Background: We genetically engineered a novel IL27p28/IL12p40 cytokine and examined its use in treatment of uveitis.
Results: IL27p28/IL12p40 ameliorated uveitis by inhibiting the differentiation and activation of Th1 and Th17 lymphocytes.
Conclusion: IL27p28/IL12p40 can potentially be used to treat CNS autoimmune diseases like uveitis.
Significance: This proof-of-concept experiment suggests that novel combinations of α/β IL12 subunits may constitute a new class of therapeutic cytokines.
Keywords: Bioengineering, Cytokine, Eye, Immunology, Immunotherapy, Inflammation, Interleukin, Lymphocyte, STAT Transcription Factor, STAT3
Abstract
IL-12 family cytokines are important in host immunity. Whereas some members (IL-12, IL-23) play crucial roles in pathogenesis of organ-specific autoimmune diseases by inducing the differentiation of Th1 and Th17 lymphocytes, others (IL-27 and IL-35) suppress inflammatory responses and limit tissue injury induced by these T cell subsets. In this study, we have genetically engineered a novel IL27p28/IL12p40 heterodimeric cytokine (p28/p40) that antagonizes signaling downstream of the gp130 receptor. We investigated whether p28/p40 can be used to ameliorate uveitis, a CNS inflammatory disease. Experimental autoimmune uveitis (EAU) is the mouse model of human uveitis and is mediated by Th1 and Th17 cells. We show here that p28/p40 suppressed EAU by inhibiting the differentiation and inflammatory responses of Th1 and Th17 cells while promoting expansion of IL-10+- and Foxp3+-expressing regulatory T cells. Lymph node cells from mice treated with p28/p40 blocked adoptive transfer of EAU to naïve syngeneic mice by immunopathogenic T cells and suppressive effects of p28/p40 derived in part from antagonizing STAT1 and STAT3 pathways induced by IL-27 and IL-6. Interestingly, IL27p28 also suppressed EAU, but to a lesser extent than p28/p40. The inhibition of uveitogenic lymphocyte proliferation and suppression of EAU by p28/p40 and IL27p28 establish efficacy of single chain and heterodimeric IL-12 family cytokines in treatment of a CNS autoimmune disease. Creation of the biologically active p28/p40 heterodimeric cytokine represents an important proof-of-concept experiment, suggesting that cytokines comprising unique IL-12 α- and β-subunit pairing may exist in nature and may constitute a new class of therapeutic cytokines.
Introduction
A critical function of the immune system is to establish tolerance against self-antigens while allowing selective immunity to foreign pathogens and preventing harmful pathogenic autoreactivity (1–3). Although the deletion of self-reactive T cells contributes to the prevention of autoimmunity, some self-reactive T cells escape central tolerance and activation of these autoreactive lymphocytes by dendritic cells presenting cognate or cross-reactive antigens is thought to be the cause of organ-specific autoimmune disease (1). Organ-specific autoimmune diseases of the CNS, such as uveitis and multiple sclerosis, are of significant public health importance in developed countries (4). They are characterized by years and even decades of repeated cycles of remission and recurrent inflammation (5). The progressive loss of irreplaceable neurons or photoreceptors caused by prolonged secretion of cytotoxic cytokines (e.g. IFN-γ, TNF-α, IL-17) in the brain or neuroretina by autoreactive T cells eventually leads to neuronal deficit, paralysis, or blindness (6, 7). Recent studies in humans and mouse models of uveitis or multiple sclerosis have identified Th1 and Th17 as important autoreactive memory T cells that initiate or fuel subsequent cycles of the remitting and recurrent inflammation (7–9). Significant effort is now focused on developing biologics that can be used to target critical transcription factors and signaling pathways utilized by autoreactive Th1 and Th17 cells to treat the disease.
The decision as to which pathway to target is informed by our current understanding that IL-12 family cytokines promote the development of naïve T cells into Th1 and Th17 subsets (10). The family comprises IL-12 (IL12p35/IL12p40), IL-23 (IL23p19/IL12p40), IL-27 (IL27p28/Ebi3), and IL-35 (IL12p35/Ebi3) and belongs to the Type 1 family of hematopoietic cytokines (11–13). Each member comprises heterodimeric ligands; an α-subunit with a helical structure similar to type 1 cytokine, IL-6; and a β-subunit structurally related to the soluble IL-6 receptor (IL-6Rα) (11, 12, 14). IL-12 and IL-27 promote the differentiation of naïve T cells into Th1 phenotype. Whereas IL-12 mediates its biological activities through the IL-12 receptor (IL12Rβ1/IL12Rβ2) and STAT4 pathway, IL-27 signals through glycoprotein 130 (gp130)2 and activates STAT1 and STAT3 pathways. In contrast, IL-6 and IL-23 skew naïve T cells toward the Th17 developmental pathway by binding to the IL-6 receptor (gp130/IL-6R) or IL-23 receptor (IL12Rβ1 and IL23R) and activating STAT3 pathways. The IL12p40 component (p40) is shared by IL-12 and IL-23, suggesting that the development of Th1 and Th17 cells can simultaneously be inhibited by antagonizing binding of p40 to IL-12Rβ1. Common usage of gp130 by IL-6 and IL-27 also suggests that therapeutic targeting of the gp130 receptor signaling can be exploited to inhibit Th1 and Th17 cells. Recent studies indicate that IL-27p28 (p28) antagonizes gp130-mediated signaling (15), but it has not been tested whether it can inhibit an autoimmune disease. Other studies showing that IL12p40 homodimer is a potent IL-12 antagonist (16) further suggest that a heterodimeric protein comprising p28 and p40 can be used to simultaneously inhibit signaling downstream of IL12Rβ1 and gp130 and thus suppress the differentiation or expansion of Th1 and Th17 cells.
Experimental autoimmune uveitis (EAU) shares essential immunopathological features with human autoimmune uveitis, and most of what we know about the pathophysiology of uveitis derives from studies of uveitis in the mouse species (17–19). In previous studies we showed that the expression of IL-17 was temporally correlated with the onset of EAU, and treatment with antibody to IL-17 reduced the severity of EAU, providing further evidence for the involvement of Th17 cells in EAU (20, 21). However, other studies have established the role of Th1 cells and that severity of EAU is correlated with increase in a population of T cells that produce IL-17 and IFN-γ. In this study, we have genetically engineered a heterodimeric protein (p28/p40) comprising p28 and p40, established that it is biologically active, and used it to simultaneously inhibit Th1 and Th17 cells and ameliorate uveitis.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 mice (6–8 weeks old) were from Jackson Laboratory (Bar Harbor, ME). Animal care and use were in compliance with the National Institutes of Health guidelines.
Construction and Expression of p28, p40, or p28/p40
The mature mouse p28 or p40 cDNA was generated by PCR and fused in-frame between the N-terminal honeybee melittin secretion signal sequence and the carboxyl terminus-encoded V5-epitope/polyhistidine tags of the insect cell expression vector pMIB/V5-His (Invitrogen). For p28/p40 bicistronic expression vector, p28 was inserted upstream of the FLAG sequence whereas p40 was cloned upstream of the V5 peptide of the bicistronic vector as shown in Fig. 1, A and B (pIRES-hrGFP-1a vector was purchased from Agilent Technologies, Santa Clara, CA). The expression construct was then transfected into High FiveTM insect cells, and stable transfectants were subjected to selection in blasticidin S (80 μg/ml). The recombinant proteins secreted by the insect cells were purified on a nickel-nitrilotriacetic acid column, followed by differential centrifugation on Centricon filtration units and Sepharose chromatography. The recombinant proteins were further characterized on denaturing SDS gels, nondenaturing gels.
FIGURE 1.
Genetic engineering of p28, p40, and p28/p40 recombinant proteins. A and B, schematic of the cDNA constructs: pMIB, 3.6-kb vector containing honeybee melittin (HBM) secretion signal sequence, C-terminal V5-epitope, and polyhistidine tag; single chain p28 or p40; heterodimeric p28/p40 (stoichiometric, bicistronic expression of p28 or p40). C, recombinant proteins characterized on denaturing SDS-PAGE (left panels) or nonreducing polyacrylamide gel (right panel). D, detection of recombinant p28, p40, and p28/p40 proteins by immunoprecipitation/Western blot analysis. Results are representative of three independent experiments.
Immunoprecipitation and Western Blot Analyses
Antibodies used for immunoprecipitation were precoupled using protein G-Sepharose beads. Immunoprecipitates were resolved by SDS-PAGE, and blots were probed with anti-FLAG or anti-V5 (Invitrogen). Preparation of whole cell lysates was as described (22). Western blotting antibodies utilized were: anti-STAT1, pSTAT1, STAT3, pSTAT3, STAT4, pSTAT4 (Cell Signaling Technology, Danvers, MA), p28, IL-12p40, V5, FLAG, and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Preimmune serum was used in parallel as controls, and signals were detected with HRP-conjugated secondary F(ab′)2 (Zymed Laboratories Inc., San Francisco, CA) using ECL system (Amersham Biosciences).
Flow Cytometry
For intracellular cytokine detection, cells were restimulated for 5 h with phorbol 12-myristate 13-acetate (20 ng/ml)/ionomycin (1 μm). Golgi-stop was added in the last hour, and intracellular cytokine staining was performed using a Cytofix/Cytoperm kit as recommended (BD Biosciences). FACS analysis was performed on a FACSCalibur (BD Biosciences) using protein-specific monoclonal antibodies and corresponding isotype control Abs (BD Biosciences) as previously described (23). Quadrant gates were set using isotype controls with <0.2% background.
Lymphocyte Proliferation Assay
AE.7 Th1 cells (1 × 105 cells/well) were stimulated with cytochrome c (5 μm) and irradiated syngeneic spleen cells (10×) for 72 h in medium containing 100 ng/ml pMIB mock control, p28, p40, or p28/p40. Cytokines (IL-6, IL-12, or IL-27) were added to some cultures (10 ng/ml), and after 72 h cultures were pulsed with [3H]thymidine (0.5 μCi/10-μl well) as described (22). Data are mean cpm ± S.E. of responses of five replicate cultures.
Induction of EAU and Histology
EAU was induced in C57BL/6 mice by active immunization with 150 μg of bovine interphotoreceptor retinoid-binding protein (IRBP) and 300 μg of human IRBP peptide (amino acid residues 1–20) in a 0.2-ml emulsion (1:1 v/v with complete Freund's adjuvant containing Mycobacterium tuberculosis strain H37Ra (2.5 mg/ml). Mice also received Bordetella pertussis toxin (0.2 μg/mouse) concurrent with immunization. All experiments comprised an EAU group (immunized with IRBP in complete Freund's adjuvant) and treatment group that was also injected (intraperitoneally) with p28, p40, or p28/p40 at 100 ng/ml. The cytokines were injected at time of immunization with IRBP and also at day 4, day 8, and day 12 after immunization. The control group received complete Freund's adjuvant alone or protein extracts from insect cells transfected with the empty vector, pMIB. For each study at least six mice were used per group, and they were matched by age and sex. Clinical disease was established and scored by fundoscopy as described previously (8).
Adoptive Transfer
EAU was induced in WT C57BL/6 mice as described above, and mice exhibiting clinical features of uveitis at day 21 after immunization were identified by fundoscopic examination. Donor mice were sacrificed, and cells isolated from spleen and lymph nodes (LN) were restimulated for 3 days with IRBP (20 μg/ml) and then transferred intravenously into naïve syngeneic recipient mice (1 × 107 cells/mouse). Ten days after cell transfer, disease was assessed by fundoscopy.
Imaging Mouse Fundus
Fundoscopic examinations were performed on eyes enucleated 21 days after immunization using a modified Karl Storz veterinary otoendoscope coupled with a Nikon D90 digital camera, as described previously (24). Briefly, mice were anesthetized by intraperitoneal injection with ketamine (1.4 mg/mouse) and xylazine (0.12 mg/mouse), and the pupils were dilated by topical administration of 1% tropicamide ophthalmic solution (Alcon, Fort Worth, TX). To avoid a subjective bias, evaluation of the fundus photographs was conducted without knowledge of the mouse identity by a masked observer. At least six images (two posterior central retinal views, four peripheral retinal views) were taken from each eye by positioning the endoscope and viewing from superior, inferior, lateral, and medial fields, and each individual lesion was identified, mapped, and recorded. The clinical grading system for retinal inflammation was as established previously (25).
Statistical Analysis
Statistical analysis was performed by Student's t test (two-tailed). EAU scores were analyzed by nonparametric Mann-Whitney U test (two-tailed). Asterisks denote p value (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.001).
RESULTS
Generation of Mouse Recombinant p28, and p40 and p28/p40
To investigate whether a novel heterodimeric protein (p28/p40) comprising p28 and p40 can suppress the differentiation and/or expansion of Th1 and Th17 cells, we genetically engineered the expression construct shown in Fig. 1A. The bicistronic vector used for expressing the heterodimeric p28/p40 protein contains an internal ribosomal entry site as well as FLAG, V5-epitope, and His tags to facilitate isolation and characterization of the recombinant α- and β-subunits of the heterodimeric protein (Fig. 1, A and B). We also cloned and expressed the p28 as well as the p40 single chain proteins to investigate whether they each possess intrinsic biological activities independent of the p28/p40 protein. The full-length mouse p28 or p40 cDNA was cloned into the 3.6-kb pMIB vector containing honeybee melittin secretion signal sequence, C-terminal V5-epitope, and polyhistidine (His) tag (Fig. 1, A and B). The constructs were transfected into High FiveTM insect cells, and secreted recombinant proteins were purified by a combination of affinity chromatography and differential centrifugation as described under “Experimental Procedures.” The purified proteins were characterized by denaturing SDS gels and nondenaturing PAGE (Fig. 1C), and coimmunoprecipitation/Western blot analysis indicated successful production of the monomeric p28 and p40 proteins, as well as the novel heterodimeric p28/p40 recombinant protein (Fig. 1 C, and D). The formation of a stable p28-p40 heterodimeric complex suggests that the α-subunit chain from a different IL-12 family cytokine can pair with any β-subunit. It is of note that we detected a contaminating insect cell protein of ∼60 kDa. High FiveTM insect cells that were transfected with the control empty pMIB vector also secrete the contaminating protein, and as shown in subsequent data (see below), it has no discernible biological activity.
p28/p40 Suppressed T Cell Proliferation and Differentiation
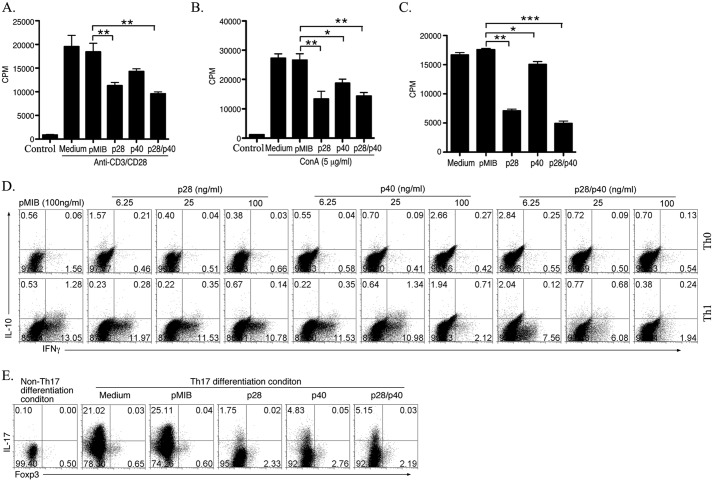
To determine whether the recombinant proteins had biological activity, we examined whether they could induce or suppress lymphocyte proliferation. However, it was important to use extracts from High FiveTM insect cells transfected with the empty pMIB vector as control to rule out the possibility that the contaminating insect protein detected in the recombinant protein preparations (see Fig. 1C, left-most panel) could have contributed to any biological effects. Thus, pMIB extract was used as control in all experiments described in this study. To investigate the effects of p28, p40, or p28/p40 on cell proliferation, we isolated naïve CD4+ T cells from the spleen and LN of C57BL/6 mice (Fig. 2, A and B) or AE.7 mouse T cells (Fig. 2C) and stimulated the cells with anti-CD3/CD28 or ConA in medium containing 100 ng/ml pMIB, p28, p40, or p28/p40. Analysis of the cells by [3H]thymidine incorporation assay revealed significant inhibition of T cell proliferation by all three proteins with p28/p40 exhibiting higher inhibitory activity (Fig. 2, A–C). To investigate the effects of the proteins on T cell effector functions, we cultured mouse primary CD4+ T cells under Th0, Th1, or Th17 polarization condition in medium containing each recombinant protein. We observed that p40 and p28/p40 substantially inhibited the development of IFN-γ-producing Th1 cells in a dose-dependent manner (Fig. 2D). However, the inhibitory effect of p28/p40 was more potent as it inhibited Th1 development even at very low concentrations (6.25 ng/ml) whereas the inhibition by p40 was observed only at high concentrations (100 ng/ml) (Fig. 2D). By contrast, p28 had no effect on the differentiation of Th1 cells (Fig. 2D). On the other hand, p28 potently suppressed development of Th17 cells. Although p40 and p28/p40 also inhibited Th17 differentiation, the inhibitory effect of p28 was most dramatic (Fig. 2E). Taken together, these results suggest that p28, p40, and p28/p40 exert distinct suppressive effects on the generation of Th17 and Th1 cells. These results also indicate that α- or β-single chain IL-12 proteins have intrinsic biological activities and can be used to regulate T cell differentiation and effector functions.
FIGURE 2.
p28, p40, and p28/p40 suppressed lymphocyte proliferation and differentiation of Th1 and Th17 cells. A–C, primary naïve CD4+ T cells (A and B) or AE.7 T cells (C) were stimulated with anti-CD3/CD28 (A and C) or ConA (B) in medium containing pMIB, p28, p40, and p28/p40. On the 3rd day T cell proliferation was assessed by [3H]thymidine incorporation assay. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.D. D and E, some T cell receptor-stimulated mouse primary T cells were propagated under Th0, Th1, or Th17 polarization condition, and cytokine expression was assessed by intracellular cytokine-staining assay. Numbers in quadrants indicate percentages of IL-10-, IL-17-, or IFN-γ-expressing CD4+ T cells detected. Results are representative of three independent experiments.
p28/p40 and p28 Suppressed T Cell Functions by Inhibiting STAT1 and STAT3 Activation
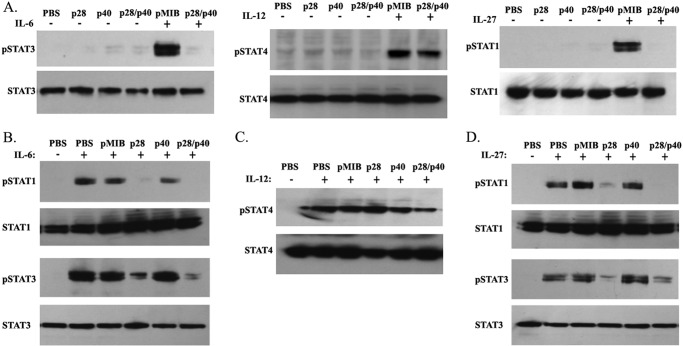
Dendritic cells influence the differentiation, proliferation, and effector functions of CD4+ T cells by secreting cytokines such as IL-6, IL-12, and/or IL-27 in peripheral tissues including draining LN and the blood. Proinflammatory activities of IL-6 and IL-12 induce proliferation of T cells through activation of STAT3 and STAT4 pathways, respectively (11, 26–29), whereas IL-27 activates STAT1/STAT3 pathways and can induce or suppress inflammation depending on developmental stage of the CD4+ T cell (28, 30, 31). We therefore investigated whether p28/p40 suppressed T cell proliferation by inhibiting STAT1, STAT3, and/or STAT4 activation. We stimulated AE.7 T cells with cytochrome c peptide/APC for 48 h in medium containing 100 ng/ml p28, p40, or p28/p40. The stimulated AE.7 T cells were then analyzed for STAT activation. Some of the AE.7 T cells that were cultured in medium containing pMIB or p28/p40 were starved for 2 h in serum-free medium containing 0.5% BSA and stimulated for 30 min with 10 ng/ml IL-6, IL-12, or IL-27. Surprisingly, Western blot analysis of the samples revealed that p28/p40, p40, or p28 could not activate STAT1, STAT3, or STAT4 (Fig. 3A). Interestingly, compared with pMIB control, addition of IL-6 or IL-27 to cells treated with p28/p40 could not activate STAT3 or STAT1, respectively. By contrast, addition of IL-12 to cultures treated with p28/p40 induced robust activation of STAT4, suggesting that the biological activities of p28/p40 derived in part from antagonizing STAT1 and/or STAT3 activation (Fig. 3A). We also stimulated AE.7 T cells as described above. APC were removed from the stimulated AE.7 T cells by use of CD4 magnetic beads. The AE.7 cells were then cultured in serum-free medium containing pMIB, p28, p40, or p28/p40 for 2 h, followed by stimulation with IL-6, IL-12, or IL-27 for 30 min. Western blot analysis revealed that the inhibitory effects of p28 or p28/p40 were mediated, in part, by inhibiting activation of STAT1 and STAT3 with no effect on STAT4 pathway (Fig. 3, B–D). This result is in line with a recent study showing that p28 antagonizes gp130-mediated signaling (15). On the other hand, p40 did not perturb STAT1, STAT3, or STAT4 activation, consistent with reports showing that whereas the p35 subunit is required for signaling, the p40 subunit is mainly required for binding IL-12Rβ1 receptor subunit (16, 32). Taken together, these results suggest that p28/p40 and p28 mediate their effects by interfering with STAT1 and STAT3 signaling during T cell priming or inhibiting differentiated T cell functions.
FIGURE 3.
p28 and p28/p40 suppressed lymphocyte proliferation through inhibition of STAT1 and STAT3 activation. A, AE.7 T cells were stimulated for 72 h with cytochrome c peptide/APC in medium containing pMIB, p28, p40, p28/p40, IL-27, IL-12, IL-6, as indicated. [3H]Thymidine incorporation assay was performed as described under “Experimental Procedures.” B, AE.7 T cells were stimulated with cytochrome c peptide/APC for 48 h, and APCs were removed by Ficoll gradient separation. The purified AE.7 T cells were then starved for 2 h in serum-free medium containing 0.5% BSA and then stimulated for 30 min with pMIB, p28, p40, p28/p40, and/or IL-6, IL-12 or IL-27. C and D, AE.7 T cells were stimulated as described above. The purified AE.7 T cells were then cultured in serum-free medium containing pMIB, p28, p40, or p28/p40 for 2 h, followed by stimulation with IL-6, IL-12, or IL-27 for 30 min. Whole cell lysates were analyzed by Western blotting using indicated Abs. Data represent three independent experiments.
p28/p40 and p28 Suppressed EAU
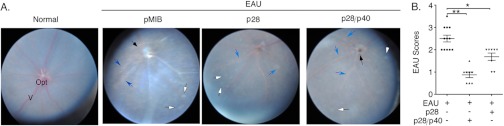
Th17, Th1, and IL-17-/IFN-γ-expressing T cells have been implicated in the pathogenesis of EAU (20, 21, 33). Next, we examined potential in vivo suppressive functions of p28/p40 in EAU. We induced EAU by immunizing C57BL/6 mice with IRBP in complete Freund's adjuvant, and disease progression was monitored by fundoscopy of control eyes or eyes of mice treated with p28, p28/p40, or pMIB as described (see “Experimental Procedures”). For assessment of disease severity and EAU scores, we used a well established semiquantitative grading scheme based on the degree of inflammatory cell infiltration and degeneration at the retina, choroid, and optic nerve disc. We examined the eyes 21 days after immunization by fundoscopy; and consistent with published reports (34, 35), fundus images of control mice or mice that received pMIB displayed severe inflammation with papilledema, retinal vasculitis, and choroidal infiltrates (Fig. 4A). By contrast, p28/p40 significantly suppressed EAU (Fig. 4A) with significantly lower EAU scores relative to control mice (Fig. 4B). Although p28 also reduced the severity of EAU (Fig. 4A), the EAU scores of p28-treated mice were significantly lower (Fig. 4B).
FIGURE 4.
p28 and p28/p40 suppressed the development of EAU. EAU was induced in C57BL/6 mice, and disease progression was analyzed by fundoscopy. Assessment of disease severity (A) and EAU scores (B) were based on changes at the optic nerve disc and retinal vessels or tissues. Black arrowhead, inflammation with blurred optic disc margins and enlarged juxtapapillary area; blue arrows, retinal vasculitis with moderate cuffing; white arrows, yellow-whitish retinal and/or choroidal infiltrates; Opt, optic nerve; V, vitreous.
p28/p40 Suppressed EAU by Inhibiting Th1/Th17 While Inducing Expansion of Tregs
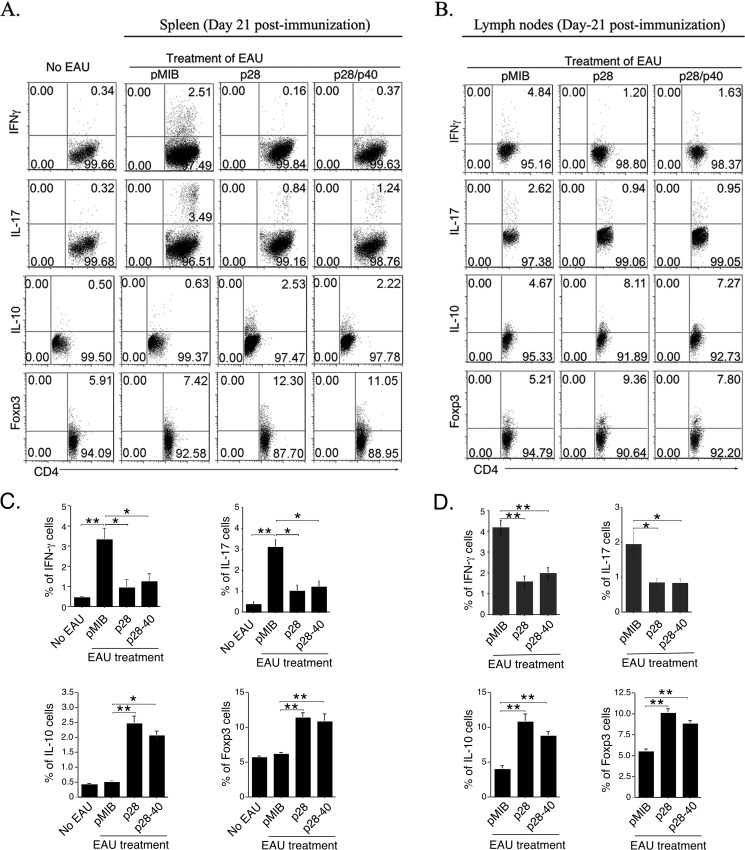
In view of the involvement of Th17, Th1, and IL-17-/IFN-γ-expressing Tregs in the etiology of uveitis (20, 21, 33), we isolated cells from the draining lymph nodes and spleens of mice 21 days after immunization with IRBP and examined whether the reduction in EAU severity induced by p28 and p28/p40 derived, in part, from decrease in the frequency of Th17 and Th1 cells. In line with published reports, EAU in control mice was accompanied by substantial increase of IL-17-expressing (Th17) and IFN-γ-expressing (Th1) cells in spleen (Fig. 5A) and LN (Fig. 5B). However, we observed much lower percentages of Th1 and Th17 in these tissues of mice treated with either p28 or p28/p40 (Fig. 5, A and B), and the decrease in Th1 and Th17 cells was accompanied by a corresponding increase in Foxp3+ and IL-10-expressing Tregs (Fig. 5, A and B).
FIGURE 5.
Suppression of EAU by p28 or p28/p40 correlated with decrease in Th1/Th17 cells and expansion of Tregs. A and B, CD4+ T cells in spleen (A) or lymph nodes (B) of mice (day 21 after immunization) treated with pMIB, p28, or p28/p40 were analyzed by the intracellular cytokine staining. Numbers in quadrants indicate percentage of cells expressing CD4, Foxp3, IL-10, IL-17, and/or IFN-γ. C and D, statistical analysis of IFN-γ-, IL-17-, IL-10-, or Foxp3-expressing T cells in the spleen (C) or LN (D). Results are representative of three independent experiments. *, p < 0.05; **, p < 0.01; error bars, S.D.
p28/p40- or p28-treated Cells Blocked Adoptive Transfer of EAU by Uveitogenic T Cells
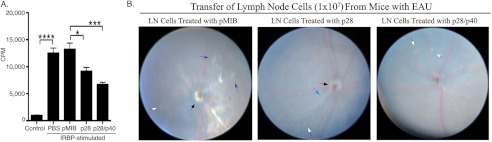
To confirm that the amelioration of EAU mediated by p28 or p28/p40 derived from direct effects on lymphocytes, we first examined whether p28 or p28/p40 could directly inhibit the proliferation of IRBP-specific pathogenic T cells. We isolated cells from draining LN of mice with EAU and restimulated the cells ex vivo for 3 days in medium containing IRBP. Thymidine incorporation assay showed significantly reduced proliferation of the uveitogenic T cells derived from p28- or p28/p40-treated cells; and consistent with data presented above, the inhibitory effect of p28/p40 is significantly greater than that observed with p28, pMIB, or PBS (Fig. 6A). We also examined whether T cells from p28- or p28/p40-treated EAU mice were capable of transferring EAU. Cells isolated from LN of EAU mice treated with pMIB, p28, or p28/p40 and restimulated ex vivo for 3 days with IRBP were transferred (1 × 107 cells/mouse) into age- and sex-matched unimmunized mice, and disease development was assessed by fundoscopy. Fundus images acquired 10 days after adoptive cell transfer revealed features characteristic of full-blown EAU in mice that received pMIB-treated cells. In contrast, transfer of p28- or p28/p40-treated cells induced very mild or no uveitis (Fig. 6B), suggesting that these cells could not efficiently transfer EAU to syngeneic naïve mice. Consistent with the greater growth-inhibitory effects of p28/p40, transfer of p28/p40-treated cells was least efficient in transferring EAU (Fig. 6B).
FIGURE 6.
T cells from mice treated with p28 or p28/p40 were less efficient in transferring EAU to naïve syngeneic mice. A, LN cells from pMIB-, p28-, or p28/p40-treated EAU mice (day 21 after immunization) were restimulated for 3 days in medium containing IRBP, and proliferation was assessed by the [3H]thymidine incorporation assay. B, IRBP-stimulated T cells from pMIB-, p28-, or p28/P40-treated EAU mice were transferred (1 × 107 cells/mouse) into naïve syngeneic C57BL/6 recipient mice. Ten days after adoptive transfer, fundoscopy was performed on the enucleated eyes.
DISCUSSION
In this study, we genetically engineered a novel IL-12 family cytokine comprising the p28 α-chain of the antiinflammatory IL-27 and p40 β-chain of the proinflammatory IL-12 cytokine. We also produced p28 and p40 single chain proteins as part of an overall goal of developing a new class of therapeutic cytokines. We show that a novel p28/p40 recombinant protein forms a stable heterodimeric complex (Fig. 1C) and have demonstrated that p28/p40 and the single chain p28 and p40 proteins are biologically active, as demonstrated by their ability to suppress the proliferation of primary T cell and the well characterized AE.7 Th1 cell line (Fig. 2, A–C). However, p28/p40 and the p28 subunit had the most suppressive activity whereas p40 exhibited the least suppressive activity. We provide data suggesting that mechanisms underlying suppressive effects of p28/p40 and p28 derived in part from inhibiting the activation of STAT1 and STAT3 pathways induced by IL-6 and IL-27, both of which signal through gp130 receptor. This is consistent with a recent report showing that p28 competes with IL-6 for binding to the IL-6 receptor (15). Although IL-27 is thought to suppress chronic inflammation by inhibiting TCR/CD28-mediated IL-2 production (36–38) and inducing IL-10-secreting T cells (31, 39–42), IL-27 also promotes inflammation by inducing naïve T cells to differentiate into the Th1 subset. Thus, in contrast to these mechanisms by which IL-27 suppresses inflammation (36, 43, 44), the data presented here suggest that p28/p40 and p28 might mitigate EAU pathology by antagonizing gp130 signaling of differentiating Th17 cells and to inhibit IL-6-dependent proliferation of mature T-helper cells through preventing apoptosis (26). Of particular significance, our data showing that p40 and p28/p40 inhibited the differentiation and expansion of Th1 cells whereas p28 and p28/p40 suppressed the Th17 subset suggest that the combination of p28 and p28/p40 may have therapeutic value in treating inflammatory disease.
Human uveitis is a diverse group of chronic intraocular inflammatory diseases that can lead to blindness, and they are responsible for 10–15% of visual handicap (45, 46). They include Behcet disease, Vogt-Koyanagi-Harada syndrome, sympathetic ophthalmia, birdshot retinochoroidopathy, and ocular sarcoidosis (45, 46). Current therapeutic strategies for treating autoimmune diseases such as uveitis focus primarily on the use of immunosuppressive agents (e.g. cyclosporine, FK-506, daclizumab, rapamycin, infliximab) that target T-helper cells or Abs that neutralize proinflammatory cytokines; these drugs are fairly effective in ameliorating disease symptoms (45, 46). However, they are of limited value as long term therapy because autoreactive T cells that mediate uveitis are constantly primed by autoantigens that are continuously released from damaged retinal tissue, and renal toxicity or other adverse effects preclude prolonged use (47). Previous studies showing an increase in Th17 cells in the blood of patients with uveitis (20) and a tremendous increase of Th17 and Th1 cells in the blood, lymph nodes, and retina of mice with EAU (20, 21, 33) suggested a role for these T cell subsets in the etiology or recovery from uveitis. However, elucidating the mechanisms involved in uveitis has been a great challenge because of the difficulty of identifying the target antigens and the specific lymphocytes responsive to these autoantigens. Thus, most of what is known about the pathophysiology of uveitis derives from studies of EAU.
Similar to human autoimmune uveitis, Th17 and Th1 responses are elevated in EAU, and either Th17 or Th1 cells have been shown to mediate EAU independently (20, 21, 33). Moreover, elevated levels of T cells expressing both IL-17 and IFN-γ have been shown to correlate with development of EAU (21, 23). Consequently, a rational therapeutic approach has been to develop immune-modulation protocols that would simultaneously target Th1 and Th17 subsets. In this study, we have shown that although p28 can inhibit Th17 differentiation and expansion, it had minimal effect on the Th1 subset. Conversely, p40 inhibited the differentiation or expansion Th1 cells. The heterodimeric p28/p40 cytokine comprising the p28 and p40 subunits thus differs from the p28 subunit protein in that it can inhibit signaling downstream of IL12Rβ1 and also gp130 receptors and is thereby efficient in suppressing differentiation and responses of Th1 and Th17 subsets. More importantly, administering p28/p40 to mice at the time of EAU induction conferred protection from EAU by suppressing the differentiation and expansion of Th1 and Th17 cells, and the amelioration of EAU was accompanied by concomitant increase of IL-10-producing regulatory T cells or Tregs (Fig. 5, A and B). The increase of IL-10- and Foxp3-producing T cells in the spleen and LN of the mice treated with p28 or p28/p40 further suggests that Tregs with their voracious consumption of IL-2 could also have contributed to suppression of EAU by depriving effector T cells of the growth factor, IL-2, and thereby inhibiting their expansion. In addition, we found that sensitization of the autoreactive T cells to p28/p40 rendered the uveitogenic T cells less efficient at transferring uveitis to syngeneic naïve mice, underscoring the efficacy of p28/p40 in treating uveitis. Interestingly, the p28 subunit protein also conferred protection from EAU and inhibited the transfer of EAU by uveitogenic T cells in adoptive transfer experiments. However, in all EAU and adoptive transfer experiments p28/p40 was consistently more superior in inhibiting EAU and proliferation of IRBP-specific pathogenic T cells. Although p28 did not inhibit Th1 cells in ex vivo studies, it inhibited Th1 cells in the LN and spleen of mice with EAU. A plausible explanation for the difference in the in vitro and in vivo results may derive from the fact that p28 is a potent antagonist of gp130 and IL-6 signaling (15).
IL-6 is a pleiotropic cytokine involved in the physiology of virtually every organ system, and in the immune system it has been shown to regulate the balance between Th17 and Treg by inducing STAT3-mediated development of Th17 while inhibiting TGF-β-induced Treg differentiation (29). Thus, the inhibition of IL-6 signaling during inflammation would not only promote generalized T cell suppression by inhibiting the antiapoptotic/prosurvival functions of IL-6 but would tilt the Th17/Treg balance in favor of Treg development (26). It is however of note that neither p28/p40 nor p28 by itself was able to completely block the development of EAU, suggesting that the combination of p28 and p28/p40 can be more effective in suppressing both Th1- and Th17-mediated responses and uveitis.
In summary, our proof-of-principle therapeutic strategy showing that p28 and p28/p40 can suppress EAU establishes that IL-12 α- or β-subunit proteins and IL-12 cytokines comprising novel combinations of α- and β-subunits may form the basis of a new class therapeutic cytokines. Thus, combining p28 and p28/p40 cytokines may offer an attractive therapeutic strategy to simultaneously inhibit Th17, Th1, and IL-17/IFN-γ-expressing T cells in the treatment of potentially blinding uveitis and other Th17/Th1-mediated autoimmune diseases.
Acknowledgments
We thank Drs. Bernadette Marrero, Ivy Dambuza, and Li Zhang (NEI, National Institutes of Health) for critical reading of the manuscript and Sung-Hye Kim (Post-Baccalaureate Fellow, NEI, National Institutes of Health) for technical assistance.
This work was supported, in whole or in part, by the National Institutes of Health through the Intramural Research Programs of the NEI.
- gp130
- glycoprotein 130
- APC
- antigen-presenting cells
- EAU
- experimental autoimmune uveitis
- IRBP
- interphotoreceptor retinoid-binding protein
- LN
- lymph nodes
- Treg
- regulatory T cell.
REFERENCES
- 1. Gery I., Egwuagu C. E. (2002) Central tolerance mechanisms in control of susceptibility to autoimmune uveitic disease. Int. Rev. Immunol. 21, 89–100 [DOI] [PubMed] [Google Scholar]
- 2. Egwuagu C. E., Charukamnoetkanok P., Gery I. (1997) Thymic expression of autoantigens correlates with resistance to autoimmune disease. J. Immunol. 159, 3109–3112 [PubMed] [Google Scholar]
- 3. Egwuagu C. E., Charukamnoetkanok P., Gery I. (2000) Susceptibility to ocular autoimmune disease. Br. J. Ophthalmol. 84, 1083d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caspi R. R. (2010) A look at autoimmunity and inflammation in the eye. J. Clin. Invest. 120, 3073–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nussenblatt R. B. (1990) The natural history of uveitis. Int. Ophthalmol. 14, 303–308 [DOI] [PubMed] [Google Scholar]
- 6. Forrester J. V. (2007) Intermediate and posterior uveitis. Chem. Immunol. Allergy 92, 228–243 [DOI] [PubMed] [Google Scholar]
- 7. Steinman L. (2009) A molecular trio in relapse and remission in multiple sclerosis. Nat. Rev. Immunol. 9, 440–447 [DOI] [PubMed] [Google Scholar]
- 8. Oh H. M., Yu C. R., Lee Y., Chan C. C., Maminishkis A., Egwuagu C. E. (2011) Autoreactive memory CD4+ T lymphocytes that mediate chronic uveitis reside in the bone marrow through STAT3-dependent mechanisms. J. Immunol. 187, 3338–3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steinman L. (2007) A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat. Med. 13, 139–145 [DOI] [PubMed] [Google Scholar]
- 10. Zhu J., Yamane H., Paul W. E. (2010) Differentiation of effector CD4 T cell populations (*). Annu. Rev. Immunol. 28, 445–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trinchieri G. (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 [DOI] [PubMed] [Google Scholar]
- 12. Trinchieri G., Pflanz S., Kastelein R. A. (2003) The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19, 641–644 [DOI] [PubMed] [Google Scholar]
- 13. Collison L. W., Vignali D. A. (2008) Interleukin-35: odd one out or part of the family? Immunol. Rev. 226, 248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devergne O., Birkenbach M., Kieff E. (1997) Epstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietin. Proc. Natl. Acad. Sci. U.S.A. 94, 12041–12046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stumhofer J. S., Tait E. D., Quinn W. J., 3rd, Hosken N., Spudy B., Goenka R., Fielding C. A., O'Hara A. C., Chen Y., Jones M. L., Saris C. J., Rose-John S., Cua D. J., Jones S. A., Elloso M. M., Grötzinger J., Cancro M. P., Levin S. D., Hunter C. A. (2010) A role for IL-27p28 as an antagonist of gp130-mediated signaling. Nat. Immunol. 11, 1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillessen S., Carvajal D., Ling P., Podlaski F. J., Stremlo D. L., Familletti P. C., Gubler U., Presky D. H., Stern A. S., Gately M. K. (1995) Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur. J. Immunol. 25, 200–206 [DOI] [PubMed] [Google Scholar]
- 17. Nussenblatt R. B. (1991) Proctor Lecture. Experimental autoimmune uveitis: mechanisms of disease and clinical therapeutic indications. Invest. Ophthalmol. Vis. Sci. 32, 3131–3141 [PubMed] [Google Scholar]
- 18. Caspi R. R. (2011) Understanding autoimmune uveitis through animal models: the Friedenwald lecture. Invest. Ophthalmol. Vis. Sci. 52, 1872–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caspi R. R., Roberge F. G., Chan C. C., Wiggert B., Chader G. J., Rozenszajn L. A., Lando Z., Nussenblatt R. B. (1988) A new model of autoimmune disease: experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J. Immunol. 140, 1490–1495 [PubMed] [Google Scholar]
- 20. Amadi-Obi A., Yu C. R., Liu X., Mahdi R. M., Clarke G. L., Nussenblatt R. B., Gery I., Lee Y. S., Egwuagu C. E. (2007) TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 13, 711–718 [DOI] [PubMed] [Google Scholar]
- 21. Liu X., Lee Y. S., Yu C. R., Egwuagu C. E. (2008) Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 180, 6070–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oh H. M., Yu C. R., Golestaneh N., Amadi-Obi A., Lee Y. S., Eseonu A., Mahdi R. M., Egwuagu C. E. (2011) STAT3 protein promotes T-cell survival and inhibits interleukin-2 production through up-regulation of class O Forkhead transcription factors. J. Biol. Chem. 286, 30888–30897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu C. R., Oh H. M., Golestaneh N., Amadi-Obi A., Lee Y. S., Eseonu A., Mahdi R. M., Egwuagu C. E. (2011) Persistence of IL-2-expressing Th17 cells in healthy humans and experimental autoimmune uveitis. Eur. J. Immunol. 41, 3495–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paques M., Guyomard J. L., Simonutti M., Roux M. J., Picaud S., Legargasson J. F., Sahel J. A. (2007) Panretinal, high-resolution color photography of the mouse fundus. Invest. Ophthalmol. Vis. Sci. 48, 2769–2774 [DOI] [PubMed] [Google Scholar]
- 25. Haxhinasto S., Mathis D., Benoist C. (2008) The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takeda K., Kaisho T., Yoshida N., Takeda J., Kishimoto T., Akira S. (1998) Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J. Immunol. 161, 4652–4660 [PubMed] [Google Scholar]
- 27. Nishimoto N., Kishimoto T. (2006) Interleukin 6: from bench to bedside. Nat. Clin. Pract. Rheumatol. 2, 619–626 [DOI] [PubMed] [Google Scholar]
- 28. Hunter C. A. (2005) New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5, 521–531 [DOI] [PubMed] [Google Scholar]
- 29. Kimura A., Kishimoto T. (2010) IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 40, 1830–1835 [DOI] [PubMed] [Google Scholar]
- 30. Batten M., Li J., Yi S., Kljavin N. M., Danilenko D. M., Lucas S., Lee J., de Sauvage F. J., Ghilardi N. (2006) Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol. 7, 929–936 [DOI] [PubMed] [Google Scholar]
- 31. Yoshimura T., Takeda A., Hamano S., Miyazaki Y., Kinjyo I., Ishibashi T., Yoshimura A., Yoshida H. (2006) Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J. Immunol. 177, 5377–5385 [DOI] [PubMed] [Google Scholar]
- 32. Ling P., Gately M. K., Gubler U., Stern A. S., Lin P., Hollfelder K., Su C., Pan Y. C., Hakimi J. (1995) Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J. Immunol. 154, 116–127 [PubMed] [Google Scholar]
- 33. Luger D., Silver P. B., Tang J., Cua D., Chen Z., Iwakura Y., Bowman E. P., Sgambellone N. M., Chan C. C., Caspi R. R. (2008) Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 205, 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan C. C., Caspi R. R., Ni M., Leake W. C., Wiggert B., Chader G. J., Nussenblatt R. B. (1990) Pathology of experimental autoimmune uveoretinitis in mice. J. Autoimmun. 3, 247–255 [DOI] [PubMed] [Google Scholar]
- 35. Caspi R. R. (2003) Experimental autoimmune uveoretinitis in the rat and mouse. Curr. Protoc. Immunol. 15, 15.6.1–15.6.2 [DOI] [PubMed] [Google Scholar]
- 36. Owaki T., Asakawa M., Kamiya S., Takeda K., Fukai F., Mizuguchi J., Yoshimoto T. (2006) IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J. Immunol. 176, 2773–2780 [DOI] [PubMed] [Google Scholar]
- 37. Villarino A. V., Stumhofer J. S., Saris C. J., Kastelein R. A., de Sauvage F. J., Hunter C. A. (2006) IL-27 limits IL-2 production during Th1 differentiation. J. Immunol. 176, 237–247 [DOI] [PubMed] [Google Scholar]
- 38. Villarino A. V., Tato C. M., Stumhofer J. S., Yao Z., Cui Y. K., Hennighausen L., O'Shea J. J., Hunter C. A. (2007) Helper T cell IL-2 production is limited by negative feedback and STAT-dependent cytokine signals. J. Exp. Med. 204, 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Awasthi A., Carrier Y., Peron J. P., Bettelli E., Kamanaka M., Flavell R. A., Kuchroo V. K., Oukka M., Weiner H. L. (2007) A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8, 1380–1389 [DOI] [PubMed] [Google Scholar]
- 40. Fitzgerald D. C., Zhang G. X., El-Behi M., Fonseca-Kelly Z., Li H., Yu S., Saris C. J., Gran B., Ciric B., Rostami A. (2007) Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 8, 1372–1379 [DOI] [PubMed] [Google Scholar]
- 41. Stumhofer J. S., Laurence A., Wilson E. H., Huang E., Tato C. M., Johnson L. M., Villarino A. V., Huang Q., Yoshimura A., Sehy D., Saris C. J., O'Shea J. J., Hennighausen L., Ernst M., Hunter C. A. (2006) Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 7, 937–945 [DOI] [PubMed] [Google Scholar]
- 42. Stumhofer J. S., Silver J. S., Laurence A., Porrett P. M., Harris T. H., Turka L. A., Ernst M., Saris C. J., O'Shea J. J., Hunter C. A. (2007) Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8, 1363–1371 [DOI] [PubMed] [Google Scholar]
- 43. Yu C. R., Mahdi R. M., Ebong S., Vistica B. P., Gery I., Egwuagu C. E. (2003) Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J. Biol. Chem. 278, 29752–29759 [DOI] [PubMed] [Google Scholar]
- 44. Matsumoto A., Seki Y., Watanabe R., Hayashi K., Johnston J. A., Harada Y., Abe R., Yoshimura A., Kubo M. (2003) A role of suppressor of cytokine signaling 3 (SOCS3/CIS3/SSI3) in CD28-mediated interleukin 2 production. J. Exp. Med. 197, 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nussenblatt R. B., Fortin E., Schiffman R., Rizzo L., Smith J., Van Veldhuisen P., Sran P., Yaffe A., Goldman C. K., Waldmann T. A., Whitcup S. M. (1999) Treatment of noninfectious intermediate and posterior uveitis with the humanized anti-Tac mAb: a phase I/II clinical trial. Proc. Natl. Acad. Sci. U.S.A. 96, 7462–7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jabs D. A., Nussenblatt R. B., Rosenbaum J. T. (2005) Standardization of uveitis nomenclature for reporting clinical data: results of the First International Workshop. Am. J. Ophthalmol. 140, 509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nussenblatt R. B., Thompson D. J., Li Z., Chan C. C., Peterson J. S., Robinson R. R., Shames R. S., Nagarajan S., Tang M. T., Mailman M., Velez G., Roy C., Levy-Clarke G. A., Suhler E. B., Djalilian A., Sen H. N., Al-Khatib S., Ursea R., Srivastava S., Bamji A., Mellow S., Sran P., Waldmann T. A., Buggage R. R. (2003) Humanized anti-interleukin-2 (IL-2) receptor α therapy: long-term results in uveitis patients and preliminary safety and activity data for establishing parameters for subcutaneous administration. J. Autoimmun. 21, 283–293 [DOI] [PubMed] [Google Scholar]