Background: Lubricin is an abundant mucin-like glycoprotein in synovial fluid and a major component responsible for joint lubrication.
Results: Lewis x and sulfated O-glycans enable lubricin to bind L-selectin. Lubricin binds to polymorphonuclear granulocytes (PMN) in an L-selectin-dependent and -independent manner.
Conclusion: PMN isolated from peripheral blood and synovial fluid keep a coat of lubricin.
Significance: Lubricin may play a role in PMN-mediated inflammation.
Keywords: Arthritis, Fluorescence, Glycomics, Mass Spectrometry (MS), Proteomics, L-selectin, Lubricin, Polymorphonuclear Granulocytes, Rheumatoid Arthritis, Synovial Fluid
Abstract
Lubricin (or proteoglycan 4 (PRG4)) is an abundant mucin-like glycoprotein in synovial fluid (SF) and a major component responsible for joint lubrication. In this study, it was shown that O-linked core 2 oligosaccharides (Galβ1–3(GlcNAcβ1–6)GalNAcα1-Thr/Ser) on lubricin isolated from rheumatoid arthritis SF contained both sulfate and fucose residues, and SF lubricin was capable of binding to recombinant L-selectin in a glycosylation-dependent manner. Using resting human polymorphonuclear granulocytes (PMN) from peripheral blood, confocal microscopy showed that lubricin coated circulating PMN and that it partly co-localized with L-selectin expressed by these cells. In agreement with this, activation-induced shedding of L-selectin also mediated decreased lubricin binding to PMN. It was also found that PMN recruited to inflamed synovial area and fluid in rheumatoid arthritis patients kept a coat of lubricin. These observations suggest that lubricin is able to bind to PMN via an L-selectin-dependent and -independent manner and may play a role in PMN-mediated inflammation.
Introduction
Lubricin is encoded by the PRG4 (proteoglycan 4) gene and synthesized by superficial zone chondrocytes and synoviocytes. Translated products of PRG4 have been referred to as superficial zone protein, megakaryocyte-stimulating factor precursor, camptodactyly-arthropathy-coxa vara-pericarditis protein, and hemangiopoietin (1–7). Alternative exon splicing of PRG4 transcripts and post-translational modifications lead to the differences in protein primary structure (4, 6). There are seven different isoforms of lubricin, six of which are identified in humans (1, 4, 6–9). They may have different biological functions. As a lubricating glycoprotein, the expression of lubricin has been found in synovial fluid (SF)3 (10), superficial layer of articular cartilage (11), tendons (12), and menisci (13). In addition, lubricin has been detected in blood and urine (7, 14). However, its extra-articular function is still poorly understood. In this study, we use “lubricin” when referring to the PRG4 gene products expressed in articular joints as well as in plasma.
Human lubricin is a secreted mucinous glycoprotein consisting of 1404 amino acids (1). Lubricin contains multiple protein domains, which probably contribute to its diverse biological properties, such as promoting growth in both pure and mixed megakaryocyte colonies, cytoprotection, matrix binding, dimerization, and inhibition of adhesion (1, 15, 16). The largest central mucin-like domain consists of 59 imperfectly repeated sequences of EPATTPK. This domain is flanked by a C-terminal hemopexin domain and two somatomedin B-like domains at the N terminus. In addition, a single putative glycosaminoglycan attachment site (D220EAGSG225) and heparin binding site (K134RSPKPPNKKKTKKV148) have been reported within the human lubricin primary sequence (1, 4, 11, 17).
Lubricin is responsible for the boundary lubrication of articular cartilage (18, 19). The O-glycans of lubricin have been assigned this function because removal of the ultimate and penultimate sugars (NeuAc and Gal residues) resulted in a loss of boundary lubricating ability (6). Recent studies indicate that lubricin also plays an important role in controlling adhesion-dependent synovial growth (20), preventing protein deposition onto cartilage from synovial fluid and inhibiting the adhesion of synovial cells to the cartilage surface (1, 3, 12). Loss-of-function mutations in the PRG4 gene causes the camptodactyly-arthropathy-coxa vara-pericarditis syndrome in humans, a syndrome of precocious joint failure associated with non-inflammatory synovial hyperplasia and subintimal fibrosis of the joint capsule (3). Lubricin is found heterogeneously expressed in the synovium of rheumatoid arthritis (RA) and osteoarthritis (OA), implying a role in the pathogenesis of these diseases (21, 22). RA patients varied with respect to lubricin expression, where the patients with low levels of lubricin had more aggressive joint disease (22). A relationship between the pathogenesis of OA and down-regulation of lubricin has been suggested in several animal models (23–26). Furthermore, treatment of rat joints with injections of recombinant lubricin has indicated its protective effects on chondrocytes, suggesting the benefits of lubricating molecules as a possible therapy for OA (1, 16).
One of the hallmarks of RA is the infiltration of inflammatory cells, such as T cells, B cells, and macrophages, in the synovial tissue (27). The recruitment of leukocytes toward inflammatory foci is preceded by a highly coordinated sequence of interactions between leukocytes and endothelial cells, a process termed the adhesion cascade (28, 29). Polymorphonuclear granulocytes (PMN) or neutrophils are one of the first immune cells to be recruited at inflammatory foci. PMN, like other leukocytes, use L-selectin to roll along the endothelium in the initial phase of the adhesion cascade. In RA, PMN are rarely found in the synovial tissue. Instead, they accumulate in the synovial cavity (30). In particular, PMN have been reported at the pannus/cartilage border, where the joint destruction takes place, suggesting that these cells play an important role in the RA pathogenesis (31–33).
In our previous study, the O-glycans of lubricin were investigated (34). Approximately 30–35% of the total molecular mass of lubricin counts for O-glycosylation. Among them, core 1 O-linked oligosaccharides (Galβ1–3GalNAcα1-) and sialylated core 1 (Neuα2–3Galβ1–3GalNAcα1- and Neuα2–3Galβ1–3(NeuAcα2–6)GalNAcα1-) were the predominant structures on lubricin. In addition, core 2 structures, such as NeuAcα2–3Galβ1–3(NeuAcα2–3Galβ1–3/4GlcNAcβ1–6)GalNAcα1-, were also found in a low amount together with a small proportion of sulfated core 2 oligosaccharides. The presence of sulfated glycans indicates a potential L-selectin binding ability, especially if the sulfate is linked to the 6-positon of GlcNAc on either extended core 1- or core 2-branched O-glycans (34). The optimal recognition by the C-type lectin domain of L-selectin requires sialylation, fucosylation, and sulfation (e.g. 6-sulfo sialyl Lewis x (6-sulfo sLex, NeuAcα2–3Galβ1-4(Fucα1–3)(6S)GlcNAcβ1-)) of N- or O-linked glycans (35–38).
In this study, the linkage position of sulfation and potential fucosylation of lubricin were addressed. The ability of L-selectin and human PMN from peripheral blood and SF to bind lubricin were also investigated in order to discover a potential role of lubricin in inflammation.
EXPERIMENTAL PROCEDURES
Human Synovial Tissue and Cells
Synovial fluid samples from 10 RA patients (six rheumatoid factor (RF)-positive and four RF-negative) were collected during aseptic aspiration of knee joints at the Rheumatology Clinic, Sahlgrenska University Hospital (Gothenburg, Sweden). The RF-positive RA patients (age 58.8 ± 16.6; DAS28 3.7 ± 0.8) were treated with methotrexate (5), biologicals (3), or corticosteroids (3); the RF-negative patients (age 67.5 ± 8.3; DAS28 3.7 ± 1.3) were treated with methotrexate (4). In addition, synovial tissue specimens were obtained from RA patients during joint replacement surgery at the Orthopedic Clinic, Sahlgrenska University Hospital. All patients gave informed consent, and the procedure was approved by the Ethics Committee of Gothenburg University. All RA patients fulfilled the American College of Rheumatology 1987 revised criteria for RA (39). One synovial fluid sample from a psoriatic arthritis patient was obtained from Royal Prince Alfred Hospital (Sydney, Australia) with the patient's written consent.
Enrichments of Native and Recombinant Lubricin
Collected synovial fluid were clarified by centrifugation at 10,000 × g for 10 min and stored at −80 °C before use. The acidic proteins were purified as described previously (34). Lubricin-containing fractions were precipitated with 80% ethanol for 16 h at −20 °C. The precipitate was collected by centrifugation at 12,100 × g for 20 min and resuspended in phosphate-buffered saline (PBS) at pH 7.4 after air drying. Protein concentration was determined by BCA protein assay kit (Thermo Scientific, San Jose, CA) using bovine serum albumin (BSA) as a standard.
Stable CHO cells expressing full-length lubricin (accession number NM_005807) in pcDNA3.1 vector was generated by zeocin selection (40, 41). Serum-free medium, which contained recombinant lubricin, was enriched as native lubricin.
Oligosaccharide Characterization
Purified lubricin was reduced with 10 mm dithiothreitol (DTT) at 95 °C for 20 min and alkylated with 25 mm iodoacetamide for 1 h at room temperature in the dark before applying to a 3–8% Tris acetate gel (NuPAGE, Invitrogen). The gels were then transferred onto PVDF membrane (Immobilon P membranes, Millipore, Billerica, MA) using a semidry method as described previously (42).
O-Linked oligosaccharides were released by reductive β-elimination from the Direct Blue 71-stained PVDF membrane corresponding to the area where lubricin was detected. In brief, membrane strips were incubated with 50 μl of 1.0 m NaBH4 in 100 mm NaOH for 16 h at 50 °C. Reactions were quenched with 1 μl of glacial acetic acid, and samples were desalted and dried for capillary graphitized carbon LC-MS and LC-MS/MS in negative ion mode using an LTQ XL ion trap mass spectrometer (Thermo Scientific) (42). Oligosaccharides were identified from their MS/MS spectra using UniCarb-DB database (2011 version) (43) and validated manually. The annotated structures on lubricin are available on the UniCarb-DB database (available on the World Wide Web).
The Mann-Whitney U test was used for comparing two groups (RF-positive and RF-negative) for the degree of O-glycosylation. A p value of 0.05 was considered to indicate statistical significance.
Western Blotting, Inhibition ELISA, and Co-immunoprecipitation
Samples were separated by electrophoresis either with 3–8% Tris acetate gel or with gradient SDS-agarose polyacrylamide composite gel (SDS-AgPAGE). Proteins blotted onto PVDF membranes were blocked with TBS-T buffer (Tris-buffered saline, pH 7.4, with 0.01% Tween 20) containing 1% BSA. After washing, membranes were incubated for 1 h at room temperature with mouse anti-lubricin antibody (mAb 13 and mAb 378, Pfizer Research, Cambridge, MA), mouse anti-sialyl Lewis x (CD15s or mAb CSLEX1, BD Biosciences), mouse anti-chondroitin sulfate (mAb CS56, Sigma-Aldrich), or recombinant human L-selectin/Fc (human IgG1) chimera (R&D Systems, Minneapolis, MN) and subsequently incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated rabbit anti-mouse immunoglobulins (DakoCytomation, Glostrup, Denmark) and goat anti-human IgG (Fc-specific, Sigma-Aldrich), respectively. After washing, immobilized antibodies were visualized by SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific).
To desialylate O-glycans, the reduced and alkylated samples were incubated with 5 milliunits of sialidase A (Prozyme Inc., Oxford, UK) at 37 °C for 16 h. The reaction was stopped by heating at 95 °C for 10 min in SDS-loading buffer. To characterize bound lubricin, human PMN were freshly isolated from heparinized peripheral blood from healthy volunteers as described previously (44). Cell pellet was heated at 95 °C for 10 min in the presence of SDS-loading buffer. The resultant samples were subjected to electrophoresis directly.
To investigate the potential association of synovial lubricin with proteoglycan, synovial fluid was treated with either chondroitinase ABC from Proteus vulgaris (Seikagaku Corp., Tokyo, Japan) in 0.1 m NH4Ac (pH 8.0) or hyaluronidase from Streptomyces hyalurolyticus (Sigma-Aldrich) in 0.1 m NH4Ac (pH 5.9) at 37 °C overnight. The resultant samples were separated by 3–8% Tris acetate gel after reduction and alkylation. Bovine articular cartilage aggrecan was used as a positive control (Sigma-Aldrich).
Inhibition ELISA was carried out by coating 96-well microtiter plates (Nunc, Roskilde, Denmark) with 2 μg/ml recombinant L-selectin in 0.1 m sodium carbonate buffer, pH 9.5. After washing and blocking with 1% BSA in TBS-T buffer, synovial lubricin with or without inhibitors were added and incubated at 37 °C for 1 h. Bound lubricin was detected by mAb 13 and HRP-conjugated rabbit anti-mouse immunoglobulins. Color was developed by using tetramethyl benzidine buffer (Sigma-Aldrich) as substrate for 10 min and measured at 450-nm wavelength. 100 μg/ml bovine fetuin (Sigma-Aldrich), 100 μg/ml porcine gastric mucins (Sigma-Aldrich), and 50 μg/ml 6-sulfo Lex (Dextra, Reading, UK) were used as inhibitors.
For immunoprecipitation, purified lubricin was reduced with 10 mm DTT at 56 °C for 45 min. The DTT and other impurities were removed by a spin filter with a 300-kDa cut-off (Millipore). The retained sample was then incubated with recombinant human L-selectin/Fc chimera at room temperature for 1 h in the presence of 1 mm CaCl2. Then Dynabeads® Protein G (Invitrogen) were transferred to the mixture and incubated at room temperature for another 2 h. After washing, the beads were heated at 70 °C for 20 min in the presence of SDS-loading buffer containing 10 mm DTT and 5% SDS.
Immunofluorescence and Confocal Microscopy
Human synovial tissue specimens from five RA patients were processed for immunohistochemical staining as described previously (45). In brief, paraformaldehyde-fixed paraffin-embedded 4-μm sections of human synovial tissue were deparaffinized and rehydrated and subjected to an antigen retrieval procedure. Nonspecific binding was blocked using a serum-free protein block (DakoCytomation). After blocking, rabbit anti-lubricin (PA3–118) antibodies or normal rabbit serum (DakoCytomation) in TBS containing 1% BSA were used in sequential staining with Alexa Fluor 555-conjugated goat anti-rabbit IgG (Invitrogen), respectively.
Peripheral PMN were isolated as described from buffy coats from healthy volunteers (46). After dextran sedimentation at 1 × g and hypotonic lysis of the remaining erythrocytes, the PMN obtained by centrifugation in a Ficoll-Paque gradient were washed twice in Krebs-Ringer phosphate buffer. The cells were resuspended in Krebs-Ringer phosphate buffer and stored on ice as resting cells (1 × 107/ml).
For confocal immunofluorescence microscopy analysis, peripheral resting PMN were stained with an allophycocyanin-labeled anti-L-selectin antibody (clone DREG56, BioLegend, San Diego, CA) and rabbit anti-lubricin diluted in blocking buffer for 90 min on ice. After washing, cells were stained with a secondary fluorescein isothiocyanate (FITC)-labeled swine anti-rabbit antibody (DakoCytomation) for 80 min on ice. Following a final wash, cells were air-dried on microscopic slides overnight protected from light.
For studies on synovial PMN, SF samples from three RA patients were collected and passed through a 40-μm nylon cell strainer (BD Biosciences). The cell suspensions were washed in PBS and subjected to centrifugation at 1500 rpm for 10 min at room temperature.
The pelleted synovial cells were resuspended in PBS, spread onto glass slides, and fixed in 70% ethanol for 10 min. The fixed cells were subjected to the same double immunofluorescence protocol as the synovial tissue specimen mentioned above, staining for lubricin.
The stained cell and tissue specimens were washed and mounted in Prolong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain (Invitrogen). Normal mouse IgG and normal rabbit serum were used as negative controls to set the background fluorescence levels. Images were collected using a confocal microscope (Zeiss LSM700, Oberkochen, Germany) and analyzed using Zen image analysis software 2009 (Zeiss).
Flow Cytometric Analysis
Resting PMN were incubated in Krebs-Ringer phosphate buffer alone or in the presence of 10 ng/ml human recombinant TNF-α (Sigma-Aldrich) at 37 °C for 20 min. After washing, cells were incubated with different antibodies for 1 h on ice. For L-selectin detection, phycoerythrin-labeled anti-L-selectin antibody (CD62L, BD Biosciences) was used. After washing, bound lubricin was detected by the rabbit anti-lubricin antibody followed by a FITC-labeled swine anti-rabbit antibody (DakoCytomation). Samples were washed and analyzed by a flow cytometer (Accuri C6, BD Biosciences). When indicated, purified SF lubricin (50 μg/ml) was added to cells on ice for 90 min prior to washing and the addition of antibodies.
Lubricin LC-MS Identification
Coomassie-stained bands were destained, carbamidomethylated, digested with trypsin, and extracted from gel pieces as described previously (34). The resultant peptides were subjected to nano-LC-electrospray ionization MS/MS analysis with an LTQ Orbitrap XL mass spectrometer (Thermo Scientific). Peptide MS/MS spectra were searched against UniProt and the NCBI human protein database by using Mascot software (version 2.2.04, Matrix Science Inc.). Only peptides with a mass deviation lower than 10 ppm were accepted, and two peptides with manual inspection were used for protein identification.
RESULTS
Lewis x and Sulfated O-Glycans Enable Synovial Lubricin to Bind L-selectin
The presence of sulfated O-glycans of synovial lubricin makes it a potential ligand for selectin binding (34). We were therefore interested to see if human SF lubricin can bind to L-selectin.
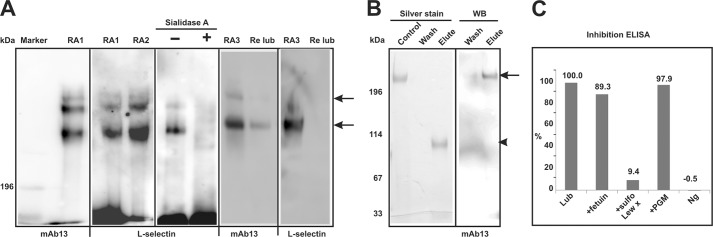
Synovial lubricin was purified from RA patients (n = 10). As shown in Fig. 1A, using lubricin-specific antibody (mAb 13), SF lubricin was detected as two bands both with an apparent molecular mass of >200 kDa when separated on SDS-agarose polyacrylamide composite gel under non-reducing conditions. Both bands were identified as lubricin by LC-MS after tryptic digestion (see below). The upper band is supposedly a dimer of SF lubricin, similar to that reported for bovine SF lubricin (47).
FIGURE 1.
Lubricin binds to recombinant human L-selectin/Fc chimera. A, RA lubricin samples (RA1 and RA2) were separated by SDS-AgPAGE under non-reducing conditions. Recombinant L-selectin/Fc chimera bound to lubricin, whereas sialidase A treatment abolished this interaction (RA1). SF lubricin and L-selectin/Fc chimera were detected by mAb 13 and goat anti-human IgG (Fc-specific) antibodies, respectively. Recombinant lubricin (Re lub) was also analyzed in the same way. No L-selectin was found bound to recombinant lubricin in comparison with native lubricin (RA3). B, immunoprecipitation was verified by silver stain and lubricin-specific antibody (mAb 13). In breakthrough, no L-selectin/Fc chimera was detectable by silver stain. In elutant, eluted L-selectin/Fc chimera could be detected by silver stain, whereas lubricin was detectable by immunoblot (WB). The arrow indicates synovial lubricin, and the arrowhead indicates L-selectin/Fc chimera. C, inhibition ELISA with synovial lubricin and L-selectin. 96-well microtiter plates were coated with L-selectin. Synovial lubricin with or without inhibitors (fetuin, porcine gastric mucins (PGM), or 6-sulfo Lex (sulfo Lew x)) is shown. Bound lubricin was detected by mAb 13. Treatment with 1% BSA instead of lubricin was used as negative control (Ng).
The separated SF lubricin was probed with recombinant human L-selectin/IgG chimera. As shown in Fig. 1A, RA SF lubricin from different patients was consistently shown to bind recombinant L-selectin. No binding was detected when SF lubricin was incubated with secondary antibody alone (goat anti-human IgG, Fc-specific; data not shown). Because interaction with L-selectin is sialylation-dependent, as expected, desiaylation of lubricin by sialidase A diminished the interaction (Fig. 1A, Sialidase A). Sulfation is also required for high affinity L-selectin binding. When recombinant lubricin, expressed in CHO cells that do sialylate glycan but lack sulfation due to an inability to make core 2 structures, was incubated with L-selectin (Fig. 1A), no binding was detected, indicating that sulfation on O-glycan of lubricin was necessary for L-selectin binding. Although the presence of both sialylated and sulfated O-glycans on lubricin has been reported in our previous study (34), high affinity L-selectin ligand, 6-sulfo sLex, was not detected on lubricin. Because our result indicated L-selectin binding despite the lack of this epitope, the level of L-selectin affinity was investigated further. Thus, an inhibition ELISA was performed (Fig. 1C). The results indicated that the binding of lubricin to L-selectin was inhibited by 6-sulfo Lex (90%), whereas bovine feutin (mainly sialylated glycans) and porcine gastric mucin (sulfated non-sialylated glycans) could only inhibit slightly (10%). These data indicate that lubricin is a moderate binder to L- selectin, between the level of a nonspecific sialylated and sulfated glycoprotein but not as good as previously reported high affinity ligand (48, 49).
L-selectin/IgG was also shown to capture lubricin in immunoprecipitation experiments. Silver staining of eluted sample revealed a band around 114 kDa representative of eluted L-selectin/IgG (Fig. 1B, Silver stain), and Western blot with mAb 13 revealed that lubricin was selectively enriched from SF (Fig. 1B, WB).
Presence of Lubricin in Serum and Binding to Peripheral PMN
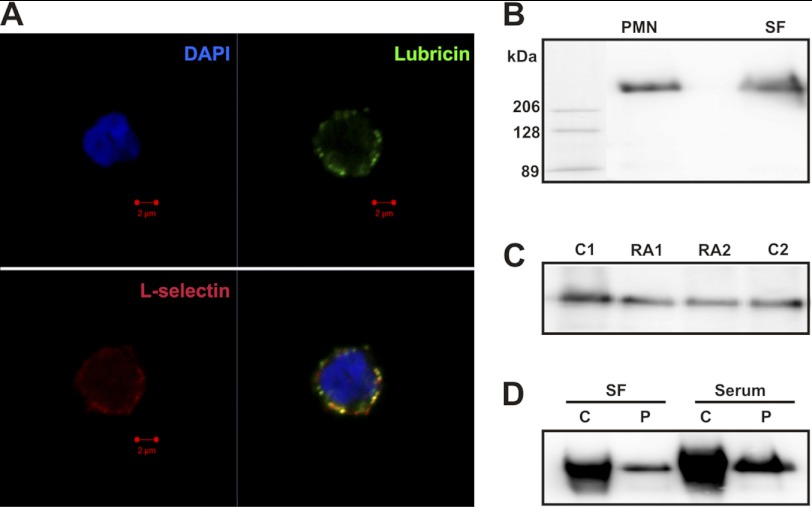
In order to study the potential binding of lubricin to native L-selectin on leukocytes, we first investigated the presence of lubricin on freshly prepared PMN from peripheral blood. Using confocal microscopy, we found that lubricin coated the surface of circulating PMN (Fig. 2A, green). That lubricin associates with peripheral PMN in healthy subjects was further shown by immunoblotting of cell lysates (Fig. 2, B and C) and flow cytometry (Fig. 3) of isolated PMN samples.
FIGURE 2.
Lubricin binds to peripheral PMN and partially co-localizes with L-selectin. A, confocal immunofluorescence analysis of L-selectin and lubricin on the surface of resting PMN from peripheral blood of healthy donors. Lubricin (green) on the cell surface of PMN partially co-localizes (yellow) with L-selectin (red). Nuclei were stained with DAPI (blue). B, presence of serum lubricin in lysates of peripheral PMN. Isolated peripheral PMN were lysed and separated on SDS-agarose polyacrylamide composite gel using purified SF lubricin as a positive control. C, both serum and synovial lubricin have similar size and protein level. 4 μl of serum from a healthy donor (C1 and C2) and RA patients (RA1 and RA2) were separated on SDS-AgPAGE. D, serum lubricin (Serum) from one RA patient before (C; 1 μl) and after purification (P) was separated by SDS-AgPAGE. For comparison, the same patient's SF lubricin (SF) before (C; 0.5 μl) and after purification (P) was used as a control.
FIGURE 3.
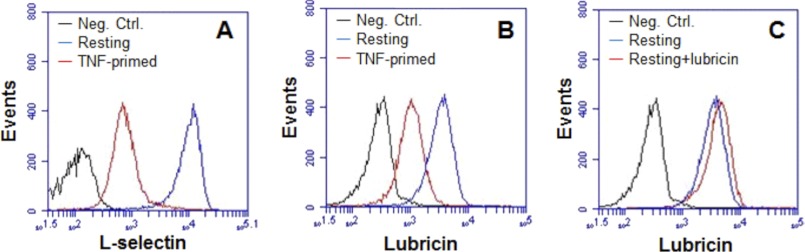
L-selectin-tethered lubricin is shed off upon activation. The levels of L-selectin and lubricin on peripheral PMN from healthy controls were monitored by flow cytometry. A, level of L-selectin on PMN before (blue line) and after (red line) stimulation with TNF. B, level of lubricin on PMN before (blue line) and after (red line) stimulation with TNF. C, the association between resting PMN and lubricin in the absence (blue line) and presence (red line) of exogenous lubricin purified from SF. Neg. Ctrl., negative control.
The presence of lubricin in circulation was confirmed by Western blotting of serum from both healthy and RA patients (Fig. 2C). The result indicated that lubricin has a similar size and protein level in both RA and healthy sera. In addition, there was no apparent difference between synovial fluid and serum lubricin in size. As shown in Fig. 2D, the higher loading of lubricin in SF and serum manifests as increased polydispersity, whereas after purification and a lower amount of lubricin loaded, both SF lubricin and serum lubricin display similar apparent molecular mass. This indicated that lubricin from these two compartments displayed similar size. Preliminary data also indicated similar glycosylation on both proteins (data not shown).
We hypothesized that lubricin association with PMN is mediated by binding to L-selectin on the surface of these cells. Because L-selectin is cleaved off from activated PMN (44), we expected to find decreased lubricin levels on the surface-activated cells. We found that both the surface expression of L-selectin and the coating of lubricin were dramatically decreased after TNF activation, indicating that release of L-selectin and lubricin was mediated by the TNF signaling pathway (Fig. 3, A and B). Because lubricin is able to bind to L-selectin, it is possible that some lubricin binds to PMN via L-selectin, whereas other lubricin binds through unknown receptors. Confocal microscopy of resting peripheral PMN displayed partial colocalization (Fig. 2A, yellow) of these molecules, which strengthens the notion that lubricin binding to the surface of peripheral PMN is at least in part mediated via L-selectin. The addition of exogenous lubricin, purified from SF, to resting peripheral PMN resulted in a minor increase of lubricin binding (Fig. 3C).
These data demonstrate that lubricin is present not only in joints but also in serum and that circulating PMN are coated with lubricin under physiological conditions. Some, but not all, of this lubricin is removed from the cell surface concomitant with L-selectin shedding during cellular activation, strongly indicating that lubricin association with resting PMN is mediated by both L-selectin and by unknown receptors.
As mentioned above, in flares of RA, large numbers of neutrophils accumulate in the SF. Therefore, we next addressed whether lubricin could be associated with synovial PMN as well.
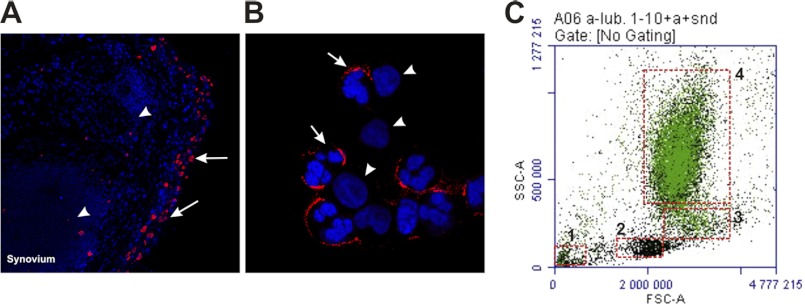
Synovial leukocytes were freshly isolated from SF of RA patients, and the PMN were identified on basis of their particular multilobulated nuclei. Most synovial PMN were coated with lubricin (Fig. 4, B (arrow) and C) in a similar manner as PMN from peripheral blood. Confocal immunofluorescence using RA synovial tissue specimens revealed positive cytoplasmic staining of lubricin in cells of the synovial lining (Fig. 4A). No continuous staining of the apical surface of the lining layer was found in these specimens. These findings suggest that adherence of lubricin to synovial PMN is specific and not just a casual coating of cells and tissues in the synovial cavity. In the synovium, only synovial fibroblasts were found to be lubricin-positive (Fig. 4A, arrows). Lubricin is absent on lymphocytes in synovium (Fig. 4A, arrowhead) and monocytes or lymphocytes in synovial fluid (Fig. 4B, arrowhead). Flow cytometric analysis of a synovial fluid sample isolated from an RA patient showed that all PMN were lubricin-positive, and the lubricin negative cells were identified as monocytes or lymphocytes (Fig. 4C). Taken together, both peripheral and synovial PMN are covered by lubricin, some of which co-localize with L-selectin.
FIGURE 4.
The presence of synovial lubricin in the synovial lining layer and on the surface of synovial PMN. Immunofluorescence analysis was carried out on synovial tissue specimens (A) and isolated synovial PMN (B) from RA patients. A, in the RA synovium, a large number of leukocytes (arrowheads) were present in germinal center-like structures in the sublining. Lubricin was only found within synoviocytes of the synovial lining layer (arrows). B, synovial PMN were isolated by centrifugation and washed with PBS. Lubricin was found on the surface of most PMN (arrows). C, representative flow cytometry plot of synovial cells stained with anti-lubricin antibody. The isolated cells were recognized as PMN (70%; 4), monocytes (5%; 3), lymphocytes (7%; 2), and nonspecific cells (1) by forward-side scatter. A majority of the PMN were coated with lubricin (green).
O-Linked Oligosaccharides of Synovial Lubricin Are Likely to Be Responsible for L-selectin Binding
Because binding to L-selectin requires 6-sulfo sLex and lubricin is able to bind to L-selectin, O-glycans of lubricin should contain 6-sulfo sLex, as expected. However, in our previous study, no fucosylated O-glycans or 6-sulfo sLex-containing O-glycans were found on synovial lubricin. Furthermore, the linkage and position of sulfate groups were not determined. The sulfate could link to C3 or C6 of Gal or C6 of GlcNAc residue resulting in 3′-sulfo, 6′-sulfo, and 6-sulfo O-glycan, respectively (34).
To characterize the position and linkage of sulfate groups as well as other modifications, such as fucosylation, O-linked oligosaccharide of synovial lubricin isolated from RA patients was released by reductive β-elimination as described previously (34); the entire oligosaccharide mixture was analyzed by porous graphitized carbon LC-electrospray ionization MS/MS.
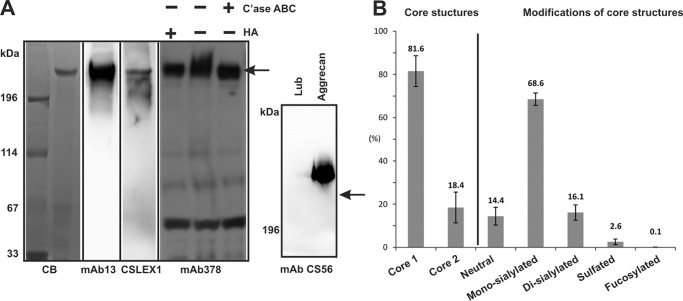
Core 1 type O-glycans were the predominant O-glycan structures (81.6%) on synovial lubricin from RA patients (n = 10). About 73% of core 1 and 50% of core 2 O-glycans were monosialylated. There were more monosialylated structures (68.5%) than disialylated structures (16.1%) (Fig. 6B). In comparison with RF-negative RA patients (n = 4), RF-positive patients (n = 6) have higher core 2 (21.3 ± 6.2% versus 14.2 ± 6.2%) and lower core 1 (78.7 ± 6.2% versus 85.8 ± 6.2%). However, the difference was not significant. RF-positive RA patients have significantly higher sulfated O-glycans than RF-negative ones (3.2 ± 1.2 versus 1.6 ± 1.6%, p = 0.038, Mann-Whitney test).
FIGURE 6.
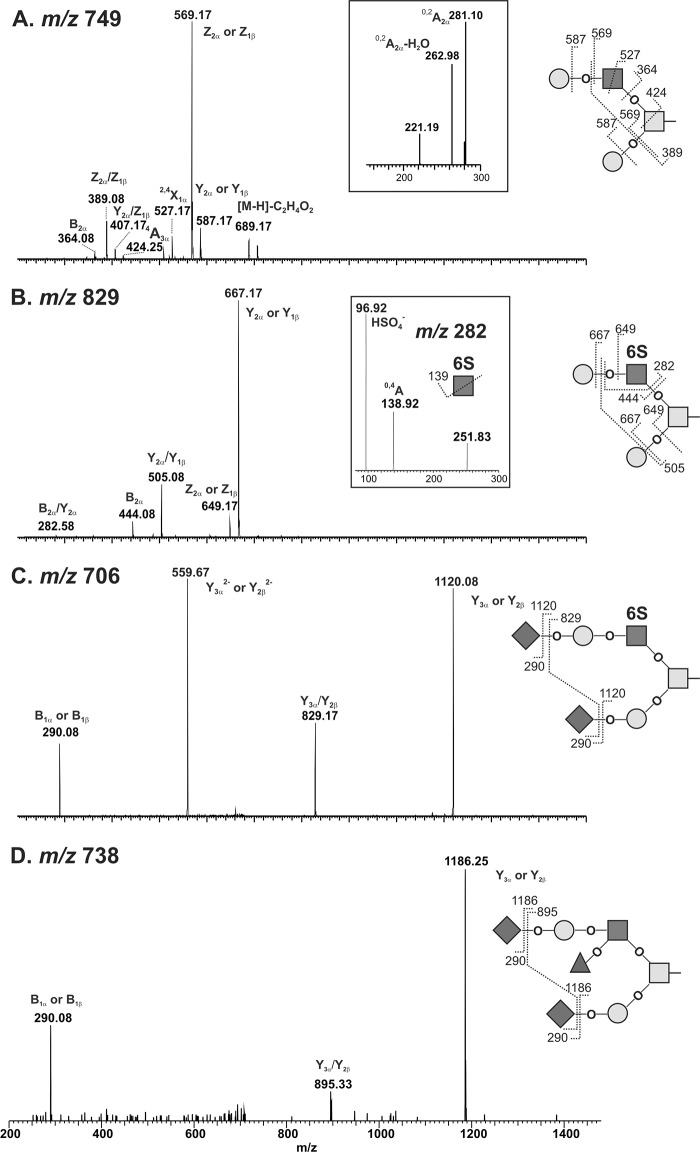
Synovial lubricin contains sLex epitope but no detectable chondroitin sulfate. A, SF lubricin has the sLex epitope. Lubrin was separated by 3–8% NuPAGE and probed with antibodies specific to lubricin (mAb 13 and mAb 378), chondroitin sulfate (mAb CS56), and sLex epitope (CSLEX1), respectively. A significant amount of sLex epitope could be detected by CSLEX-1. SF lubricin was negative to chondroitin sulfate-specific antibody (mAb CS56), although positive control bovine aggrecan showed very strong binding. However, when SF lubricin was treated with hyaluronidase (HA) and chondroitinase ABC (C'ase ABC), the size of lubricin was slightly reduced, as indicated by the lubricin-specific antibody (mAb 378). The arrow indicates synovial lubricin localization. B, distribution of O-linked glycans on SF lubricin (n = 10 RA patients, including four RF-negative and six RF-positive). Core 1 O-glycans include neutral and mono-/disialylated core 1 O-glycans (m/z 384, 675, and 966, respectively). Neutral O-glycans include one core 1 O-glycan (m/z 384) and two core 2 type O-glycans ions at m/z 587 and 749. Monosialylated O-glycans contain ions at m/z 675, 878, 1040, and 1120, whereas disialylated structures include ions at m/z 966, 1331, 1411, and 1477. Sulfated O-glycans consist of ions at m/z 667, 829, 1120, and 1411. Fucosylated O-glycan only represents ion at m/z 1477. The number represents the percentage relative to total ion density. Error bars, S.D.
In the mass spectrum analysis, oligosaccharides corresponding to sialylated and sulfated core 2 structures were detected (Fig. 5). The biosynthetic precursor of the non-sulfated core 2 structure with the composition Hex2HexNAc2 ([M − H]− ions of m/z 749) was identified as a single isomer (Fig. 5A) by LC-MS/MS. The presence of the 4A3α fragment ion (m/z 424) was consistent with an N-acetyllactosamine elongation on the C-6 of the GalNAcol. The 0,2A2α and 0,2A2α-H2O fragment ions (m/z 281 and 263) indicated Hex1HexNAc1 contained in a type 2 (Galβ1–4GlcNAcβ1-) glycan (Fig. 5A, inset) (50). Thus, this structure was annotated as the core 2 O-glycans, Galβ1-4GlcNAcβ1–6(Galβ1–3)GalNAcα1- (50). Sulfated structures were also detected and sequenced after desialylation. To determine the position and linkage of the sulfated core 2 O-glycan, the [M − H]− ions of m/z 829 were chosen for fragmentation. The presence of ions at m/z 282, 444, and 667 in MS/MS spectrum (Fig. 5B) indicated that the sulfate group was linked to GlcNAc and not to the Gal residue. The MS3 spectrum showed fragmentions at m/z 97 (HSO4−) and 139 (0,4AGlcNAc, OCHCH2SO4−), which further confirmed the presence of sulfate (m/z 97) and sulfate residue linked to C-6 of GlcNAc but not to C-3 of GlcNAc (Fig. 5B, inset) (51). Therefore, ions at m/z 829 were assigned as 6-sulfo core 2 type O-glycans. Sialylated sulfated core 2 O-glycans, such as [M − H]− ions with m/z 1120 and 1411, were assigned as mono- and disialylated 6-sulfo core 2 O-glycans, respectively, based on the sequence obtained after desialylation (Fig. 5C). On synovial lubricin isolated from RA patients, the sulfated O-glycans accounted for about 14.6% of core 2 type and 2.6% of the total O-glycans based on mass spectrometric intensities (Fig. 6B).
FIGURE 5.
Sialylated and sulfated core 2 O-glycans on SF lubricin determined by LC-MS in the negative ion mode. A, MS/MS spectrum of a desialylated core 2 oligosaccharide with a [M − H]− ion of m/z 749. The inset shows the expanded region of linkage-specific 0,2A ions for type 2 (Galβ1–4GlcNAc) linkage. B, MS/MS spectrum of a desialylated sulfated core 2 oligosaccharide with an [M − H]− ion of m/z 829. The inset shows the MS3 spectrum of the fragment ion at m/z 282, which is consistent with GlcNAc6S; C, MS/MS spectrum of sialylated and sulfated core 2 O-glycan with an [M − 2H]2− ion of m/z 706. D, MS/MS spectrum of an sLex-containing core 2 O-glycan with an [M − 2H]2− ion of m/z 738. Proposed structures and fragments are featured as insets in each spectrum. The nomenclature of fragments is based on the rules introduced by Domon and Costello (68).
A low intense parent ion of a disialylated, fucosylated O-glycan ([M − 2H]2− ion of m/z 738, NeuAc2Hex2HexNAc2deHex1) indicated a potential sLex epitope on synovial lubricin (Fig. 5D). The MS/MS spectrum of the parent ion of m/z 738 showed that it was a core 2 type O-glycan. This structure was only detectable in some samples (n = 7 of 10). Due to the low abundance of this glycan (0.7% of core 2 type and less than 0.1% of total O-glycans), limited structural information was obtained by MS/MS. When using CSLEX-1 mAb (mouse anti-sLex monoclonal antibody) probed with synovial lubricin, positive staining was observed (Fig. 6A). It demonstrated the presence of the sLex epitope and low abundance of this epitope. Further modification of this structure, such as sulfation, which leads to 6-sulfo sLex, was not detectable by LC-MS. The central mucin-like domain of human synovial lubricin consists of 59 imperfectly tandem repeat units (EPATTPK) with dense O-linked oligosaccharides attached to serine or threonine residues. Based on mass spectrometric intensities, even with the most optimistic estimation of the glycosylation with two glycosylation sites per tandem repeat, the amount of sLex will be less than one epitope per lubricin molecule.
There were also two potential N-glycosylation sites (Asn206 and Asn1159) on lubricin. The second N-glycosylation site has been identified recently from human blood plasma lubricin (52). In this study, non-glycosylated peptide (N1159GTLVAFR, [M + 2H]2+ ion of m/z 439.7403) was detected in synovial lubricin (Table 1), indicating that the second N-glycosylation site on synovial lubricin was not fully occupied, if occupied at all. The peptide containing the first N-glycosylation site was not detected. The sequence of lubricin and the proteomic data indicated that N-glycosylation was low and cannot provide the high avidity required for L-selectin binding (53, 54).
TABLE 1.
Proteomic analysis of enriched synovial lubricin
Reduced and alkylated sample was separated by 3–8% Tris acetate NuPAGE. Protein bands were visualized by Coomassie Blue (Fig. 6A, CB). Gel pieces were subjected to LC-MS/MS analysis after trypsin digestion. Peptides from exons 2–12 were detected. Exon 1 is supposed to be the signal peptide. In addition to the canonical repeat unit (EPAPTTPK, 33 repeats), two additional non-canonical repeats (KPAPTTPK, four repeats; ETAPTTPK, three repeats) were also observed without O-glycosylation.
| Lubricin peptide sequence | Amino acid positions | Exon(s) |
|---|---|---|
| CGEGYSR | 34–40 | 2 |
| VCTAELSCK | 63–71 | 2–3 |
| CFESFER | 74–80 | 3 |
| (K)APPPSGASQTIK | 118–130 | 4 |
| NSAANRELQK | 187–196 | 5 |
| ETSLTVNK | 273–280 | 6 |
| ETTVETK | 281–287 | 6 |
| ETQSIEK | 309–315 | 6 |
| EPAPTTPK | 403–410, 434–441, 450–457, 473–480, 497–504, 559–566, 567–574, 583–590, 607–614, 679–686, 687–694, 695–702, 719–726, 764–771, 772–779, 788–795, 833–840 | 6 |
| KPAPTTPK | 442–449, 489–496, 575–582, 599–606 | 6 |
| ETAPTTPK | 615–622, 703–710, 825–832 | 6 |
| KPTSTKKPK | 1030–1038 | 6 |
| VPNQGIIINPMLSDETNICNGKPVDGLTTLR | 1128–1158 | 6–7 |
| NGTLVAFR | 1159–1166 | 7 |
| GHYFWMLSPFSPPSPAR | 1167–1201 | 7–8 |
| DSQYWR | 1213–1218 | 9 |
| (FTNDIK)DAGYPKPIFK | 1219–1234 | 9 |
| GFGGLTGQIVAALSTAK | 1235–1251 | 9 |
| NWPESVYFFK | 1254–1263 | 9 |
| (R)GGSIQQYIYK | 1264–1274 | 10 |
| RPALNYPVYGETTQVR | 1285–1300 | 10 |
| IQYSPAR | 1318–1324 | 10 |
| GVLHNEVK | 1331–1338 | 11 |
| KPDGYDYYAFSK | 1361–1372 | 11 |
| DQYYNIDVPSR | 1373–1383 | 12 |
| VWYNCP | 1399–1404 | 12 |
In addition, we failed to identify chondroitin sulfate (CS) on lubricin using the CS-specific mAb CS-56, which readily detected CS in bovine aggrecan (Fig. 6A, mAb CS56), which was confirmed by LC-MS after chondroitinase ABC treatment using bovine aggrecan as a control (data not shown). However, we found that the apparent polydispersity of lubricin was lower after chondroitinase ABC treatment of synovial fluid (Fig. 6A). A similar result was obtained when synovial lubricin was treated with hyaluronidase (Fig. 6A, HA) indicating that residual hyaluronidase activity of chondroitinase ABC and synovial hyaluronic acid influence the migration of lubricin in SDS-AgPAGE.
Taken together, all of these data indicate that sLex epitopes and CS side chains are not the prime ligand for L-selectin binding of lubricin but rather the cluster of sialylated and sulfated oligosaccharides on the tandem repeat of lubricin, which all together provide a patch for L-selectin binding.
Proteomic Analysis of Human Synovial Lubricin
To identify SF lubricin, the bands corresponding to lubricin were excised, trypsinized, and subjected to proteomic analysis. The LC-MS identified 74.9% peptides of the non-mucin domain (residues 1–199 and 1141–1404) (Table 1). We also observed the non-glycosylated mucin repeat unit tryptic peptide, EPAPTTPK (m/z 417.71322+) from the mucin domain (residues 200–1140), indicating that not all potential O-glycosylation sites were occupied. This shows that the amount of glycan epitope sLex is even less on lubricin compared with if all threonines are occupied in the mucin domain.
There are five splice variants that have been reported on lubricin (4, 7). Isoform A consists of exons 1–12 (Swiss-Prot number Q92954-1); isoform B (Q92954-2) lacks exon 2; isoform C (Q92954-3) lacks exons 4 and 5; isoform D (Q92954-4) lacks exons 2, 4, and 5; and isoform F (Q92954-6), also known as hemangiopoietin, lacks exon 5. Our proteomic results identified peptides derived from all exons except exon 1 (Table 1). These data suggest that if only a single isoform is present in synovial fluid, it is isoform A, but it is more likely that we are detecting a mixture of several isoforms.
DISCUSSION
Because the expression level of lubricin in SF has been associated with disease stage of arthritis, lubricin may serve as a potential arthritic biomarker like glycosaminoglycans and cartilage proteins (e.g. aggrecan, heparin sulfate, and cartilage oligomeric matrix protein) (55, 56). Lubricin is a mucin-like glycoprotein. The diverse O-glycan structures and multiple protein domains of lubricin endow properties not only for boundary lubrication but also for other biological functions, including protection of chondrocytes.
In this study, lubricin was found to be able to bind to L-selectin (Figs. 1–3), suggesting that lubricin is a novel L-selectin ligand. There are three groups of L-selectin ligands: the orthodox ligands with 6-sulfo sLex structure on sialomucins and glycoproteins (i.e. GlyCAM-1 (glycosylation cell adhesion molecule-1), CD34, podocalyxin, and MAdCAM-1 (mucosal vascular addressin)); ligands with clusters of sLex and Tyr-SO3 like PSGL-1 (P-selectin-glycoprotein ligand-1) and endoglycan; and proteoglycans with predominantly heparan or chondroitin sulfate modifications (57–59). However, lubricin is different from those identified L-selectin ligands. We did not find extended core 1 O-glycan structures. Only a low amount of sLex could be found on core 2 O-glycan (Figs. 5D and 6A). However, the 6-sulfo sLex epitope was absent on lubricin isolated from 10 RA patients. This suggests that L-selectin may actually recognize a conformational “clustered saccharide patch” rather than a linear defined epitope on lubricin. Thus, the interaction between L-selectin and lubricin is suggested to be weaker than high affinity L-selectin ligands. Inhibition ELISA (Fig. 1C) confirmed that when using 6-sulfo Lex, the binding of lubricin to L-selectin was inhibited significantly. A similar clustered saccharide patch could be found in the closely packed oligosaccharides of mucins or in densely modified heparan/chondroitin sulfate glycosaminoglycans (60, 61). However, our result showed that lubricin was devoid of heparan/chondroitin sulfate (Fig. 6A). Although the apparent polydispersity was reduced upon chondroitinase ABC treatment, this was probably due to the trace hyaluronidase activity of chondroitinase ABC. When samples were treated with hyaluronidase, a similar result as with chondroitinase ABC treatment was obtained, indicating the presence of interaction between lubricin and undigested hyaluronic acid in the sample. This result is consistent with a recent study in which a protein-rich lubricin fraction was devoid of chondroitin sulfate (17). Therefore, the sialylated and sulfated glycans may play a key role in the interaction with L-selectin.
In this study, the sulfated O-linked oligosaccharides present on lubricin from RA patients account for 2.6% of total O-glycans and 14.1% of core 2 O-glycans (Fig. 6B). All sulfate groups on lubricin are linked to the 6-position of GlcNAc on core 2 O-glycan structures as determined by MS3 spectrum (Fig. 2B). There are two 6-O-sulfotransferases, GlcNAc6ST-1 and -2, responsible for transferring the sulfate group to the GlcNAc residue and also essential for L-selectin ligand biosynthesis. GlcNAc6ST-2 (also known as LSST and HEC-GlcNAc6ST) is restrictedly expressed in high endothelial cells. A study has shown that the expression of GlcNAc6ST-2 was detected in endothelia in RA tissue but not in OA (62). However, in our previous study, sulfated core 2 type O-glycans were also found in OA patients (34). Thus, GlcNAc6ST-1 is more likely to be responsible for sulfation of synovial lubricin.
To our knowledge, this is the first report of the presence of lubricin on the surface of both peripheral and synovial PMN, and in part, this interaction was executed by association with L-selectin on the cell surface (Figs. 2–4). Upon stimulation with TNF-α, most lubricin was shed off together with L-selectin. In the circulation, the presence of L-selectin-tethered lubricin may be expected to compete with membrane-bound higher affinity L-selectin ligands and influence the rolling of leukocytes on the surface of endothelial cells. In joints, the presence of negatively charged lubricin on cartilage protects cartilage surfaces and underlying superficial zone chondrocytes by inhibiting protein deposit and cell overgrowth (20). Most PMN coated with lubricin may be electrostatically repelled from cartilage surfaces in a similar manner. Defects of the replenishing layer of lubricin on the synovial lining (e.g. down-regulation or increasing protease activity in SF) may result in loss of protection. In this scenario, cells in synovial fluid (e.g. PMN) may adhere to cartilage, leading to destruction of cartilage. It has been reported that low expression levels of lubricin were associated with a strong synovial stromal activation (22), characterized by the infiltration of T cells and macrophages, and was associated with a higher destructive potential and a worse prognosis (63). Prg4 knock-out mice exhibited a degenerative phenotype, including synovial hyperplasia and abnormal cartilage surface (64).
There are two things of particular importance in this study: first, the presence of lubricin in plasma and, second, that not only L-selectin but also another receptor(s) on PMN is able to bind to lubricin. Although the presence in plasma has been reported (7, 14), the origin and properties (e.g. splicing variant, glycosylation) of this lubricin have not been fully characterized. In this study, serum lubricin was demonstrated to be of a size similar to that of SF lubricin, and the protein levels in both compartments were similar. It is not clear whether serum lubricin has the same origin as synovial lubricin, but it has been indicated that intra-articular injection of recombinant lubricin constructs will deposit on an area of post-traumatic arthritis damage in a rat model (65). The origin and biological functions of serum lubricin are worthy of further investigation.
That lubricin binds PMN also in a non-L-selectin-dependent manner suggests that an additional PMN receptor(s) is also involved. Siglec-9 (sialic acid-binding immunoglobulin-like lectin 9) could be one of these receptors, which recognizes both α2,3- and α2,6-linked NeuAc (66) and is up-regulated on neutrophils in RA patients (67).
In summary, we demonstrated the presence of sLex epitopes on lubricin. These glycoepitopes enable lubricin to bind to L-selectin. We also detected the presence of lubricin on both peripheral and synovial PMN, indicating a role in inflammation by influencing the interaction between PMN and other cells.
Acknowledgment
We thank C. R. Flannery (Wyeth Research, Cambridge, MA) for providing lubricin-specific antibody (mAb 13).
This work was supported by European Union Marie Curie Programme Grant PIRG-GA-2007-205302. The mass spectrometers were purchased with Swedish Research Council Grant LTQ, 342-2004-4434 and Knut och Alice Wallenberg's Stiftelse Grant LTQ XL, KAW2007.0118. The confocal microscope was purchased with Swedish Research Council Grant 521-2007-4263 (to J. B.). Additional support was from Swedish Research Council Grant 521-2009-3443, the King Gustav V Memorial Foundation, and the Swedish state (LUA/ALF).
- SF
- synovial fluid
- CS
- chondroitin sulfate
- DAS28
- disease activity score using 28 joint counts to assess the severity of RA
- Fuc
- fucose
- OA
- osteoarthritis
- PMN
- human polymorphonuclear granulocyte(s)
- RA
- rheumatoid arthritis
- RF
- rheumatoid factor
- SDS-AgPAGE
- SDS-agarose polyacrylamide composite gel electrophoresis
- sLex
- sialyl Lewis x.
REFERENCES
- 1. Flannery C. R., Hughes C. E., Schumacher B. L., Tudor D., Aydelotte M. B., Kuettner K. E., Caterson B. (1999) Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem. Biophys. Res. Commun. 254, 535–541 [DOI] [PubMed] [Google Scholar]
- 2. Kuroda M., Wang X., Sok J., Yin Y., Chung P., Giannotti J. W., Jacobs K. A., Fitz L. J., Murtha-Riel P., Turner K. J., Ron D. (1999) Induction of a secreted protein by the myxoid liposarcoma oncogene. Proc. Natl. Acad. Sci. U.S.A. 96, 5025–5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marcelino J., Carpten J. D., Suwairi W. M., Gutierrez O. M., Schwartz S., Robbins C., Sood R., Makalowska I., Baxevanis A., Johnstone B., Laxer R. M., Zemel L., Kim C. A., Herd J. K., Ihle J., Williams C., Johnson M., Raman V., Alonso L. G., Brunoni D., Gerstein A., Papadopoulos N., Bahabri S. A., Trent J. M., Warman M. L. (1999) CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat. Genet. 23, 319–322 [DOI] [PubMed] [Google Scholar]
- 4. Ikegawa S., Sano M., Koshizuka Y., Nakamura Y. (2000) Isolation, characterization, and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet. Cell Genet. 90, 291–297 [DOI] [PubMed] [Google Scholar]
- 5. Jay G. D., Britt D. E., Cha C. J. (2000) Lubricin is a product of megakaryocyte-stimulating factor gene expression by human synovial fibroblasts. J. Rheumatol. 27, 594–600 [PubMed] [Google Scholar]
- 6. Jay G. D., Tantravahi U., Britt D. E., Barrach H. J., Cha C. J. (2001) Homology of lubricin and superficial zone protein (SZP). Products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J. Orthop. Res. 19, 677–687 [DOI] [PubMed] [Google Scholar]
- 7. Liu Y. J., Lu S. H., Xu B., Yang R. C., Ren Q., Liu B., Li B., Lu M., Yan F. Y., Han Z. B., Han Z. C. (2004) Hemangiopoietin, a novel human growth factor for the primitive cells of both hematopoietic and endothelial cell lineages. Blood 103, 4449–4456 [DOI] [PubMed] [Google Scholar]
- 8. DuRaine G., Neu C. P., Chan S. M., Komvopoulos K., June R. K., Reddi A. H. (2009) Regulation of the friction coefficient of articular cartilage by TGF-β1 and IL-1β. J. Orthop. Res. 27, 249–256 [DOI] [PubMed] [Google Scholar]
- 9. Grad S., Lee C. R., Wimmer M. A., Alini M. (2006) Chondrocyte gene expression under applied surface motion. Biorheology 43, 259–269 [PubMed] [Google Scholar]
- 10. Swann D. A., Silver F. H., Slayter H. S., Stafford W., Shore E. (1985) The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem. J. 225, 195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schumacher B. L., Hughes C. E., Kuettner K. E., Caterson B., Aydelotte M. B. (1999) Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J. Orthop. Res. 17, 110–120 [DOI] [PubMed] [Google Scholar]
- 12. Rees S. G., Davies J. R., Tudor D., Flannery C. R., Hughes C. E., Dent C. M., Caterson B. (2002) Immunolocalization and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix Biol. 21, 593–602 [DOI] [PubMed] [Google Scholar]
- 13. Schumacher B. L., Schmidt T. A., Voegtline M. S., Chen A. C., Sah R. L. (2005) Proteoglycan 4 (PRG4) synthesis and immunolocalization in bovine meniscus. J. Orthop. Res. 23, 562–568 [DOI] [PubMed] [Google Scholar]
- 14. Su J. L., Schumacher B. L., Lindley K. M., Soloveychik V., Burkhart W., Triantafillou J. A., Kuettner K., Schmid T. (2001) Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma 20, 149–157 [DOI] [PubMed] [Google Scholar]
- 15. Englert C., McGowan K. B., Klein T. J., Giurea A., Schumacher B. L., Sah R. L. (2005) Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis Rheum. 52, 1091–1099 [DOI] [PubMed] [Google Scholar]
- 16. Jones A. R., Gleghorn J. P., Hughes C. E., Fitz L. J., Zollner R., Wainwright S. D., Caterson B., Morris E. A., Bonassar L. J., Flannery C. R. (2007) Binding and localization of recombinant lubricin to articular cartilage surfaces. J. Orthop. Res. 25, 283–292 [DOI] [PubMed] [Google Scholar]
- 17. Lord M. S., Estrella R. P., Chuang C. Y., Youssef P., Karlsson N. G., Flannery C. R., Whitelock J. M. (2012) Not all lubricin isoforms are substituted with a glycosaminoglycan chain. Connect. Tissue Res. 53, 132–141 [DOI] [PubMed] [Google Scholar]
- 18. Swann D. A., Sotman S., Dixon M., Brooks C. (1977) The isolation and partial characterization of the major glycoprotein (LGP-I) from the articular lubricating fraction from bovine synovial fluid. Biochem. J. 161, 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jay G. D. (1992) Characterization of a bovine synovial fluid lubricating factor. I. Chemical, surface activity, and lubricating properties. Connect. Tissue Res. 28, 71–88 [DOI] [PubMed] [Google Scholar]
- 20. Rhee D. K., Marcelino J., Baker M., Gong Y., Smits P., Lefebvre V., Jay G. D., Stewart M., Wang H., Warman M. L., Carpten J. D. (2005) The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Invest. 115, 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jüsten H. P., Grünewald E., Totzke G., Gouni-Berthold I., Sachinidis A., Wessinghage D., Vetter H., Schulze-Osthoff K., Ko Y. (2000) Differential gene expression in synovium of rheumatoid arthritis and osteoarthritis. Mol. Cell. Biol. Res Commun 3, 165–172 [DOI] [PubMed] [Google Scholar]
- 22. Ungethuem U., Haeupl T., Witt H., Koczan D., Krenn V., Huber H., von Helversen T. M., Drungowski M., Seyfert C., Zacher J., Pruss A., Neidel J., Lehrach H., Thiesen H. J., Ruiz P., Blass S. (2010) Molecular signatures and new candidates to target the pathogenesis of rheumatoid arthritis. Physiol. Genomics 42A, 267–282 [DOI] [PubMed] [Google Scholar]
- 23. Young A. A., McLennan S., Smith M. M., Smith S. M., Cake M. A., Read R. A., Melrose J., Sonnabend D. H., Flannery C. R., Little C. B. (2006) Proteoglycan 4 down-regulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res. Ther. 8, R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elsaid K. A., Jay G. D., Chichester C. O. (2007) Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 56, 108–116 [DOI] [PubMed] [Google Scholar]
- 25. Elsaid K. A., Fleming B. C., Oksendahl H. L., Machan J. T., Fadale P. D., Hulstyn M. J., Shalvoy R., Jay G. D. (2008) Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 58, 1707–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Teeple E., Elsaid K. A., Fleming B. C., Jay G. D., Aslani K., Crisco J. J., Mechrefe A. P. (2008) Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop. Res. 26, 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Firestein G. S. (2003) Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 [DOI] [PubMed] [Google Scholar]
- 28. Butcher E. C. (1991) Leukocyte-endothelial cell recognition. Three (or more) steps to specificity and diversity. Cell 67, 1033–1036 [DOI] [PubMed] [Google Scholar]
- 29. Springer T. A. (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration. The multistep paradigm. Cell 76, 301–314 [DOI] [PubMed] [Google Scholar]
- 30. Pillinger M. H., Burg N. D., Abramson S. B. (2004) in Rheumatoid arthritis (St. Clair E. W., Pisetsky D. S., Haynes B. F., eds) pp. 161–175, Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 31. Edwards S. W., Hallett M. B. (1997) Seeing the wood for the trees. The forgotten role of neutrophils in rheumatoid arthritis. Immunol. Today 18, 320–324 [DOI] [PubMed] [Google Scholar]
- 32. Mohr W., Westerhellweg H., Wessinghage D. (1981) Polymorphonuclear granulocytes in rheumatic tissue destruction. III. An electron microscopic study of PMNs at the pannus-cartilage junction in rheumatoid arthritis. Ann. Rheum. Dis. 40, 396–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bromley M., Woolley D. E. (1984) Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 27, 857–863 [DOI] [PubMed] [Google Scholar]
- 34. Estrella R. P., Whitelock J. M., Packer N. H., Karlsson N. G. (2010) The glycosylation of human synovial lubricin. Implications for its role in inflammation. Biochem. J. 429, 359–367 [DOI] [PubMed] [Google Scholar]
- 35. Kawashima H., Petryniak B., Hiraoka N., Mitoma J., Huckaby V., Nakayama J., Uchimura K., Kadomatsu K., Muramatsu T., Lowe J. B., Fukuda M. (2005) N-Acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. 6, 1096–1104 [DOI] [PubMed] [Google Scholar]
- 36. Yeh J. C., Hiraoka N., Petryniak B., Nakayama J., Ellies L. G., Rabuka D., Hindsgaul O., Marth J. D., Lowe J. B., Fukuda M. (2001) Novel sulfated lymphocyte homing receptors and their control by a Core1 extension β1,3-N-acetylglucosaminyltransferase. Cell 105, 957–969 [DOI] [PubMed] [Google Scholar]
- 37. Rosen S. D. (2004) Ligands for L-selectin. Homing, inflammation, and beyond. Annu. Rev. Immunol. 22, 129–156 [DOI] [PubMed] [Google Scholar]
- 38. Mitoma J., Bao X., Petryanik B., Schaerli P., Gauguet J. M., Yu S. Y., Kawashima H., Saito H., Ohtsubo K., Marth J. D., Khoo K. H., von Andrian U. H., Lowe J. B., Fukuda M. (2007) Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat. Immunol. 8, 409–418 [DOI] [PubMed] [Google Scholar]
- 39. Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31, 315–324 [DOI] [PubMed] [Google Scholar]
- 40. Thomsson E., Persson L., Grahn A., Snäll J., Ekblad M., Brunhage E., Svensson F., Jern C., Hansson G. C., Backstrom M., Bergström T. (2011) Recombinant glycoprotein E produced in mammalian cells in large scale as an antigen for varicella-zoster-virus serology. J. Virol. Methods 175, 53–59 [DOI] [PubMed] [Google Scholar]
- 41. Bäckström M., Link T., Olson F. J., Karlsson H., Graham R., Picco G., Burchell J., Taylor-Papadimitriou J., Noll T., Hansson G. C. (2003) Recombinant MUC1 mucin with a breast cancer-like O-glycosylation produced in large amounts in Chinese hamster ovary cells. Biochem. J. 376, 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schulz B. L., Packer N. H., Karlsson N. G. (2002) Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal. Chem. 74, 6088–6097 [DOI] [PubMed] [Google Scholar]
- 43. Hayes C. A., Karlsson N. G., Struwe W. B., Lisacek F., Rudd P. M., Packer N. H., Campbell M. P. (2011) UniCarb-DB. A database resource for glycomic discovery. Bioinformatics 27, 1343–1344 [DOI] [PubMed] [Google Scholar]
- 44. Björkman L., Raynes J. G., Shah C., Karlsson A., Dahlgren C., Bylund J. (2010) The proinflammatory activity of recombinant serum amyloid A is not shared by the endogenous protein in the circulation. Arthritis Rheum. 62, 1660–1665 [DOI] [PubMed] [Google Scholar]
- 45. Ekwall A. K., Eisler T., Anderberg C., Jin C., Karlsson N., Brisslert M., Bokarewa M. I. (2011) The tumor-associated glycoprotein podoplanin is expressed in fibroblast-like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res. Ther. 13, R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bøyum A., Løvhaug D., Tresland L., Nordlie E. M. (1991) Separation of leucocytes. Improved cell purity by fine adjustments of gradient medium density and osmolality. Scand. J. Immunol. 34, 697–712 [DOI] [PubMed] [Google Scholar]
- 47. Schmidt T. A., Plaas A. H., Sandy J. D. (2009) Disulfide-bonded multimers of proteoglycan 4 PRG4 are present in normal synovial fluids. Biochim. Biophys. Acta 1790, 375–384 [DOI] [PubMed] [Google Scholar]
- 48. Kannagi R. (2002) Regulatory roles of carbohydrate ligands for selectins in the homing of lymphocytes. Curr. Opin. Struct. Biol. 12, 599–608 [DOI] [PubMed] [Google Scholar]
- 49. Hemmerich S., Leffler H., Rosen S. D. (1995) Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J. Biol. Chem. 270, 12035–12047 [DOI] [PubMed] [Google Scholar]
- 50. Karlsson N. G., Schulz B. L., Packer N. H. (2004) Structural determination of neutral O-linked oligosaccharide alditols by negative ion LC-electrospray-MSn. J. Am. Soc. Mass Spectrom. 15, 659–672 [DOI] [PubMed] [Google Scholar]
- 51. Karlsson N. G., Karlsson H., Hansson G. C. (1996) Sulfated mucin oligosaccharides from porcine small intestine analyzed by four-sector tandem mass spectrometry. J. Mass Spectrom. 31, 560–572 [DOI] [PubMed] [Google Scholar]
- 52. Liu T., Qian W. J., Gritsenko M. A., Camp D. G., 2nd, Monroe M. E., Moore R. J., Smith R. D. (2005) Human plasma N-glycoproteome analysis by immunoaffinity subtraction, hydrazide chemistry, and mass spectrometry. J. Proteome Res. 4, 2070–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scudder P. R., Shailubhai K., Duffin K. L., Streeter P. R., Jacob G. S. (1994) Enzymatic synthesis of a 6′-sulfated sialyl-Lewis x which is an inhibitor of L-selectin binding to peripheral addressin. Glycobiology 4, 929–932 [DOI] [PubMed] [Google Scholar]
- 54. Poppe L., Brown G. S., Philo J. S., Nikrad P. V., Shah B. (1997) Conformation of sLe(x) tetrasaccharide, free in solution and bound to E-, P-, and L-selectin. J. Am. Chem. Soc. 119, 1727–1736 [Google Scholar]
- 55. Kamphorst J. J., van der Heijden R., DeGroot J., Lafeber F. P., Reijmers T. H., van El B., Tjaden U. R., van der Greef J., Hankemeier T. (2007) Profiling of endogenous peptides in human synovial fluid by NanoLC-MS. Method validation and peptide identification. J Proteome Res. 6, 4388–4396 [DOI] [PubMed] [Google Scholar]
- 56. Catterall J. B., Stabler T. V., Flannery C. R., Kraus V. B. (2010) Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res. Ther. 12, R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Khan A. I., Landis R. C., Malhotra R. (2003) L-Selectin ligands in lymphoid tissues and models of inflammation. Inflammation 27, 265–280 [DOI] [PubMed] [Google Scholar]
- 58. Leppänen A., Parviainen V., Ahola-Iivarinen E., Kalkkinen N., Cummings R. D. (2010) Human L-selectin preferentially binds synthetic glycosulfopeptides modeled after endoglycan and containing tyrosine sulfate residues and sialyl Lewis x in core 2 O-glycans. Glycobiology 20, 1170–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leppänen A., Yago T., Otto V. I., McEver R. P., Cummings R. D. (2003) Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J. Biol. Chem. 278, 26391–26400 [DOI] [PubMed] [Google Scholar]
- 60. Varki A. (1994) Selectin ligands. Proc. Natl. Acad. Sci. U.S.A. 91, 7390–7397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koenig A., Norgard-Sumnicht K., Linhardt R., Varki A. (1998) Differential interactions of heparin and heparan sulfate glycosaminoglycans with the selectins. Implications for the use of unfractionated and low molecular weight heparins as therapeutic agents. J. Clin. Invest. 101, 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pablos J. L., Santiago B., Tsay D., Singer M. S., Palao G., Galindo M., Rosen S. D. (2005) A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-alpha/beta and TNF-α in cultured endothelial cells. BMC Immunol. 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ilgner S., Stiehl P. (2002) Strong LFA-1 and VCAM-1 expression in histological type II of rheumatoid arthritis. Cell. Mol. Biol. 48, OL243–OL249 [PubMed] [Google Scholar]
- 64. Coles J. M., Zhang L., Blum J. J., Warman M. L., Jay G. D., Guilak F., Zauscher S. (2010) Loss of cartilage structure, stiffness, and frictional properties in mice lacking PRG4. Arthritis Rheum. 62, 1666–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vugmeyster Y., Wang Q., Xu X., Harrold J., Daugusta D., Li J., Zollner R., Flannery C. R., Rivera-Bermúdez M. A. (2012) Disposition of human recombinant lubricin in naive rats and in a rat model of post-traumatic arthritis after intra-articular or intravenous administration. AAPS J. 14, 97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Angata T., Varki A. (2000) Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. Evidence for co-evolution with sialic acid synthesis pathways. J. Biol. Chem. 275, 22127–22135 [DOI] [PubMed] [Google Scholar]
- 67. von Gunten S., Yousefi S., Seitz M., Jakob S. M., Schaffner T., Seger R., Takala J., Villiger P. M., Simon H. U. (2005) Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood 106, 1423–1431 [DOI] [PubMed] [Google Scholar]
- 68. Domon B., Costello C. E. (1988) A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 5, 397–409 [Google Scholar]