/I stimulation. However, THC and CBD elevated intracellular calcium, regardless of the stimulation level with P/I, suggesting that the cannabinoid-induced calcium increase provides an appropriate signal for activation in suboptimally stimulated T cells but an anergic-like signal as a result of excessive calcium in optimally stimulated T cells. Overall, these data demonstrate differential modulation by cannabinoids of a HIV antigen-specific response and identify a possible mechanism responsible for this effect.
Introduction
Plant-derived cannabinoids, such as THC and CBD, are well-established to possess similar immunomodulatory activity despite their distinct pharmacology. THC exhibits affinity for both known cannabinoid receptors and is psychotropic, but CBD exhibits weak affinity for cannabinoid receptors and is therefore, not psychotropic [1, 2]. THC and CBD are primarily characterized as immunosuppressive, as evidenced by decreased mitogen-induced T cell proliferation, T cell activation, and production of IL-2, IFN-γ, and other cytokines [3–7]. Interestingly, depending on experimental conditions, such as cannabinoid concentration, cell types, and specific immune stimuli, enhancement of immune response by cannabinoids has also been reported in a number of studies [8–13]. In particular, cannabinol, another plant-derived cannabinoid, has been shown to positively and negatively regulate IL-2 production by T cells depending on the magnitude of stimulation with P/I or anti-CD3/CD28 [12, 13]. Inhibition of transcriptional activity of transcription factors, NFAT and AP-1, and disruption of intracellular calcium concentration in resting T cells by cannabinoids have been suggested, at least in part, as mechanisms for cytokine suppression [3, 6, 14–16].
Cannabinoids also modulate host resistance to viral and microbial pathogens. For example, THC and other cannabinoids have been reported to suppress production of Th1-promoting cytokines, IFN-γ and IL-12, post-Legionella pneumophila challenge [17]. In a mouse influenza model, our laboratory has shown that THC increased viral load in the lung, in part, through decreasing infiltration of T lymphocytes to the lung [18]. Suppression of CTL function by THC has also been demonstrated in an allogeneic model [19]. In the case of HIV infection, marijuana is used by ∼25% of HIV patients to ameliorate AIDS-associated nausea, pain, and wasting [20]. The clearance of virus in the acute infection stage correlates with the appearance of HIV-specific CTL [21], which directly act on target cells presenting the same antigens and induce apoptosis of target cells while secreting cytokines, such as IFN-γ, TNF-α, and IL-2, to promote the cytolytic activity [22, 23]. A more robust immune response after acute infection will lead to a lower viral set point, which might slow the progression to AIDS and vice versa [24]. However, the effect of cannabinoids on immune function of immunocompromised HIV patients is still unclear. To date, only a few studies have examined the impact of short-term or chronic THC on HIV viral load, CD4+ and CD8+ lymphocyte counts using patients or nonhuman primate models [25–27].
Here, a novel mouse model of HIVgp120 antigen presentation was established, in which proliferation and differentiation of CD8+ T cells, as well as an antigen-specific IFN-γ response by CTL, were elicited. The model is designed to elicit a broad antiviral immune response against multiple gp120-derived antigenic epitopes, so it closely resembles part of the host antiviral immunity in the initial phase of HIV infection. HIV envelope protein gp120 was used as a representative HIV protein in the model because cellular and humoral immune responses against gp120 have been reported previously [28–30]. A C57BL/6 mouse-derived DC line, DC2.4, and the T lymphoma cell line, EL4, were transduced with gp120 (DC2.4gp120 and EL4gp120, respectively) and used as APCs and target cells to restimulate CTL, respectively. The effect of THC on CTL elicitation and IFN-γ production was investigated in response to DC2.4gp120 stimulation and demonstrated differential modulation by THC. The cellular and molecular mechanisms for the differential THC effects were determined using P/I or anti-CD3/CD28 for stimulation and confirmed with CBD. These studies provide a possible mechanism to explain the widely reported discrepancy regarding cannabinoid effects on immune responses in the literature [3–6, 8, 10–13, 16, 31]. Consistent with previous reports [25–27], our findings also suggest possible enhancement of immune function by cannabinoids in response to HIV antigens.
MATERIALS AND METHODS
Chemicals and reagents
THC and CBD were obtained from the National Institute on Drug Abuse (Bethesda, MD, USA). RPMI-1640 media, Pen/Strep, MEM NEAA, sodium pyruvate, and L-glutamine were obtained from Gibco Invitrogen (Carlsbad, CA, USA). Restriction endonucleases and ligase were purchased from New England Biolabs (Ipswich, MA, USA). Unless otherwise specified, all other reagents were purchased from Sigma (St. Louis, MO, USA).
Animals
Female C57BL/6 or B6C3F1 mice were purchased from Charles River (Portage, MI, USA). Rooms were kept on a 12-h light/dark cycle at 21–24°C and 40–60% humidity. All experiments followed the guidelines set forth by Michigan State University Institutional Animal Care and Use Committee.
Cell culture conditions
DC2.4 (a kind gift from Dr. Kenneth Rock at University of Massachusetts, Worcester, MA, USA), EL4, DC2.4gp120, and EL4gp120 cells were maintained in complete RPMI media (10% BCS; HyClone, Logan, UT, USA), 100 U/ml/100 μg/ml Pen/Strep, respectively. HEK293T cells were cultured in DMEM with 10% BCS and Pen/Strep. Splenocytes were isolated aseptically from female C57BL/6 mouse spleens unless specified otherwise and made into single-cell suspensions. In the elicitation phase of the gp120 response (see below), “CTL media” (RPMI plus 5% BCS, Pen/Strep, 2 mM MEM NEAA, 2 mM sodium pyruvate, 2 mM L-glutamine, and 50 μM 2-ME), supplemented with 5 ng/ml mouse rIL-12 were used (R&D Systems, Minneapolis, MN, USA). In the effector phase, 2% BCS and 1 ng/ml mouse rIL-12 were substituted in the CTL media. In the other experiments, splenocytes were cultured in complete RPMI media with 2% BCS, Pen/Strep, and 50 μM 2-ME. Human leukocyte packs were obtained commercially from anonymous donors (Gulf Coast Regional Blood Center, Houston, TX, USA). PBMCs were enriched from each pack by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ). Human PBMCs were cultured in complete RPMI media with 2% BCS and Pen/Strep.
Lentiviral plasmid construction
HIV gp120 cDNA, derived from the HIV-1 IIIB (HXBc2) strain, was amplified from plasmid pVRC2000-gp120 [32] (a kind gift from Dr. Norman Letvin, Beth Israel Deaconess Medical Center, Boston, MA, USA) using primers gp-FP and gp-RP (Supplemental Table 1). To generate pLexPuro-gp120, the gp120 amplicon was digested with SpeI and NotI (underlined in gp-FP and gp-RP, respectively) and ligated into the pLexMCS plasmid containing the IRES-puror gene cassette (Trans-Lentiviral pLex packaging system, Open Biosystems, Huntsville, AL, USA). A similar plasmid, pLexNeo-gp120, was made by replacing the IRES-puror cassette of pLexMCS with an IRES-neor cassette and inserting gp120 as above. The IRES-neor cassette was amplified from plasmid pIRESneo3 (Clontech Laboratories, Mountain View, CA, USA) using primers IRES5′ and Neo3′ (Supplemental Table 1) with respective XhoI and HpaI sites. Each ligate was transformed into competent Escherichia coli cells, Stbl3 (Gibco Invitrogen) or 10-beta (C3019; New England Biolabs). Transformants were selected on LB plus ampicillin plates. Individual colonies were expanded in LB broth plus ampicillin and 0.5% glycerol. Plasmids were isolated using a HiSpeed Plasmid Maxi kit (Qiagen, Valencia, CA, USA).
DC2.4gp120 and EL4gp120 cell line construction
Packaging plasmids (Trans-Lentiviral pLex packaging system, Open Biosystems) were cotransfected with pLexPuro-gp120 or pLexNeo-gp120 into HEK293T cells, according to the manufacturer's protocol. Lentiviral particles were collected and incubated with 6 mg/ml polybrene at room temperature for 5 min. The polybrene/particle (gp120-puror or gp120-neor) mixtures were added to DC2.4 or EL4 cells, respectively, in 24-well plates (750 μl/well) and centrifuged at 800 g for 2 h. Media were replaced with complete RPMI media and incubated for 48 h. Transduced cells were cultured in 1.5 μg/ml puromycin for DC2.4gp120 cells and 400 μg/ml G418 for EL4gp120 cells. Cloning by limiting dilution was performed by seeding heterogeneous mixtures of transduced cells at approximately one cell/three wells in 96-well U-bottom plates (200 μl/well) to select individual clones, which were cultured for screening by real-time PCR (primer/probe sets in Supplemental Table 1) and Western blotting.
Cytokine real-time PCR
Splenocytes (5×106 cells) were treated with VH (0.1% ethanol) or CBD for 30 min at 37°C and activated with 10× P/I (40 nM/0.5 μM) or 1× P/I (4 nM/0.05 μM) for 2, 6, or 24 h at 37°C. Total RNA was isolated using the TRI Reagent method as reported previously [6]. Equal amounts of RNA were reverse-transcribed with random primers using the high-capacity cDNA RT kit (Applied Biosystems, Foster City, CA, USA). Real-time PCR amplification and detection were performed with Taqman primer/probe sets for mouse IL-2 (Mm00434256_m1; Applied Biosystems) or IFN-γ (Mm00801778_m1; Applied Biosystems) on a 7900 HT Fast Real-Time PCR system (Applied Biosystems). Fold-change values were calculated using the ΔΔCt method [33].
Western blotting analysis
Cytosolic protein was isolated from DC2.4gp120 and EL4gp120 clones. Briefly, cells were washed with PBS, lysed with lysis buffer [1% Igepal CA-630, 0.5% sodium deoxycholate, 50 mM Tris, 150 mM NaCl, pH 8.0, supplemented with EDTA containing protease inhibitors (Complete, Mini protease inhibitor cocktail tablets; Roche Applied Science, Indianapolis, IN, USA)] at ≤4 × 107 cells/ml on ice for 5 min, and centrifuged at 25,000 g at 4°C for 5 min. Supernatants were taken, and 20 μg protein for each sample was loaded/lane into a 7% denaturing polyacrylamide gel. Proteins from parental DC2.4 or EL4 were negative controls, and 500 ng purified gp120 protein (HIV-196ZM651 gp120; U.S. National Institutes of Health AIDS Research and Reference Reagent Program) was the positive control. Nuclear protein was isolated from splenocytes (5×107 cells) that were treated with VH or CBD for 30 min at 37°C and then activated with 10× or 1× P/I for 30 min at 37°C as described previously [34]. Protein extract was then loaded into an 8% denaturing polyacrylamide gel to ensure resolution of several bands that correspond to NFAT2 [35]. All protein samples were transferred to nitrocellulose membranes using a semidry electroblotter in transfer buffer (0.025 M Tris, 0.2 M glycine, 10% methanol for NFAT or 20% methanol for gp120). Membranes were blocked in 5% milk or 1% BSA in Tris-buffered saline in Tween (0.01 M Tris, 0.15 M NaCl, 0.1% Tween-20) overnight at 4°C. gp120 was detected using a polyclonal HIV-1 gp120 antibody (Prosci, Poway, CA, USA), and NFATc1 (NFAT2) was detected using an anti-mouse NFAT2 antibody (Affinity Bioreagents/Thermo Scientific, Rockford, IL, USA), followed by HRP-conjugated sheep anti-mouse IgG (GE Healthcare, Buckinghamshire, UK) and West Pico chemiluminescent substrate development (Affinity Bioreagents/Thermo Scientific).
Elicitation of splenocytes with DC2.4gp120 cells
DC2.4gp120 cells were X-ray irradiated (35 gray) to prevent proliferation, washed three times, and adjusted to 1 × 106 cells/ml. Splenocytes (1×107 cells/ml) were treated with THC (1, 5, 10, and 15 μM) or VH (0.1% ethanol) for 30 min and then cocultured with DC2.4gp120 cells (100 μl each cell type in 96 U-bottom plates) for 5 days of elicitation in 5% CTL media.
Enumeration of IFN-γ-secreting cells by ELISPOT
After 5 days of coculture, elicited effector splenocytes (2×107 cells/ml) were restimulated with EL4gp120 or parental EL4 target cells (1×106 cells/ml) in 2% CTL media. Effectors and targets (50 μl each) were plated in ELISPOT wells (Multiscreen-HA filter plate, Millipore, Billerica, MA, USA) overnight. A CTL-only group (no target restimulation) was included as a control to assess background, nonspecific CTL activity. ELISPOT was performed as described previously [36]. Anti-mouse IFN-γ (10 μg/ml) and biotin-conjugated anti-mouse IFN-γ (1 μg/ml; BD PharMingen, San Diego, CA, USA) were used as coating and secondary antibody, respectively. IFN-γ-secreting cells were enumerated using the Cellular Technology ImmunoSpot system (Cellular Technology, Shaker Heights, OH, USA).
Immunofluorescence analysis
Splenocytes (5×106 cells/ml) were labeled with 5 μM CellTrace Violet dye (cell proliferation kit, Gibco Invitrogen) following the manufacturer's protocol. Proliferation was assessed on Days 1–5 after coculture of labeled splenocytes with DC2.4gp120 cells, and IFN-γ production was assessed after restimulation as described above. In some studies, splenocytes were treated with VH (0.1% ethanol) or CBD at various concentrations for 30 min and then activated with 1× P/I for 18–24 h at 37°C. Cells were incubated with LIVE/DEAD Fixable Near-IR or Aqua Dead Cell Stain (Gibco Invitrogen) to assess cell viability, according to the manufacturer's instructions. FcRs were blocked with purified rat anti-mouse CD16/CD32 (BD PharMingen) in FACS buffer (1× HBSS containing 1% BSA and 0.1% sodium azide) and stained for extracellular and intracellular proteins using the following antibodies from BioLegend (San Diego, CA, USA): PE/Cy7- or PE/Cy5-CD8α (clone 53-6.7), FITC-CD62L (clone MEL-14), allophycocyanin/Cy7-CD3 (clone 145-2c11), PE/Cy7-CD4 (clone GK1.5), FITC-CD49b (clone DX5), allophycocyanin-IL-2 (clone JES6-5H4), and PE-IFN-γ (clone XMG1.2), following protocols described previously [37]. Brefeldin A (BioLegend) was added during the last 6 h of restimulation incubation to block cytokine secretion, allowing for the identification of cytokine-producing T cells. Violet staining from each day was compared with the Day 1 control, and a decrease of fluorescence intensity is indicative of cell proliferation. Single-stain controls were included in all experiments to compensate for fluorescence interference between detectors. Cells were assessed on a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed with FlowJo v8.8.6 (Tree Star, Ashland, OR, USA) or Kaluza 1.1 (Beckman Coulter, Miami, FL, USA) software. Linear regression was performed using GraphPad Prism version 4.0a (Graphpad Software, San Diego, CA, USA).
T cell purification
T cells were purified from splenocytes by negative selection using the Pan T cell isolation kit, according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA, USA). Purity of T cells was determined using immunofluorescence analysis with anti-CD3 antibody (clone 145-2c11; BD PharMingen) and generally exceeded 98%. Purified T cells were used subsequently for ELISA analysis.
ELISA
In 48-well plates, splenocytes (8×105 cells/well) or human PBMCs (2×106 cells/well) were treated with VH, THC, or CBD for 30 min at 37°C and then activated with 10× or 1× P/I for 18–24 h at 37°C. In some studies, cells were activated with anti-CD3 plus anti-CD28 antibodies (BD Pharmingen). For optimal stimulation, plates were coated with 0.1 μg/well-purified NA/LE hamster anti-mouse CD3e antibody (clone 145-2C11) overnight at 4°C, washed twice with RPMI, and overlaid with splenocytes in the presence of 0.8 μg/well-purified NA/LE hamster anti-mouse CD28 antibody (clone 37.51) for 2 days at 37°C. For suboptimal stimulation, splenocytes were activated with 0.8 μg/well of each of anti-CD3 and anti-CD28. Quantification of IL-2 and IFN-γ was determined by ELISA, as described previously [6]. Purified mouse rIL-2 or rIFN-γ or human IL-2 (BD Pharmingen) served as standards. Capture antibodies were purified anti-mouse IL-2 or IFN-γ or anti-human IL-2, and detection antibodies were biotinylated anti-mouse IL-2 or IFN-γ or anti-human IL-2 (BD Pharmingen). Samples were read at 450 nm using the BioTek Synergy HT (BioTek, Winooski, VT, USA).
Calcium determinations
Splenocytes (2×107 cells) were incubated with 2 μM Fluo-3 (Gibco Invitrogen) and 5 μM Fura Red (Gibco Invitrogen) for 30–60 min at room temperature in the dark. Cells were analyzed on a FACSCalibur (BD Biosciences) using the kinetic acquisition setting over 5-min intervals. Baseline analysis was conducted for 1 min, after which, cells received various treatments (VH or THC or CBD at 1 min; cellular activation at 2 min). Kinetic analyses were performed with FlowJo software.
Statistical analysis
Differences between means of each treatment group were determined using parametric ANOVA. When significant differences were detected, treatment groups were compared with the appropriate control using Dunnett's two-tailed t test. Following a two-way ANOVA, all groups were compared using Bonferroni's test. For cytokine PCR data, Grubb's outlier test was performed for each treatment group using ΔCt (Cttarget gene−Ct18S). Fold-change values were transformed using natural log (fold-change+1) for statistical analysis using GraphPad Prism version 4.0a.
RESULTS
Expression of gp120 protein in transduced DC2.4 and EL4 clones
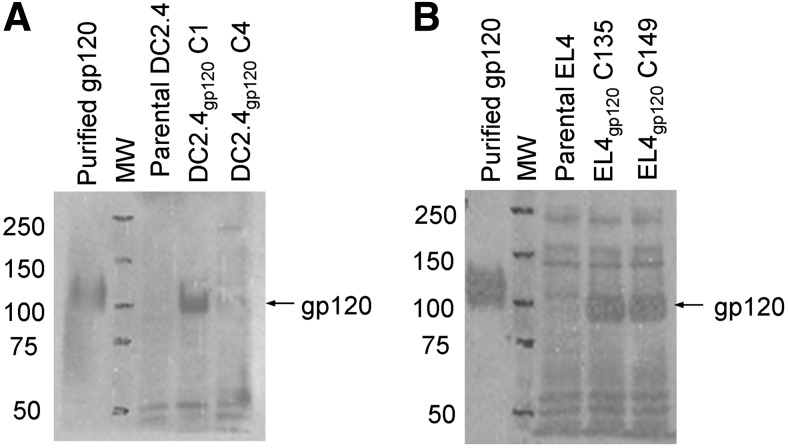
DC2.4 and EL4 cells were transduced with pLexPuro-gp120 and pLexNeo-gp120, respectively. Importantly, because any endogenously expressed cellular proteins are processed and presented in the context of MHC, any peptide recognized as nonself has the potential of eliciting an immune response, including gp120 and the antibiotic-resistance genes. Thus, each cell line (i.e., DC2.4gp120 and EL4gp120) was engineered to express a different antibiotic resistance gene to ensure that the elicited immune response was specific to gp120. Transduced DC2.4gp120 clone 1 (C1) expressed high levels of the gp120 transcript (data not shown) and gp120 protein compared with parental DC2.4 or another clone (C4; Fig. 1A). Two tested EL4gp120 clones (C135 and C149) expressed high levels of the gp120 transcript (data not shown) and protein compared with parental EL4 (Fig. 1B). Subsequent experiments were conducted with DC2.4gp120 C1 for elicitation and EL4gp120 C135 as target cells in the effector phase. Furthermore, the presence of the gp120 transcript and protein in DC2.4gp120 C1 and EL4gp120 C135 was verified after cells had been passaged several times in culture, confirming stable insertion of the cassette into the genome of clones (data not shown). The clones will be referred to as DC2.4gp120 and EL4gp120.
Figure 1. Expression of gp120 protein in transduced DC2.4gp120 and EL4gp120 clones.
(A and B) Cytosolic proteins were isolated from transduced DC2.4gp120 clones (C1) and C4 (A) and EL4gp120 clones C135 and C149 (B). Each sample (20 μg) was assessed by Western blotting for gp120 expression. Protein extract from parental DC2.4 or EL4 was included as a negative control, and purified gp120 protein (500 ng) was included as a positive control. Data are representative of at least three separate experiments.
Elicitation of gp120-specific CD8+ T cell response
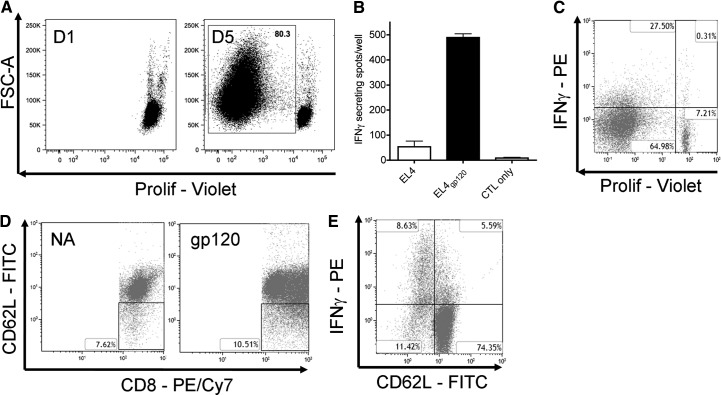
Following coculture stimulation with DC2.4gp120, proliferation of CD8+ T cells in splenocytes was observed, and ∼80% of total viable CD8+ T cells had divided at least once by Day 5 (Fig. 2A). IFN-γ ELISPOT was performed as a proof-of-principle experiment to demonstrate a gp120-specific CTL response. DC2.4gp120-elicited splenocytes showed a greater IFN-γ response against EL4gp120 than against parental EL4 target cells (Fig. 2B), which were used to assess the contribution to the IFN-γ response from putative antigens derived from BCS, EL4 tumor antigens, or antibiotic-resistance peptides. The IFN-γ response was low when there was no antigenic restimulation (CTL-only group in Fig. 2B), demonstrating the requirement of target-cell restimulation for measuring effector activity. The same trend of gp120-specific CTL response was confirmed by intracellular IFN-γ staining (data not shown). Furthermore, almost all viable IFN-γ+CD8+ T cells had proliferated (Fig. 2C). Finally, the percent of the CD62LlowCD8+ T cell population increased after elicitation with DC2.4gp120 for 5 days compared with naïve CD8+ T cells from the splenocytes (Fig. 2D), and most IFN-γ+CD8+ cells were also the CD62Llow population (Fig. 2E).
Figure 2. Establishment of an in vitro mouse model in which proliferation, differentiation, and gp120-specific CTL were elicited.
(A) CellTrace Violet-labeled splenocytes (1×106 cells/well) from C57Bl/6 mice were incubated with irradiated DC2.4gp120 cells (1×105 cells/well). On Days 1 and 5 (D1 and D5) after initiation of the coculture, cells were stained for viability and CD8 and assessed by flow cytometry for proliferation (Prolif) of CD8+ T cells. Cells were gated on singlet, viable, lymphocyte, and CD8+ populations. The percent of the proliferated CD8+ T cell population on D5 is indicated in the plot. FSC-A, Forward-scatter area. (B) Splenocytes were cocultured with irradiated DC2.4gp120 cells for 5 days as described in A. Elicited cells were assayed for CTL activity against EL4gp120 or parental EL4 target cells. IFN-γ secretion was measured by ELISPOT. Each well received 1 × 106 effectors and 5 × 104 target cells. No target cells were added to the CTL-only group. (C) Labeled splenocytes were cocultured with irradiated DC2.4gp120 cells as described in A and restimulated with EL4gp120 or parental EL4 target cells at a ratio of 20:1 as in B. Brefeldin A was added during the last 6 h of incubation. Cells were stained for viability, surface CD8, and intracellular IFN-γ. Gates were set as in A. Cells were analyzed for IFNγ and CD8+ T cell proliferation in one plot. (D) DC2.4gp120-elicited (D5) and naive splenocytes were stained for viability, CD8, and CD62L. Gates were used as above. The percent of CD62Llow cells within CD8+ T cells is presented in each plot. (E) After EL4gp120 restimulation, samples were analyzed for IFN-γ and CD62L expression on CD8+ T cells in one plot. (A–E) Data are representative of at least three experiments. Three replicates were concatenated for each treatment in the flow cytometric analysis.
Differential effects of cannabinoids on CD8+ T cell IFN-γ production and proliferation
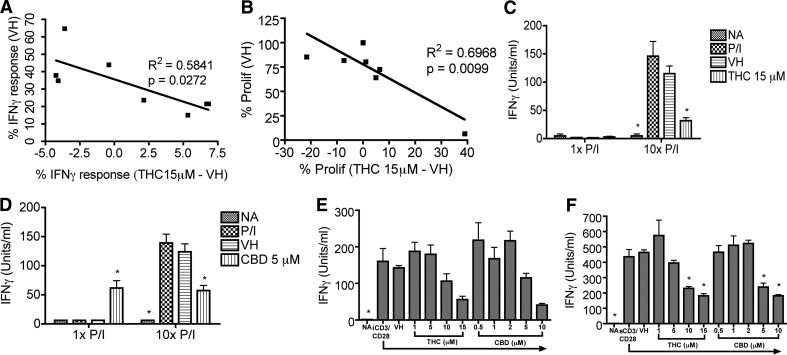
THC was added at the initiation of the coculture, and IFN-γ release by CTL was suppressed in some experiments but enhanced in other experiments in a concentration-dependent manner (data not shown). The overall THC effect was modest, but the trend toward suppression or enhancement was clear. An inverse correlation between the THC effect and the level of IFN-γ response, represented by the percent of IFN-γ+CD8+ T cells in the VH group, was drawn based on the data from eight separate experiments (Fig. 3A; P=0.0272; R2=0.5841).
Figure 3. Differential effects of cannabinoids on IFN-γ production and CD8+ T cell proliferation.
(A and B) CellTrace Violet-labeled splenocytes (1×106 cells/well) were cocultured with irradiated DC2.4gp120 cells (1×105 cells/well) in the presence of THC (1, 5, 10, 15 μM) or VH (0.1% ethanol) or no treatment. On D5, elicited cells (1×106 cells/well) were restimulated with EL4gp120 or parental EL4 target cells (5×104 cells/well). Brefeldin A was added during the last 6 h of incubation. Cells were stained for viability, surface CD8, and intracellular IFN-γ. Cells were gated on singlet, viable, lymphocyte, and CD8+ populations. The percent of IFN-γ+ cells within the CD8+ T cell population (A) and the percent of the proliferated CD8+ T cell population (B) were analyzed. The inverse correlation between the magnitude of IFN-γ response in VH and the THC effect on the response (A) and the correlation between the magnitude of proliferation in VH and the THC effect were evaluated (B) using data collected from eight independent experiments. (C and D) B6C3F1 splenocytes (8×105 cells/well) were treated with THC (15 μM; C) or CBD (5 μM; D) for 30 min and then activated with P/I (10× P/I 40 nM/0.5 μM, 10× P/I; or 1× P/I 4 nM/0.05 μM, 1× P/I) for 18–24 h. (E and F) B6C3F1 splenocytes (8×105 cells/well) were treated with THC or CBD at various concentrations for 30 min and then activated with iCD3/CD28 (E) or sCD3/CD28 (F) for 2 days. (C–F) IFN-γ in the supernatant was quantified by ELISA. Data are presented as the mean units/ml ± se of triplicate cultures. *P < 0.05 as compared with respective VH. Data are representative of at least two separate experiments.
In light of the differential modulation on the CTL IFN-γ response by THC (Fig. 3A) and the correlation between IFN-γ response and CD8+ T cell proliferation by Day 5 (Fig. 2A and C), we sought to investigate the effect of THC on CD8+ T cell proliferation during the elicitation phase. Similar effects on the IFN-γ response and proliferation were observed: THC decreased the percent of proliferating CD8+ T cells under conditions in which CD8+ T cell proliferation was high in the VH group but increased the percent of proliferating CD8+ T cells under conditions in which CD8+ T cell proliferation was low (Fig. 3B; P=0.0099; R2=0.6968).
The differential effects of THC on the IFN-γ response were reminiscent of previous reports in the literature in which positive and negative modulatory effects of cannabinoids have been observed [3, 5, 6, 10–13, 16, 31]. However, the mechanisms for such differential effects of cannabinoids have produced significant confusion in the literature and are poorly understood. Thus, we sought to determine if the correlation between magnitude of stimulation and the cannabinoid-mediated effect was broadly observed using two plant-derived cannabinoids (THC and CBD) and readily titratable T cell activators. THC and CBD were compared, as they possess different affinities for CB1 and CB2 [1, 2]. We anticipated that the effects of THC and CBD would be similar, as we had demonstrated previously that cannabinoid-mediated modulation of T cell function occurred independent of cannabinoid receptors [6, 7, 37]. In response to “optimal” stimulation with 10× P/I (40 nM/0.5 μM) or iCD3/CD28, IFN-γ was robustly induced, which was suppressed by THC and CBD, similar to previous findings [6, 7, 16] (Fig. 3C–E). In contrast, IFN-γ production following “suboptimal” stimulation using 1× P/I (4 nM/0.05 μM) was low and was enhanced by CBD but not THC (Fig. 3C and D). Interestingly, in response to sCD3/CD28, which was used previously as a suboptimal activation for IL-2 [12, 13], IFN-γ production was robustly induced and then suppressed by THC and CBD (Fig. 3F).
Differential effects of cannabinoids on IL-2 response
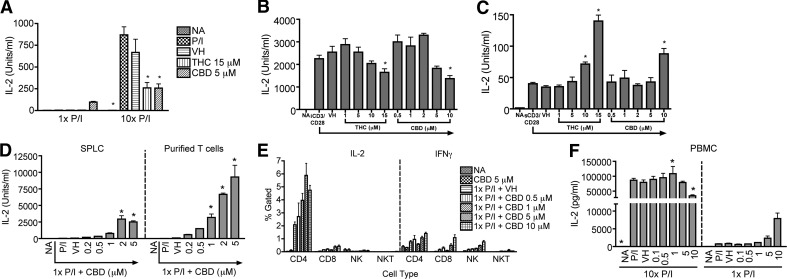
With the demonstration that THC differentially modulated CD8+ T cell proliferation in response to DC2.4gp120 stimulation, IL-2 production in the presence of P/I or anti-CD3/CD28 and the effect of cannabinoids were evaluated. Optimal stimulation—10× P/I or iCD3/CD28—induced robust IL-2 production, which was suppressed significantly by THC and CBD, similar to previous reports [6, 7, 13, 16] (Fig. 4A and B). In response to 1× P/I, CBD but not THC enhanced IL-2 production (Fig. 4A), consistent with the differential cannabinoid effects on the IFN-γ response. Again, with the use of sCD3/CD28 as a suboptimal stimulation for IL-2, as done previously [12, 13], IL-2 production was low and was enhanced by THC and CBD (Fig. 4C).
Figure 4. Differential effects of cannabinoids on IL-2 production from different populations.
(A) B6C3F1 splenocytes (8×105 cells/well) were treated with THC (15 μM), CBD (5 μM), or VH (0.1% ethanol) for 30 min and then activated with P/I (10× P/I 40 nM/0.5 μM, 10× P/I; or 1× P/I 4 nM/0.05 μM, 1× P/I) for 18–24 h. (B and C) B6C3F1 splenocytes (8×105 cells/well) were treated with THC or CBD at the indicated concentrations for 30 min and then activated with iCD3/CD28 (B) or sCD3/CD28 (C) for 2 days. (D) B6C3F1 splenocytes (SPLC) or T cells purified from the splenocytes were treated with 0.2–5 μM CBD for 30 min and then activated with 1× P/I for 48 h. (E) B6C3F1 splenocytes (8×105 cells/well) were treated with 0.5–10 μM CBD for 30 min, followed by activation with 1× P/I for 18–24 h. Cells were also treated with Brefeldin A, 4 h prior to harvest. Cells were stained for viability and extracellular markers, CD4, CD8, CD3, and CD49b, as well as intracellular IL-2 and IFN-γ. NK = CD49b+; NKT = CD3+CD49b+. (F) PBMCs were enriched from human leukocyte packs by density gradient centrifugation. PBMCs (2×106 cells/well) were treated with 0.1–10 μM CBD for 30 min and then activated with 1× or 10× P/I for 18–24 h. (A–D and F) IL-2 in the supernatant was quantified by ELISA. Data are presented as the mean units/ml ± se of triplicate cultures. *P < 0.05 as compared with respective VH. (A–F) Data are representative of at least three separate experiments.
Additional experiments were conducted to decipher the mechanisms by which cannabinoids differentially affected cytokine production with a focus on 1× P/I and CBD. As P/I likely activated all cells in the splenocyte population, a comparison between splenocytes and purified T cells in response to 1× P/I was performed and demonstrated that the CBD-induced enhancement of the IL-2 response also occurred in purified T cells (Fig. 4D). IFN-γ production was also enhanced in purified T cells in response to 1× P/I plus CBD (data not shown). Moreover, FACS analysis of the cytokine-producing cells in splenocytes in response to 1× P/I plus CBD demonstrated that CD4+ T cells produced most of the IL-2, whereas CD4+ and CD8+ T cells and NK cells all contributed to IFN-γ production (Fig. 4E). Finally, to determine whether the differential effects of cannabinoids also occur in human PBMCs, human IL-2 production was quantified. CBD suppressed IL-2 production when using 10× P/I and enhanced IL-2 production when using 1× P/I (Fig. 4F).
Differential effects of cannabinoids on cytokine production were correlated with effects on NFAT nuclear translocation
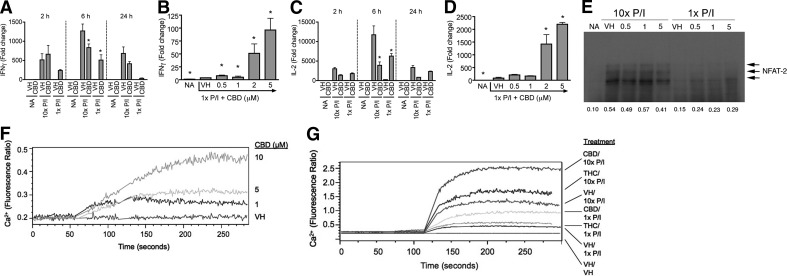
The differential effects of cannabinoids on cytokine production were also observed at the steady-state mRNA level as CBD suppressed or enhanced IFN-γ and IL-2 mRNA expression in response to 10× or 1× P/I, respectively, at several time-points following stimulation (Fig. 5A and C). In addition, CBD-induced IFN-γ and IL-2 mRNA expression was increased in a concentration-dependent manner in response to 1× P/I (Fig. 5B and D). The concentration-dependent suppression of IFN-γ and IL-2 mRNA expression by CBD on 10× P/I-stimulated splenocytes at 24 h has been reported previously [6]. These results suggested that CBD-induced modulation of cytokine production might occur at the level of transcription. NFAT family members NFAT1 (NFATc2) and NFAT2 (NFATc1) are the two predominant NFAT proteins expressed by T cells [38], and of the two, NFAT2 is the primary form induced under lower calcium or anergic conditions [39]. As CBD enhanced cytokine production in response to suboptimal cellular activation, and NFAT plays an important role in IFN-γ and IL-2 transcription [40–43], the effect of CBD on NFAT2 nuclear translocation was examined. 10× P/I induced nuclear translocation of NFAT2, which was modestly suppressed by CBD, whereas 1× P/I-stimulated NFAT2 translocation was enhanced by CBD (Fig. 5E).
Figure 5. Cannabinoids differentially regulated IL-2 and IFN-γ steady-state mRNA expression by modulating nuclear translocation of NFAT2 and increased intracellular calcium regardless of activation level.
(A–D) B6C3F1 splenocytes (5×106 cells) were treated with CBD (0.5–5 μM) or VH (0.1% ethanol) for 30 min and then activated with P/I (10× P/I 40 nM/0.5 μM, 10× P/I; or 1× P/I 4 nM/0.05 μM, 1× P/I) for various times. Total RNA was isolated and reverse-transcribed, and real-time PCR was performed for IFN-γ (A and B) and IL-2 (C and D). Data are expressed as the mean fold change ± se of triplicate cultures. *P < 0.05 as compared with respective VH. (E) B6C3F1 splenocytes (5×106 cells) were treated with 0.5–5 μM CBD or VH (0.1% ethanol) for 30 min, followed by activation with 1× or 10× P/I for 30 min. Nuclear protein was isolated and loaded into an 8% SDS-PAGE gel. NFAT2 is detected as multiple bands in the 100- to 130-kDa range. (F and G) B6C3F1 splenocytes (2×107 cells) were incubated with Fura Red and Fluo-3 for 30–60 min in the dark. Cells were then analyzed by flow cytometry, during which time they received [CBD (1–10 μM) in F] or [THC (10 μM), CBD (5 μM) or VH (0.1% ethanol) for 1 min followed by 1× P/I, 10× P/I, or VH (0.1% DMSO) for 3 additional min in G]. Results are representative of at least two separate experiments.
Cannabinoids enhanced intracellular calcium regardless of the magnitude of cellular activation
Intracellular calcium is critical for activation of NFAT [40, 42]; therefore, we hypothesized that the effect of cannabinoids on intracellular calcium might correlate with the differential regulation of NFAT translocation and cytokine production. THC has been shown to induce intracellular calcium in resting lymphocytes [14, 15], and as seen in Fig. 5F, CBD also induced intracellular calcium in resting splenocytes in a concentration-dependent manner. 1× P/I modestly induced intracellular calcium, and THC and CBD further elevated the level of intracellular calcium (Fig. 5G); 10× P/I robustly induced intracellular calcium, but unlike the effects of CBD or THC on NFAT nuclear translocation and cytokine production, THC and CBD further enhanced the intracellular calcium above the level induced by P/I alone (Fig. 5G).
DISCUSSION
HIV patients use marijuana to mitigate AIDS-associated adverse effects [20], but there are no suitable mouse models that address the effect of cannabinoids, the active components of marijuana, on initial anti-HIV immune responses. The novel mouse model described here mimics the T cell response against HIV-associated antigens in the early stages of infection when T cells are critical in eliminating the virus and was used to investigate the modulation of the T cell response by cannabinoids. Although only one HIV protein, gp120, was studied in our model, broad T cell responses against multiple viral epitopes of this protein are elicited. In our model, different engineered cell lines were used, which provided the ability to study the response specific to gp120 antigens shared by DC2.4gp120 and EL4gp120 cells but not to antigens from BCS, parental cells, or antibiotic-resistance markers. As demonstrated, DC2.4gp120 was capable of eliciting CD8+ T cell proliferation, differentiation, and therefore, gp120-specific IFN-γ production from CTL, indicating that APCs, CD8+ T cells, and target cells all play functional roles in the model system.
The present studies demonstrated that THC differentially modulated IFN-γ production from gp120-specific CTL and proliferation of CD8+ T cells in response to DC2.4gp120 elicitation. This is the first report of the differential effect of cannabinoids on an antigen-specific T cell response. The experimental factors that control the magnitude of cellular activation in this in vitro system are unknown. Across eight independent experiments, a general trend was observed such that the magnitude of the immune response, as measured by IFN-γ and CD8+ T cell proliferation, appeared to be one important determinant of whether THC produced enhancement or suppression. Moreover, the suppression or enhancement produced by THC on the IFN-γ response was consistent with its effect on CD8+ T cell proliferation, indicating that THC modulated CD8+ T cell effector function, in part, via regulating CD8+ T cell proliferation first.
As a result of the complexity of the factors involved in the gp120-induced response in this cognate model (i.e., magnitude of gp120 expression, gp120 processing and presentation on MHC, initiation of TCR signaling), more direct activators of T cell effector function were used to study the differential immune modulation by cannabinoids. The pharmacological activators, P/I and anti-CD3/CD28, facilitated a more readily controlled titration of the stimulus used to induce the immune response. In addition, CBD was used in several studies to demonstrate that the differential effect of cannabinoids was not specific to THC. THC exhibits affinity for CB1 and CB2, whereas CBD does not exhibit high affinity for either cannabinoid receptor [1, 2]. In fact, we anticipated that CBD and THC would produce similar effects, as we have demonstrated previously that many effects of cannabinoids on T cells are mediated independently of CB1 or CB2 [6, 7, 37]. With one exception (cannabinoid effect in response to 1× P/I), the effects of THC and CBD were, in fact, similar. The effect of CBD on IFN-γ and IL-2 production was, in part, dictated by the magnitude of stimulation delivered through P/I or anti-CD3/CD28 treatment. Under conditions of relatively high stimulation (10× P/I, iCD3/CD28, or sCD3/CD28-only for IFN-γ), CBD suppressed cytokine production, whereas under conditions of relatively low stimulation (1× P/I or sCD3/CD28-only for IL-2), CBD enhanced cytokine production. The effect of THC on IFN-γ and IL-2 production followed the same pattern as CBD, with the exception that THC did not enhance cytokine production in response to 1× P/I. It was also unexpected that sCD3/CD28 induced robust IFN-γ production, as we showed previously that iCD3/CD28 was a stronger activation stimulus than sCD3/CD28 for IL-2 induction in splenocytes [12, 13], but THC and CBD still produced similar effects on sCD3/CD28-stimulated IFN-γ. It is possible that suboptimal stimulation with sCD3/CD28 leads to T cell anergy or AICD, which requires IFN-γ for the production of caspases and also results in decreases in IL-2 production and proliferation to regulate T cell homeostasis [44–47].
These results are consistent with previous reports that cannabinoids suppressed or enhanced immune function, but a clear mechanism has not been established. We hypothesize that the increase in intracellular calcium by cannabinoids prior to activation contributes to the downstream effects. That is, the combination of cannabinoids with suboptimal cellular activation provides an appropriate or optimal calcium signal to induce cytokine production, whereas the combination of cannabinoids with an optimal level of cellular activation might provide an anergic or cell death signal to suppress cytokine production, which has been suggested for immune suppression by CBD previously [16, 48]. Thus, it is unexpected that THC does not induce IL-2 or IFN-γ in response to 1× P/I, especially as THC has been reported to increase intracellular calcium in lymphocytes [15, 49, 50]. In these studies, we demonstrated that CBD and THC increased calcium in splenocytes, regardless of the magnitude of activation. It is possible that as THC enhanced the calcium level to a lesser extent than CBD in the presence of 1× P/I, the induction on cytokine production was so modest that it is below the level of detection. It was notable that differential regulation of IL-2 production by CBD was also observed in human PBMCs, implying that the differential effect of cannabinoids occurs in humans. Further investigations are needed to determine whether similar trends in the cannabinoid-mediated effects also occur in immunocompromised HIV patients.
As shown here and in previous studies, cannabinoids increased intracellular calcium in resting and activated splenocytes, suggesting that the differential regulation of NFAT nuclear translocation by cannabinoids must occur at a signal distal to increased intracellular calcium [15, 49, 50]. It is possible that cannabinoids in combination with optimal or suboptimal stimulation might differentially produce oscillatory calcium signals, which have been shown to differentially activate NFAT and IL-2 [51]. Alternatively, the “switch factor” for cannabinoid-induced modulation of NFAT2 nuclear translocation and subsequent cytokine production that must be distal to the increase in intracellular calcium could be cytoplasmic NFAT-regulating factors, such as stromal interaction molecule or Orai, or nuclear kinases that induce nuclear export of NFAT, such as dual-specificity tyrosine-phosphorylation regulated kinase 1A (reviewed in ref. [52]). Another possibility is that the induction of different transcription factors distal to the increase in intracellular calcium dictates the effects on cytokine production. For example, whether the cells would undergo anergy or AICD may depend on the NFAT:AP-1 ratio, as a high AP-1 level is required for AICD [53, 54]. The putative differential effects of cannabinoids on AP-1 are presently unknown, but AP-1 activity has been shown to be suppressed by cannabinoid treatment under optimal stimulation conditions [3, 4, 34].
Overall, these studies demonstrated that cannabinoids suppressed or enhanced HIVgp120-specific T cell responses. Such differential effects by cannabinoids also occurred in response to other stimuli, such as P/I or anti-CD3/CD28, and demonstrated that cannabinoids differentially regulated NFAT nuclear translocation and cytokine production. Despite these opposing effects, cannabinoids elevated intracellular calcium regardless of the presence or absence, or even the magnitude, of cellular activation. These data indicate that the intracellular calcium level resulting from the combination of cannabinoid treatment and cellular activation, at least in part, determined the overall T cell response. In particular, cannabinoids in combination with suboptimal stimulation led to an optimal calcium level and increased nuclear translocation of NFAT and therefore, enhanced the T cell response. On the other hand, cannabinoids plus optimal stimulation led to excessive intracellular calcium, which reduced NFAT nuclear translocation and resulted in suppression of the T cell response. Together, these data provide one potential mechanism to explain the many dichotomous reports in the literature in which cannabinoids induce or suppress the same immune response [3–6, 8, 10–13, 16, 31]. Our observations also identify the need for more in-depth investigations on evaluating the effect of cannabinoid use on immune competence of HIV patients.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by U.S. National Institutes of Health grants DA007908, DA020402, and 1F32DA031067-01. The authors thank Sonia Dantas and Sara Fox for assisting in the construction of the pLex-gp120 plasmids. The authors also acknowledge Robert Crawford for immunofluorescence analysis and Kimberly Hambleton for assistance with submission of the manuscript.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- 1× PMA/ionomycin
- 4 nM phorbol ester/0.05 μM ionomycin
- 10× PMA/ionomycin
- 40 nM phorbol ester/0.5 μM ionomycin
- AICD
- activation-induced cell death
- BCS
- bovine calf serum
- CB1/2
- cannabinoid receptor 1/2
- CBD
- cannabidiol
- CD62L
- CD62 ligand
- Ct
- comparative threshold
- FP
- forward primer
- HEK
- human embryonic kidney
- HIVgp120
- HIV envelope glycoprotein 120
- iCD3/CD28
- immobilized anti-CD3 plus soluble anti-CD28 antibodies
- MCS
- multiple cloning site
- NA/LE
- no azide/low endotoxin
- NEAA
- nonessential amino acids
- neor
- neomycin resistance
- NFAT
- NF of activated T cells
- P/I
- PMA (phorbol ester)/ionomycin
- Pen/Strep
- penicillin and streptomycin
- puror
- puromycin resistance
- RP
- reverse primer
- sCD3/CD28
- soluble anti-CD3 plus soluble anti-CD28 antibodies
- THC
- Δ9-tetrahydrocannabinol
- VH
- vehicle
AUTHORSHIP
W.C. wrote the manuscript and designed and performed experiments. B.L.F.K. contributed to writing the manuscript and designed and performed experiments. S.T.P. was involved in writing Materials and Methods and edited the manuscript. S.T.P., L.A.T., and N.R.L. assisted in performing experiments. S.O.S. and R.R. provided critical materials and reviewed the manuscript. N.E.K. designed the study and edited the manuscript.
DISCLOSURES
The authors report no conflict of interest. This document has been reviewed by the National Health and Environmental Effects Research Laboratory of the U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents reflect the views of the agency nor does mention of trade names or commercial products constitute the endorsement of recommendation for use.
REFERENCES
- 1. Matsuda L. A., Lolait S. J., Brownstein M. J., Young A. C., Bonner T. I. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 [DOI] [PubMed] [Google Scholar]
- 2. Munro S., Thomas K. L., Abu-Shaar M. (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65 [DOI] [PubMed] [Google Scholar]
- 3. Yea S. S., Yang K. H., Kaminski N. E. (2000) Role of nuclear factor of activated T-cells and activator protein-1 in the inhibition of interleukin-2 gene transcription by cannabinol in EL4 T-cells. J. Pharmacol. Exp. Ther. 292, 597–605 [PubMed] [Google Scholar]
- 4. Condie R., Herring A., Koh W. S., Lee M., Kaminski N. E. (1996) Cannabinoid inhibition of adenylate cyclase-mediated signal transduction and interleukin 2 (IL-2) expression in the murine T-cell line, EL4.IL-2. J. Biol. Chem. 271, 13175–13183 [DOI] [PubMed] [Google Scholar]
- 5. Srivastava M. D., Srivastava B. I., Brouhard B. (1998) Δ9 Tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology 40, 179–185 [DOI] [PubMed] [Google Scholar]
- 6. Kaplan B. L., Springs A. E., Kaminski N. E. (2008) The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT). Biochem. Pharmacol. 76, 726–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Springs A. E., Karmaus P. W., Crawford R. B., Kaplan B. L., Kaminski N. E. (2008) Effects of targeted deletion of cannabinoid receptors CB1 and CB2 on immune competence and sensitivity to immune modulation by Δ9-tetrahydrocannabinol. J. Leukoc. Biol. 84, 1574–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakano Y., Pross S., Friedman H. (1993) Contrasting effect of Δ-9-tetrahydrocannabinol on IL-2 activity in spleen and lymph node cells of mice of different ages. Life Sci. 52, 41–51 [DOI] [PubMed] [Google Scholar]
- 9. Derocq J. M., Segui M., Marchand J., Le Fur G., Casellas P. (1995) Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett. 369, 177–182 [DOI] [PubMed] [Google Scholar]
- 10. Pross S. H., Nakano Y., Widen R., McHugh S., Newton C. A., Klein T. W., Friedman H. (1992) Differing effects of Δ-9-tetrahydrocannabinol (THC) on murine spleen cell populations dependent upon stimulators. Int. J. Immunopharmacol. 14, 1019–1027 [DOI] [PubMed] [Google Scholar]
- 11. Jbilo O., Derocq J. M., Segui M., Le Fur G., Casellas P. (1999) Stimulation of peripheral cannabinoid receptor CB2 induces MCP-1 and IL-8 gene expression in human promyelocytic cell line HL60. FEBS Lett. 448, 273–277 [DOI] [PubMed] [Google Scholar]
- 12. Jan T. R., Kaminski N. E. (2001) Role of mitogen-activated protein kinases in the differential regulation of interleukin-2 by cannabinol. J. Leukoc. Biol. 69, 841–849 [PubMed] [Google Scholar]
- 13. Jan T. R., Rao G. K., Kaminski N. E. (2002) Cannabinol enhancement of interleukin-2 (IL-2) expression by T cells is associated with an increase in IL-2 distal nuclear factor of activated T cell activity. Mol. Pharmacol. 61, 446–454 [DOI] [PubMed] [Google Scholar]
- 14. Rao G. K., Zhang W., Kaminski N. E. (2004) Cannabinoid receptor-mediated regulation of intracellular calcium by Δ(9)-tetrahydrocannabinol in resting T cells. J. Leukoc. Biol. 75, 884–892 [DOI] [PubMed] [Google Scholar]
- 15. Rao G. K., Kaminski N. E. (2006) Cannabinoid-mediated elevation of intracellular calcium: a structure-activity relationship. J. Pharmacol. Exp. Ther. 317, 820–829 [DOI] [PubMed] [Google Scholar]
- 16. Kaplan B. L., Rockwell C. E., Kaminski N. E. (2003) Evidence for cannabinoid receptor-dependent and -independent mechanisms of action in leukocytes. J. Pharmacol. Exp. Ther. 306, 1077–1085 [DOI] [PubMed] [Google Scholar]
- 17. Klein T. W., Newton C. A., Nakachi N., Friedman H. (2000) Δ 9-Tetrahydrocannabinol treatment suppresses immunity and early IFN-γ, IL-12, and IL-12 receptor β 2 responses to Legionella pneumophila infection. J. Immunol. 164, 6461–6466 [DOI] [PubMed] [Google Scholar]
- 18. Buchweitz J. P., Karmaus P. W., Harkema J. R., Williams K. J., Kaminski N. E. (2007) Modulation of airway responses to influenza A/PR/8/34 by Δ9-tetrahydrocannabinol in C57BL/6 mice. J. Pharmacol. Exp. Ther. 323, 675–683 [DOI] [PubMed] [Google Scholar]
- 19. Karmaus P. W., Chen W., Kaplan B. L., Kaminski N. E. (2011) Δ(9)-Tetrahydrocannabinol suppresses cytotoxic T lymphocyte function independent of CB(1) and CB(2), disrupting early activation events. J. Neuroimmune Pharmacol. doi: 10.1007/s11481-011-9293-4 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prentiss D., Power R., Balmas G., Tzuang G., Israelski D. M. (2004) Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. J. Acquir. Immune Defic. Syndr. 35, 38–45 [DOI] [PubMed] [Google Scholar]
- 21. Koup R. A., Safrit J. T., Cao Y., Andrews C. A., McLeod G., Borkowsky W., Farthing C., Ho D. D. (1994) Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68, 4650–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong P., Pamer E. G. (2003) CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 21, 29–70 [DOI] [PubMed] [Google Scholar]
- 23. Brown D. M., Roman E., Swain S. L. (2004) CD4 T cell responses to influenza infection. Semin. Immunol. 16, 171–177 [DOI] [PubMed] [Google Scholar]
- 24. Mellors J. W., Rinaldo C. R., Jr., Gupta P., White R. M., Todd J. A., Kingsley L. A. (1996) Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272, 1167–1170 [DOI] [PubMed] [Google Scholar]
- 25. Abrams D. I., Hilton J. F., Leiser R. J., Shade S. B., Elbeik T. A., Aweeka F. T., Benowitz N. L., Bredt B. M., Kosel B., Aberg J. A., Deeks S. G., Mitchell T. F., Mulligan K., Bacchetti P., McCune J. M., Schambelan M. (2003) Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann. Intern. Med. 139, 258–266 [DOI] [PubMed] [Google Scholar]
- 26. Molina P. E., Winsauer P., Zhang P., Walker E., Birke L., Amedee A., Stouwe C. V., Troxclair D., McGoey R., Varner K., Byerley L., Lamotte L. (2010) Cannabinoid administration attenuates the progression of simian immunodeficiency virus. AIDS Res. Hum. Retroviruses 27, 585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winsauer P. J., Molina P. E., Amedee A. M., Filipeanu C. M., McGoey R. R., Troxclair D. A., Walker E. M., Birke L. L., Stouwe C. V., Howard J. M., Leonard S. T., Moerschbaecher J. M., Lewis P. B. (2011) Tolerance to chronic Δ-9-tetrahydrocannabinol (Δ-THC) in rhesus macaques infected with simian immunodeficiency virus. Exp. Clin. Psychopharmacol. 19, 154–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palker T. J., Matthews T. J., Langlois A., Tanner M. E., Martin M. E., Scearce R. M., Kim J. E., Berzofsky J. A., Bolognesi D. P., Haynes B. F. (1989) Polyvalent human immunodeficiency virus synthetic immunogen comprised of envelope gp120 T helper cell sites and B cell neutralization epitopes. J. Immunol. 142, 3612–3619 [PubMed] [Google Scholar]
- 29. Mirano-Bascos D., Tary-Lehmann M., Landry S. J. (2008) Antigen structure influences helper T-cell epitope dominance in the human immune response to HIV envelope glycoprotein gp120. Eur. J. Immunol. 38, 1231–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carbonetti N. H., Tuskan R. G., Lewis G. K. (2001) Stimulation of HIV gp120-specific cytolytic T lymphocyte responses in vitro and in vivo using a detoxified pertussis toxin vector. AIDS Res. Hum. Retroviruses 17, 819–827 [DOI] [PubMed] [Google Scholar]
- 31. Pross S. H., Nakano Y., McHugh S., Widen R., Klein T. W., Friedman H. (1992) Contrasting effects of THC on adult murine lymph node and spleen cell populations stimulated with mitogen or anti-CD3 antibody. Immunopharmacol. Immunotoxicol. 14, 675–687 [DOI] [PubMed] [Google Scholar]
- 32. Mo Y., Quanquin N. M., Vecino W. H., Ranganathan U. D., Tesfa L., Bourn W., Derbyshire K. M., Letvin N. L., Jacobs W. R., Jr., Fennelly G. J. (2007) Genetic alteration of Mycobacterium smegmatis to improve mycobacterium-mediated transfer of plasmid DNA into mammalian cells and DNA immunization. Infect. Immun. 75, 4804–4816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Δ Δ C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 34. Faubert B. L., Kaminski N. E. (2000) AP-1 activity is negatively regulated by cannabinol through inhibition of its protein components, c-fos and c-jun. J. Leukoc. Biol. 67, 259–266 [DOI] [PubMed] [Google Scholar]
- 35. Lyakh L., Ghosh P., Rice N. R. (1997) Expression of NFAT-family proteins in normal human T cells. Mol. Cell. Biol. 17, 2475–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu H., Kaplan B. L., Ngaotepprutaram T., Kaminski N. E. (2009) Suppression of T cell costimulator ICOS by Δ9-tetrahydrocannabinol. J. Leukoc. Biol. 85, 322–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karmaus P. W., Chen W., Crawford R. B., Harkema J. R., Kaplan B. L., Kaminski N. E. (2011) Deletion of cannabinoid receptors 1 and 2 exacerbates APC function to increase inflammation and cellular immunity during influenza infection. J. Leukoc. Biol. 90, 983–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horsley V., Pavlath G. K. (2002) NFAT: ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 156, 771–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Srinivasan M., Frauwirth K. A. (2007) Reciprocal NFAT1 and NFAT2 nuclear localization in CD8+ anergic T cells is regulated by suboptimal calcium signaling. J. Immunol. 179, 3734–3741 [DOI] [PubMed] [Google Scholar]
- 40. Jain J., McCaffrey P. G., Miner Z., Kerppola T. K., Lambert J. N., Verdine G. L., Curran T., Rao A. (1993) The T-cell transcription factor NFATp is a substrate for calcineurin and interacts with Fos and Jun. Nature 365, 352–355 [DOI] [PubMed] [Google Scholar]
- 41. Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. (1988) Identification of a putative regulator of early T cell activation genes. Science 241, 202–205 [DOI] [PubMed] [Google Scholar]
- 42. Sweetser M. T., Hoey T., Sun Y. L., Weaver W. M., Price G. A., Wilson C. B. (1998) The roles of nuclear factor of activated T cells and ying-yang 1 in activation-induced expression of the interferon-γ promoter in T cells. J. Biol. Chem. 273, 34775–34783 [DOI] [PubMed] [Google Scholar]
- 43. Sica A., Dorman L., Viggiano V., Cippitelli M., Ghosh P., Rice N., Young H. A. (1997) Interaction of NF-κB and NFAT with the interferon-γ promoter. J. Biol. Chem. 272, 30412–30420 [DOI] [PubMed] [Google Scholar]
- 44. Schwartz R. H. (1996) Models of T cell anergy: is there a common molecular mechanism? J. Exp. Med. 184, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schwartz R. H. (2003) T cell anergy. Annu. Rev. Immunol. 21, 305–334 [DOI] [PubMed] [Google Scholar]
- 46. Puga I., Rao A., Macian F. (2008) Targeted cleavage of signaling proteins by caspase 3 inhibits T cell receptor signaling in anergic T cells. Immunity 29, 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Refaeli Y., Van Parijs L., Alexander S. I., Abbas A. K. (2002) Interferon γ is required for activation-induced death of T lymphocytes. J. Exp. Med. 196, 999–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee C. Y., Wey S. P., Liao M. H., Hsu W. L., Wu H. Y., Jan T. R. (2008) A comparative study on cannabidiol-induced apoptosis in murine thymocytes and EL-4 thymoma cells. Int. Immunopharmacol. 8, 732–740 [DOI] [PubMed] [Google Scholar]
- 49. Ryan D., Drysdale A. J., Lafourcade C., Pertwee R. G., Platt B. (2009) Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J. Neurosci. 29, 2053–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Drysdale A. J., Ryan D., Pertwee R. G., Platt B. (2006) Cannabidiol-induced intracellular Ca2+ elevations in hippocampal cells. Neuropharmacology 50, 621–631 [DOI] [PubMed] [Google Scholar]
- 51. Dolmetsch R. E., Xu K., Lewis R. S. (1998) Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 [DOI] [PubMed] [Google Scholar]
- 52. Savignac M., Mellstrom B., Naranjo J. R. (2007) Calcium-dependent transcription of cytokine genes in T lymphocytes. Plügers Arch. 454, 523–533 [DOI] [PubMed] [Google Scholar]
- 53. Macian F., Garcia-Rodriguez C., Rao A. (2000) Gene expression elicited by NFAT in the presence or absence of cooperative recruitment of Fos and Jun. EMBO J. 19, 4783–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Macian F., Garcia-Cozar F., Im S. H., Horton H. F., Byrne M. C., Rao A. (2002) Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109, 719–731 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.