Background: Serotonin transporter (SERT) phosphorylation and transport activation requires cyclic GMP-dependent protein kinase (PKG).
Results: Using a novel mutant of PKG, we confirmed that PKG stimulated SERT activation and phosphorylation but did not directly phosphorylate SERT.
Conclusion: PKG participates in a signaling pathway that leads to SERT phosphorylation by an as yet unidentified kinase.
Significance: SERT activation and phosphorylation requires multiple protein kinases.
Keywords: Cyclic GMP (cGMP), Neurotransmitter Transport, p38 MAPK, Protein Kinase G (PKG), Serotonin, Analog-sensitive Kinase
Abstract
The serotonin transporter (SERT) is responsible for reuptake of serotonin (5-hydroxytryptamine) after its exocytotic release from neurons. It is the primary target for antidepressants and stimulants, including “ecstasy” (3,4-methylenedioxymethamphetamine). SERT is regulated by several processes, including a cyclic GMP signaling pathway involving nitric oxide synthase, guanylyl cyclase, and cGMP-dependent protein kinase (PKG). Here, we show that SERT was phosphorylated in a PKG Iα-dependent manner in vitro, but that SERT was not a direct substrate of PKG. We generated an analog-sensitive gatekeeper residue mutant of PKG Iα (M438G) that efficiently used the ATP analog N6-benzyl-ATP. This mutant, but not the wild type (WT) kinase, used the ATP analog to phosphorylate both a model peptide substrate as well as an established protein substrate of PKG (vasodilator-stimulated phosphoprotein). PKG Iα M438G effectively substituted for the WT kinase in stimulating SERT-mediated 5-hydroxytryptamine transport in cultured cells. Addition of either WT or mutant PKG Iα M438G to membranes containing SERT in vitro led to radiolabel incorporation from [γ-33P]ATP but not from similarly labeled N6-benzyl-ATP, indicating that SERT was phosphorylated by another kinase that could not utilize the ATP analog. These results are consistent with the proposed SERT phosphorylation site, Thr-276, being highly divergent from the consensus PKG phosphorylation site sequence, which we verified through peptide library screening. Another proposed SERT kinase, the p38 mitogen-activated protein kinase, could not substitute for PKG in this assay, and p38 inhibitors did not block PKG-dependent phosphorylation of SERT. The results suggest that PKG initiates a kinase cascade that leads to phosphorylation of SERT by an as yet unidentified protein kinase.
Introduction
Serotonin transporter (SERT)3 functions to recycle serotonin (5-HT) released during serotonergic neurotransmission by transporting 5-HT back into the presynaptic cell. SERT is the primary target for the most widely used drugs to treat depression and also is a target for psychostimulants such as cocaine and amphetamines. Attention has recently been focused on SERT regulation by protein kinases because non-synonymous SERT coding variants have been identified that are apparently altered in their response to regulation by these kinases. These variants have also been associated with psychiatric disorders, including autism and obsessive-compulsive disorder (1, 2).
In RBL cells, derived from 5-HT accumulating basophils, activation of the A3 adenosine receptor led to increased SERT activity (3). This activation was blocked by inhibitors of nitric oxide synthase (NOS) and cyclic GMP-dependent protein kinase (PKG), suggesting a pathway in which the A3 receptor stimulates Ca2+ entry, which in turn activates NOS, leading to synthesis of cGMP by soluble guanylyl cyclase. Accordingly, stimulation of WT SERT required addition of either an A3 agonist, an NO source or a cell permeant cGMP analog such as 8-Br-cGMP (4, 5). SERT was found to be phosphorylated in this process (4). In synaptosomes, this phosphorylation occurred on SERT threonine residues, and mutation of a single threonine, Thr-276, completely prevented activation and phosphorylation (4, 5). Of the major isoforms of PKG, only the non-myristoylated Iα and Iβ isoforms but not the myristoylated PKG II isoform were necessary for 8-Br-cGMP activation and phosphorylation of SERT (6). Moreover, PKG I was found to be associated in a complex with SERT (6, 7).
SERT was also reported to be stimulated by p38 mitogen-activated protein kinase (p38 MAPK) in response to A3 receptor activation (8). p38 MAPK was proposed to act by increasing SERT catalytic activity, in contrast to PKG, which was suggested to increase SERT surface expression (8). Anisomycin, a protein biosynthesis inhibitor, and 5′-N-ethylcarboxamidoadenosine, an A3 receptor agonist, activated p38 MAPK and stimulated SERT activity in RBL cells, an effect blocked by the p38 MAPK inhibitor SB203580 (9). p38 MAPK expression and anisomycin-dependent transporter stimulation were blocked by siRNA directed against p38 MAPK (9). SERT coding variants, some associated with autism spectrum disorders, were found to have increased basal transport activity (1, 2). Measurements of surface expression and response to transport stimulation by 8-Br-cGMP and anisomycin suggested that these variants differed in their regulation, consistent with transporter activation through multiple signaling pathways (2).
Despite evidence that PKG activates SERT via phosphorylation of Thr-276, this residue is an unlikely phosphorylation site for PKG. The sequence around this proposed site of SERT phosphorylation deviates from the consensus for PKG (10, 11). In addition, cysteine-scanning mutagenesis of the region around Thr-276 indicated that this residue is relatively inaccessible compared with neighboring residues and that the region is α-helical, an unlikely conformation for phosphorylation by kinases, which typically bind substrates in an extended conformation (12). For this reason, we sought to test whether SERT was a direct substrate for PKG. To examine this possibility, we generated an analog-sensitive mutant of PKG that, unlike WT kinases, could use a modified ATP analog as a phosphate donor.
Analog-sensitive kinases were developed as tools to identify targets of specific kinases and to allow for specific inhibition in vivo (13, 14). These mutants have typically been produced by replacing a bulky “gatekeeper” residue, near the binding site for the adenine ring of ATP, with one having a smaller side chain so that the kinase will also accept ATP analogs with larger substituents. Because WT kinases do not utilize these ATP analogs, γ-labeled analogs will transfer their label only to direct substrates of the mutant kinase and not to substrates of other kinases. Although many protein kinases have been modified in this way, there are no reports of an analog-sensitive mutant of PKG, and no three-dimensional structure of a PKG catalytic domain has been solved. Nevertheless, the high degree of homology between PKG and cAMP-dependent protein kinase allowed the identification of Met-438 as the likely gatekeeper residue in PKG. Here, we describe the generation of analog-sensitive PKG Iα and its use in testing direct phosphorylation of SERT by PKG.
EXPERIMENTAL PROCEDURES
Materials
ATP, 5-HT, 8-Br-cGMP, SB203580, monoclonal anti-FLAG M2 affinity agarose, and 3× FLAG peptide were purchased from Sigma. Monoclonal 16C2 anti-phosphovasodilator-stimulated phosphoprotein (VASP) antibody was from Millipore. PKG specific peptide substrate (RKRSRAE) was from GenScript. [3H]5-HT (27.1 Ci/mmol) and [γ-33P]ATP (3000 Ci/mmol) were from PerkinElmer Life Sciences. N6-benzyl-ADP, N6-benzyl-ATP, and purified His-tagged nucleoside diphosphate kinase, prepared as described (15, 16), were generous gifts from Dr. A. J. Koleske (Yale University). Ni-NTA agarose was from Qiagen. Activated p38 MAPK (rat p38α) was prepared by co-expression in Escherichia coli with constitutively active MKK6 and purified as described (17). Kinase activity of the purified p38 MAPK was evaluated by in vitro 33P labeling of myelin basic protein. Briefly, 1 μg of myelin basic protein was incubated with purified p38 MAPK (1–100 ng) in 10 mm HEPES buffer, pH 7.4, containing 0.5 μCi [γ-33P]ATP, 50 μm ATP, 5 mm MgCl2, 0.2 mm EDTA, and 1 mm DTT. After incubation at 30 °C for 10 min, the mixture was then resolved by SDS-PAGE, and radiolabeled myelin basic protein was detected using a Molecular Imager FX instrument (Bio-Rad). N-terminal His10-tagged human PKG Iα and N-terminal His6-tagged human VASP expression constructs were generated by PCR. Briefly, forward and reverse primers containing unique restriction sites were designed to introduce DNA sequences encoding 10 (for PKG Iα) or six (for VASP) His residues immediately after the initiator methionine and used to amplify a fragment of PKG Iα or full-length VASP. The PCR products were then doubly digested with restriction enzymes, excised from agarose gels, and ligated into the same restriction sites of pcDNA3 (pcDNA3-His-PKG Iα) or pcDNA3.1 (pcDNA3.1-His-VASP) constructs, respectively (6). The regions amplified by PCR were confirmed by DNA sequencing. PKG Iα M438A and M438G mutants were generated using the QuikChange site-directed mutagenesis system (Stratagene). The mutated regions were excised and subcloned back into the His10-tagged PKG Iα construct and confirmed by DNA sequencing. The C-terminal FLAG-tagged human SERT construct used here was described previously (6).
Purification of His-tagged PKG and VASP
HEK293T cells in T-75 flasks were transiently transfected using Lipofectamine 2000 (Invitrogen) with plasmids described above for expression of His10-tagged WT, M438A, or M438G PKG Iα. 40–48 h after transfection, the cells were rinsed once with PBS (137 mm NaCl, 2.7 mm KCl, 4.3 mm Na2HPO4, and 1.4 mm KH2PO4, pH 7.4) and scraped into 1 ml of lysis buffer (20 mm Tris, pH 7.5, containing 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 mm DTT, 0.5% protease inhibitor mixture (Sigma), and 100 μm phenylmethylsulfonyl fluoride). The suspension was incubated for 30 min with gentle agitation, and the resulting homogenates were then fractionated by centrifugation at 15,000 × g for 20 min at 4 °C. The supernatant fraction was collected, and His10-tagged PKG Iα was captured by incubating with 250 μl of Ni-NTA-agarose (50% suspension in lysis buffer) with gentle agitation at 4 °C overnight. The agarose was washed three times with wash buffer (50 mm HEPES, pH 7.4, containing 5 mm β-glycerophosphate, 0.1 mm Na3VO4, 10 mm MgCl2, 1 mm DTT, and 10 mm imidazole), then eluted with 500 μl elution buffer (50 mm HEPES, pH 7.4, containing 5 mm β-glycerophosphate, 0.1 mm Na3VO4, 10 mm MgCl2, 10% glycerol, and 250 mm imidazole). Purified PKG Iα was dialyzed using a Slide-A-Lyzer dialysis cassette (Pierce) against dialysis buffer (10 mm HEPES, pH 7.4, containing 100 mm NaCl, 10% glycerol, and 1 mm DTT) overnight at 4 °C to remove imidazole and stored at −80 °C in 0.1-ml aliquots.
Purification of His6-tagged VASP was performed similarly. Briefly, the transfected cells were lysed in 1 ml of lysis buffer B (25 mm Tris-HCl, pH 7.5, containing 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.5% protease inhibitor mixture (Sigma), and 100 μm phenylmethylsulfonyl fluoride), and His6-tagged VASP was then captured from the cytosolic fraction by incubating with 250 μl of Ni-NTA agarose with gentle agitation at 4 °C overnight after fractionation by centrifugation at 15,000 × g for 20 min at 4 °C. The agarose was washed three times with 50 mm HEPES buffer, pH 7.4, containing 100 mm NaCl and 20 mm imidazole, and VASP was eluted with 50 mm HEPES buffer, pH 7.4, containing 100 mm NaCl and 250 mm imidazole. Purified VASP was dialyzed against 50 mm HEPES buffer, pH 7.4, containing 100 mm NaCl and 10% glycerol and stored at −80 °C.
Defining the Substrate Selectivity for PKG Iα
A synthetic peptide library was screened with PKG as described (18). The library consisted of 180 peptides with the general sequence YAXXXXX(S/T)XXXXAGKK-biotin, where X is an equimolar mixture of the 17 amino acid residues excluding cysteine, serine, and threonine, (S/T) indicates equal amounts of serine and threonine, and K-biotin is biotinyl-ϵ-aminohexanoyllysine. For each of the nine X positions, a set of 20 peptides was utilized in which the residue was fixed as one of the 20 amino acids. Two additional peptides were included in which the central phosphoacceptor position was fixed as either serine or threonine, and all X positions were degenerate mixtures. Peptides (50 μm) and kinase (7.5 μg/ml) were incubated in reaction buffer (2 μl/well, 50 mm HEPES, pH 7.4, 10 mm MgCl2, 1 mm DTT, 225 nm 8-Br-cGMP, 50 μm ATP, 0.03 μCi/μl [γ-33P]ATP, 0.1% Tween 20) in 1536-well reaction plates for 2 h at 30 °C. Aliquots of the reaction mixture (200 nl) were then transferred to a streptavidin membrane (Promega SAM2 biotin capture membrane), which was washed, dried, and exposed to a phosphor screen.
Preparation of [33P]N6-benzyl-ATP
[33P]N6-benzyl-ATP was generated from N6-benzyl-ADP as described (15, 16). Briefly, 400 μl of Ni-NTA-agarose (50% suspension in PBS) was packed into an open column (length, 8.5 cm; diameter, 0.5 cm), 200 μg of purified His-tagged nucleoside diphosphate kinase was added in 2 ml and followed by 100 μCi [γ-33P]ATP in 2 ml of PBS. After washing with 2 ml PBS to remove ADP and excess labeled ATP, 2 μm N6-benzyl-ADP in 250 μl of PBS containing 5 mm MgCl2 was added. The column was then eluted with 5 ml PBS containing 5 mm MgCl2. Fractions were collected and counted using a Beckman LS6500 liquid scintillation counter. [33P]N6-benzyl-ATP was eluted maximally in two fractions containing ∼38 μCi of [33P]N6-benzyl-ATP. These two fractions were combined and stored at −20 °C in 0.1 ml aliquots.
Kinase Assays
Kinase activity was measured by determining the amount of 33P radioactivity incorporated from [33P]ATP or [33P]N6-benzyl-ATP into a PKG specific peptide substrate (RKRSRAE). The standard 75-μl assay mixture contained 0.15 μCi of [33P]ATP, 10 μm ATP, 15 μm PKG peptide substrate, 2 μm PKI (a synthetic peptide inhibitor of cAMP-dependent protein kinase), 1 μg of purified kinase, and 100 μm 8-Br-cGMP in 50 mm HEPES buffer, pH 7.4, containing 10 mm MgCl2, 0.1% Tween 20, and 1 mm DTT. After incubation at 30 °C for 2 min, the reaction was immediately put on ice, and 20 μl of the assay mixture was spotted onto P81 phosphocellulose paper and then quenched in 0.42% H3PO4. The paper was further washed three times in 0.42% H3PO4 for 10 min with gentle agitation and rinsed once with acetone. After air drying, radioactivity on the paper was measured with a Beckman LS6500 liquid scintillation counter. For measuring the effect of N6-benzyl-ATP on the activity of PKG Iα utilizing ATP as a co-substrate, unlabeled N6-benzyl-ATP was added to each reaction at the indicated concentrations. Saturation kinetic analyses for Km and Vmax with ATP or N6-benzyl-ATP were performed over a concentration range (0.0015–100 μm) by adding unlabeled ATP or N6-benzyl-ATP to a given amount of [33P]ATP or [33P]N6-benzyl-ATP, respectively.
Expression and Transport Assays
HeLa cells were cultured in DMEM supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin at 37 °C in a humidified 5% CO2 incubator. Cells were plated in 96-well or six-well plates and allowed to grow overnight. The confluent cells were infected with vTF7–3 and transiently transfected, using Lipofectin (Invitrogen), with expression plasmids harboring the open reading frames of hSERT, PKG Iα, or both under the control of a T7 promoter. 20–22 h after transfection, the cells were rinsed once with PBS and incubated 20 min at room temperature with or without 100 μm 8-Br-cGMP. 5-HT influx was initiated by the addition of 20 nm [3H]5-HT in PBS/CM (PBS containing 0.1 mm CaCl2 and 1 mm MgCl2), and the cells were incubated for another 10 min. The assay was terminated by washing with ice-cold PBS buffer. The cells were then solubilized with 0.1% NaOH, and the extent of [3H]5-HT accumulation inside the cells was determined with a Wallac MicroBeta plate counter (PerkinElmer Life Sciences) in 150 μl of Optifluor.
Phosphorylation of VASP in Cultured Cells
To verify the ability of the M438A and M438G PKG Iα mutants to phosphorylate an established PKG substrate in cells, VASP phosphorylation assays were performed as described previously (6). Briefly, HeLa cells co-expressing VASP and WT, M438A, or M438G PKG Iα were incubated with or without 100 μm 8-Br-cGMP for 20 min before lysis in lysis buffer B. Samples of total lysates containing 20 μg of protein were resolved by SDS-PAGE, transferred to a 0.2-μm PVDF membrane (Bio-Rad), and probed with an anti-phospho-VASP antibody. Immunoreactive bands were visualized by chemiluminescence using a UVP Epi-Chemi II imaging and analysis system.
In Vitro Phosphorylation of VASP
The ability of the M438G PKG Iα mutant to phosphorylate VASP in vitro utilizing ATP or N6-benzyl-ATP was also examined. The standard 50-μl assay mixture contained 50 μm ATP or N6-benzyl-ATP, 2 μg of purified VASP, 1 μg of purified WT or M438G PKG Iα, and 100 μm 8-Br-cGMP in 10 mm HEPES buffer, pH 7.4, containing 5 mm MgCl2, 0.2 mm EDTA, and 1 mm DTT. After incubation at 30 °C for 10 min, the reaction was immediately put on ice and stopped with the addition of SDS-PAGE sample buffer (19). Samples were then subjected to immunoblot analysis using an anti-phospho-VASP antibody. Alternately, in vitro VASP phosphorylation assays were conducted using labeled ATP or N6-benzyl-ATP. Briefly, in each reaction, 0.5 μCi of [33P]ATP or [33P]N6-benzyl-ATP was added to the assay mixture described above. After incubation, the assay mixture was resolved by SDS-PAGE, and radiolabeled VASP was detected using a Molecular Imager FX instrument (Bio-Rad).
33P Labeling of SERT
Membranes were prepared from HeLa cells expressing C-terminal FLAG-tagged WT or T276A SERT, as described previously (6). The standard 50-μl labeling mixture contained 0.5 μCi of [33P]ATP or [33P]N6-benzyl-ATP, 50 μm unlabeled ATP or N6-benzyl-ATP, 5 μg of membrane protein, 1 μg purified WT or M438G PKG Iα, and 100 μm 8-Br-cGMP in 10 mm HEPES buffer, pH 7.4, containing 5 mm MgCl2, 0.2 mm EDTA, and 1 mm DTT. The mixture was incubated at 30 °C for 10 min and dissolved in lysis buffer B. The detergent extracts were incubated with 100 μl of anti-FLAG M2 affinity agarose (50% suspension in lysis buffer B) at 4 °C overnight. After washing five times with ice-cold lysis buffer B, SERT was eluted with 50 μl of 150 ng/μl 3× FLAG peptide. The entire eluate was resolved by SDS-PAGE, and radiolabeled SERT was detected using a Molecular Imager FX instrument. As a control, WT SERT was extracted from the membrane preparation using lysis buffer B, captured by anti-FLAG M2 affinity agarose, and subjected to immunoblot analysis using an anti-SERT antibody, as described (6).
RESULTS
Generating an Analog-sensitive cGMP-dependent Protein Kinase Mutant
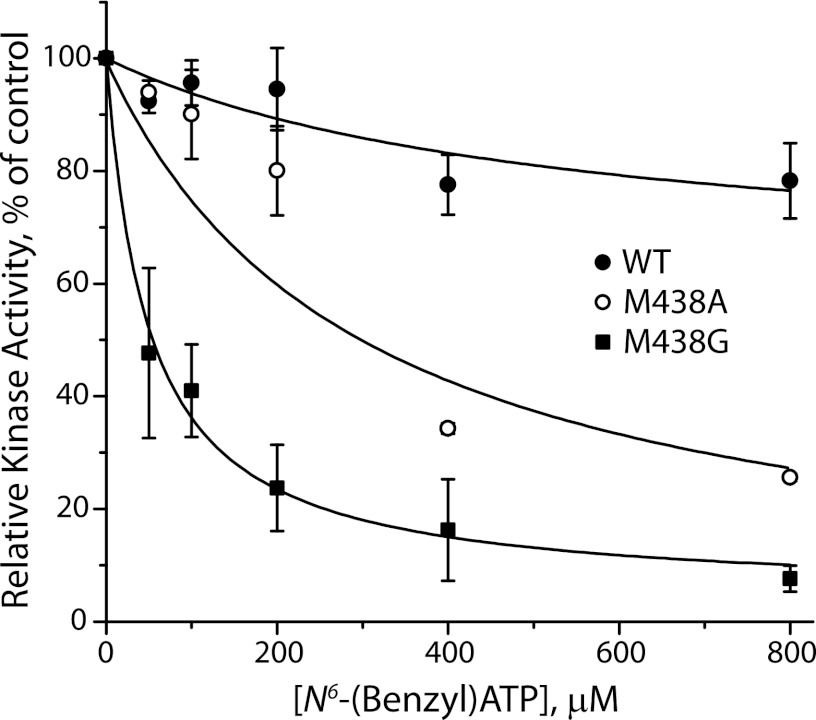
To render PKG Iα sensitive to bulky ATP analogs, we mutated the gatekeeper residue of the nucleotide binding site (Met-438 in PKG Iα) to alanine and glycine. Using unmodified [γ-33P]ATP as a substrate, these mutants were more sensitive to inhibition by unlabeled N6-benzyl-ATP than WT PKG Iα. Fig. 1 shows the effect of N6-benzyl-ATP on phosphorylation of the PKG-specific peptide substrate RKRSRAE by purified PKG Iα with [γ-33P]ATP. N6-Benzyl-ATP inhibited both M438A and M438G mutants, whereas WT kinase was essentially insensitive at analog concentrations up to 800 μm. The bulky ATP analog appeared to have higher affinity to the M438G mutant, relative to M438A, consistent with the absence of the side chain methyl group that might protrude into the ATP binding site.
FIGURE 1.
N6-benzyl-ATP inhibits kinase activity of PKG Iα gatekeeper mutants but not WT. Incorporation of [33P]ATP into the synthetic peptide PKG substrate RKRSRAE was measured, as described under “Experimental Procedures” for WT PKG Iα (closed circles) and PKG Iα gatekeeper mutants M438A (open circles) and M438G (closed squares). Unlabeled N6-benzyl-ATP was added at the indicated concentrations, and its inhibition of kinase activity was determined. The data shown are representative of two independent experiments. Control rates for kinase activity were 678 ± 37 pmol mg−1 min−1 for WT, 429 ± 40 pmol mg−1 min−1 for M438A, and 341 ± 27 pmol mg−1 min−1 for M438G.
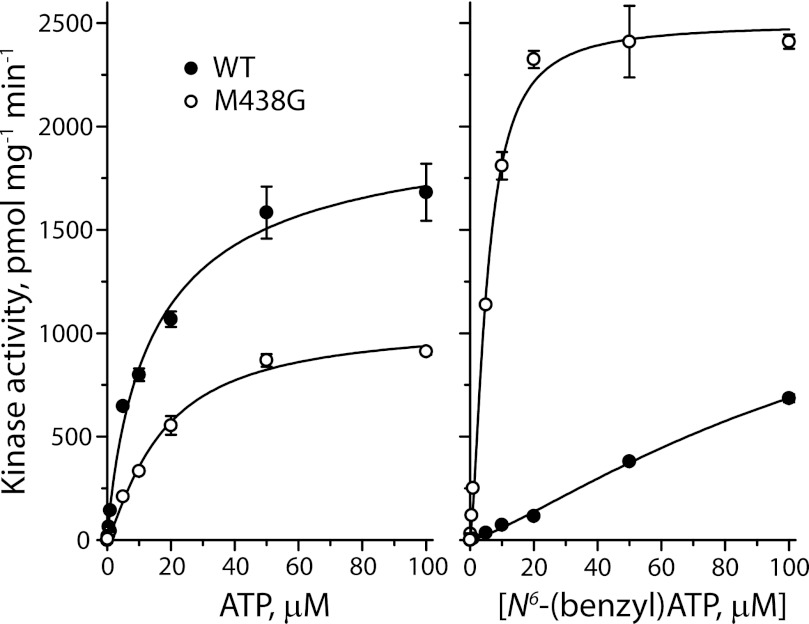
Consistent with its sensitivity to the bulky ATP analog, M438G PKG Iα was able to utilize N6-benzyl[γ-33P]ATP as a substrate in addition to [33P]ATP (Fig. 2). Although WT PKG Iα utilized ATP with a higher Vmax than the M438G mutant, the Km values for the two kinases were virtually identical (Fig. 2, left). With N6-benzyl-ATP as a substrate, however, WT PKG Iα catalyzed phosphorylation much more slowly (Fig. 2, right). The M438G mutant utilized N6-benzyl-ATP as a substrate relatively efficiently, with a Km value one-third of that measured for ATP and a Vmax slightly higher than the WT enzyme with ATP as a substrate (Table 1).
FIGURE 2.
Saturation kinetic analysis for ATP or N6-benzyl-ATP Km and Vmax. The kinase activity of wild type PKG Iα (WT, closed circles) and the M438G mutant (open circles) was measured over a concentration range of 0.0015–100 μm ATP (left) and N6-benzyl-ATP (right) by adding unlabeled ATP or N6-benzyl-ATP to a constant amount of [33P]ATP or [33P]N6-benzyl-ATP, respectively. The results show representative data from a single experiment with duplicate determinations. From these data and two additional experiments with similar results, Km and Vmax values were calculated and are listed in Table 1.
TABLE 1.
Kinetic constants for PKG Iα wild-type and M438G mutant
Experimental data were from Fig. 2 and two replicate experiments. Mean values and their S.E. were calculated from the three experiments. N.D., not determined.
| Km | Vmax | |
|---|---|---|
| μm | pmol mg−1 min−1 | |
| Wild type | ||
| ATP | 16 ± 0.5 | 2124 ± 104 |
| N6-benzyl-ATP | >900 | N.D. |
| M438G | ||
| ATP | 15 ± 1.3 | 1079 ± 60 |
| N6-benzyl-ATP | 5 ± 0.1 | 2582 ± 58 |
Analysis of PKG Substrate Specificity
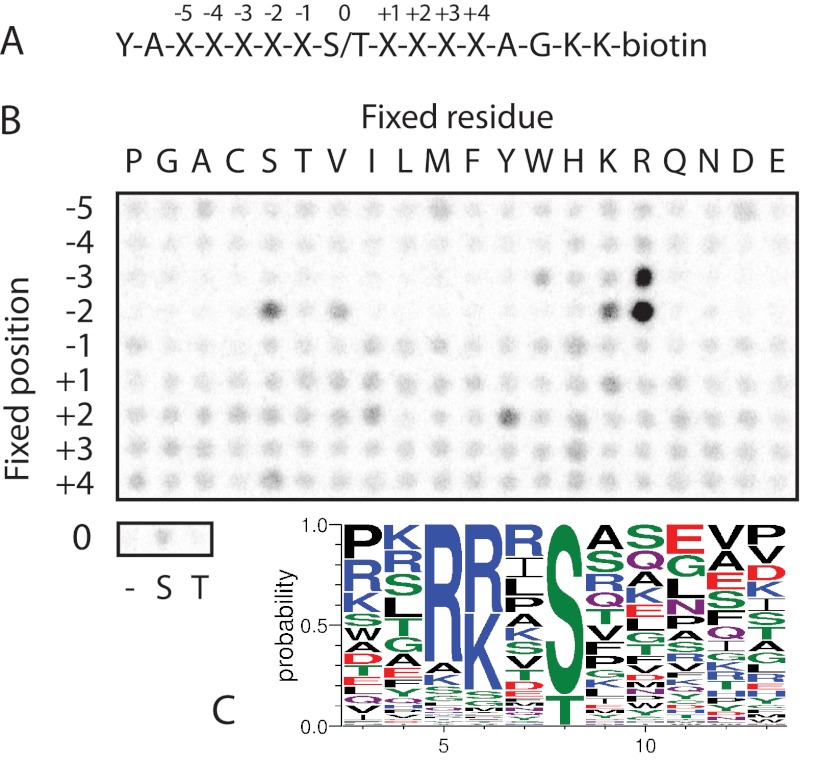
The sequence surrounding the proposed PKG phosphorylation site in SERT at Thr-276 (IWKGVKTSG) (4, 5) deviates substantially from the reported consensus sequence for PKG ((R/K)(R/K)X(S/T)B, where B is a hydrophobic residue) (10, 11). To determine whether PKG might have a preference for “non-canonical” sites such as the one found in SERT, we screened an arrayed combinatorial peptide library to systematically examine the PKG Iα substrate preference across nine positions surrounding the phosphoacceptor site (Fig. 3A). The peptide library results largely confirm the literature motif (Fig. 3B), with the strongest preferences for basic residues at the P-3 and P-2 positions, neither of which is found in the sequence surrounding Thr-276 of SERT. In addition, PKG Iα displayed a strong preference for Ser rather than Thr as the phosphoacceptor residue. Analysis of the surrounding sequence of all reported substrates of human PKG in vitro or in vivo also confirmed the strong preference for basic residues, particularly at the P-2 position, as well as the preference for Ser as the phosphoacceptor (Fig. 3C).
FIGURE 3.
The PKG consensus diverges from the sequence surrounding Thr-276 of SERT. A, sequence of the combinatorial peptide library screened with PKG Iα. B, results from peptide library screening with PKG Iα. Peptides were incubated with recombinant PKG Iα and [33P]ATP in solution in multiwell plates prior to transfer to a streptavidin-coated membrane. Spot intensities indicate the extent of phosphorylation of peptide mixtures having the indicated residue fixed at the indicated position within the context of the sequence shown in A. C, sequence logo showing the distribution of residues surrounding the 92 unique reported mammalian PKG phosphorylation sites extracted from the PhosphoSitePlus database (27), excluding autophosphorylation sites. The logo was constructed using WebLogo (version 3) (33).
Phosphorylation of Protein Substrates
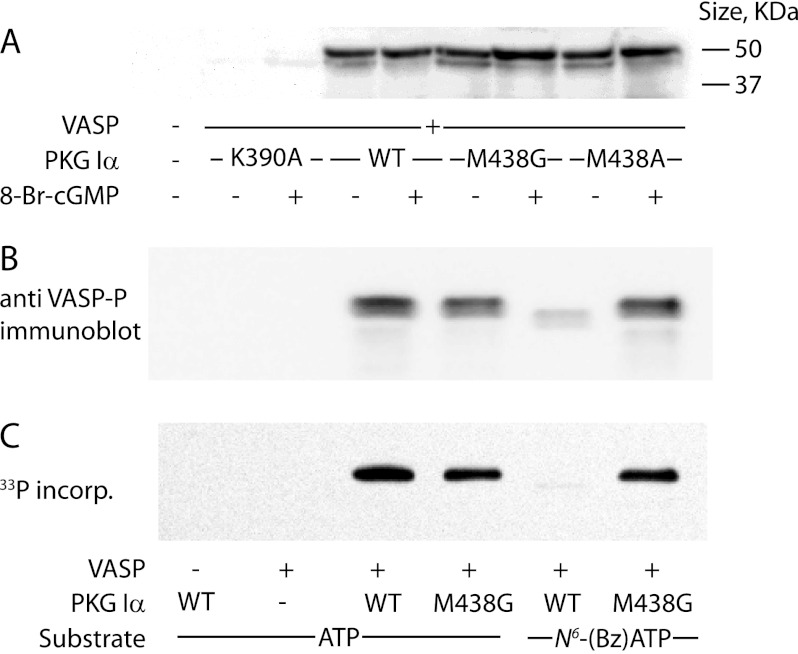
To characterize the ability of PKG Iα M438G to phosphorylate protein substrates, we used VASP, a known PKG substrate (Fig. 4). We initially examined ATP-dependent phosphorylation of VASP by WT and mutant PKG Iα (Fig. 4A). Phosphorylation was measured with an antibody that recognizes VASP phosphorylated at Ser-239. VASP contains three established PKG phosphorylation sites at Ser-157 (RRVSNAG), Ser-239 (RKVSKQE), and Thr-278 (RKATQVG) (20). Phosphorylated VASP runs as a doublet, with a faster migrating monophosphorylated species (46 kDa) and a slower migrating hyperphosphorylated species (50 kDa) (Fig. 4A). As expected, WT PKG Iα robustly phosphorylated VASP, and this activity was increased by the addition of cGMP as indicated by mobility shift. In this assay, the M438G and M438A mutants behaved identically to the WT enzyme (Fig. 4A). As a control we used the K390A mutant in which the catalytic lysine residue is mutated to abolish catalytic activity (21). We observed essentially no VASP phosphorylation using this mutant, indicating that activity observed with co-expression of WT enzyme and other mutants was unlikely to be attributable to endogenously expressed kinases.
FIGURE 4.
VASP phosphorylation by WT PKG Iα and Met-438 mutants. A, in vivo VASP phosphorylation. HeLa cells co-expressing VASP and WT, M438A, or M438G PKG Iα were treated with 8-Br-cGMP where indicated for 20 min. Cell lysates were analyzed by immunoblotting with an anti-phospho-VASP antibody. The blot shown is representative of two independent experiments. B and C, in vitro VASP phosphorylation. Purified VASP was incubated with WT PKG Iα and M438G in the presence of 50 μm ATP or N6-benzyl-ATP, as indicated, for 10 min. B, samples were analyzed by immunoblotting with anti-phospho-VASP antibody. C, using 50 μm [33P]ATP or [33P]N6-benzyl-ATP (0.5 μCi) as indicated, lysates were resolved by SDS-PAGE, and radiolabeled VASP was detected by phosphorimaging. incorp., incorporation.
In contrast to our results using ATP, phosphorylation by the WT enzyme was severely reduced with N6-benzyl-ATP as a substrate, with only low levels of partially phosphorylated VASP detected by Western blot analysis (Fig. 4B) or autoradiography (Fig. 4C). These data are consistent with the general inability of WT kinases to utilize N6-benzyl-ATP as a substrate because of the bulky side chain of the gatekeeper residue (Met-438 in PKG Iα). PKG Iα M438G, however, was as effective in phosphorylating the protein substrate using N6-benzyl-ATP as it was with ATP as shown by immunoblotting with an antibody against phospho-VASP (Fig. 4B) or by 33P autoradiography (Fig. 4C).
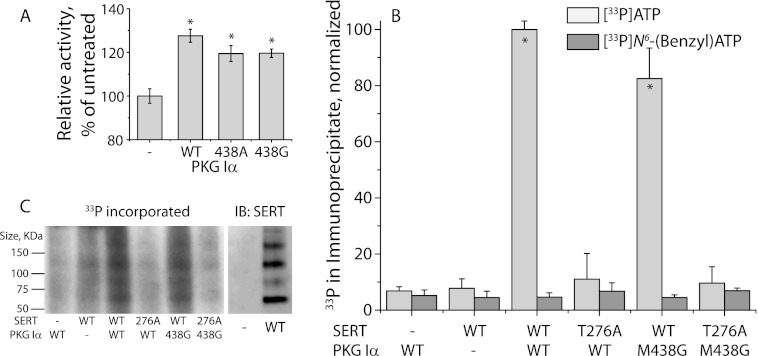
Activation of PKG Iα in cells expressing SERT stimulates 5-HT transport activity (5, 6). To evaluate the ability of PKG gatekeeper mutants to potentiate SERT activity, we utilized HeLa cells that had been grown in continuous culture until endogenous PKG activity was lost (6). In the absence of co-transfected PKG, SERT activity was not stimulated (Fig. 5A), but addition of 8-Br-cGMP led to significant (p < 0.05) stimulation when either wild type PKG Iα or a gatekeeper mutant (M438G or M438A PKG Iα) was cotransfected with SERT. The data in this experiment indicate that mutation of Met-438 to alanine or glycine did not interfere with the ability of PKG to regulate SERT, which presumably depends on cytoplasmic ATP (Fig. 5A).
FIGURE 5.
SERT is activated by M438G PKG Iα without direct phosphate transfer from N6-benzyl-ATP. A, activation of SERT by WT PKG Iα and Met-438 mutants. HeLa cells co-expressing SERT and WT or mutant PKG Iα were treated with or without 8-Br-cGMP, as indicated, for 20 min, and 5-HT influx was measured as described under “Experimental Procedures.” The results represent data (mean ± S.E.) from three independent experiments. Asterisks indicate statistically significant increases in transport activity (p < 0.05). B and C, in vitro SERT labeling. Membranes prepared from HeLa cells expressing FLAG-tagged WT or T276A SERT were incubated with purified WT or M438G PKG Iα in the presence of [33P]ATP (B and C) or [33P]N6-benzyl-ATP (B), as indicated, for 10 min and solubilized. SERT was captured by anti-FLAG agarose and eluted with FLAG peptide. A small portion of the eluate was counted by liquid scintillation spectrometry (B); the remainder was resolved by SDS-PAGE, and radiolabeled SERT was detected by phosphorimaging (C, left panel). In parallel, membranes prepared from non-transfected cells or cells expressing WT SERT were solubilized, SERT was captured and eluted as in the radiolabeled samples, and the eluate was analyzed by immunoblotting (IB) using an anti-SERT antibody (C, right panel). The results shown in B represent data from three independent experiments (means ± S.E.). Asterisks indicate statistically significant increases in phosphorylation (p < 0.05).
In vitro, addition of purified PKG Iα to membrane fragments prepared from HeLa cells expressing SERT led to incorporation of radioactivity from γ-33P-labeled ATP into the SERT immunoprecipitate (Fig. 5B, light gray bars). This incorporation required added kinase, consistent with the lack of endogenous PKG in these cells. The incorporation was reduced to background levels in a SERT mutant (T276A) previously shown to be defective in PKG-dependent phosphorylation and transport stimulation (5, 6). As with WT PKG Iα, addition of M438G PKG Iα was effective in supporting 33P incorporation into SERT, as expected from its ability to stimulate 5-HT transport (Fig. 5A). To verify that 33P was indeed incorporated into SERT, we compared the electrophoretic pattern of SERT immunoreactivity (Fig. 5C, right panel) with the incorporated 33P pattern from autoradiography of SERT immunoprecipitated after incubation with PKG and [γ-33P]ATP (Fig. 5C, left). SERT runs as a series of aggregated multimers after immunoaffinity purification when detected by Western blotting or by autoradiography (Fig. 5C). As with our results using either VASP or a peptide substrate, WT PKG Iα failed to support incorporation of 33P from N6-benzyl-[γ-33P]ATP into SERT (Fig. 5B). However, although M438G PKG Iα can utilize N6-benzyl-[γ-33P]ATP as a phosphate donor for peptide and VASP phosphorylation, this gatekeeper mutant failed to incorporate 33P into SERT from the ATP analog (Fig. 5B, dark gray bars). This result indicates that PKG Iα is unlikely to directly phopshorylate SERT, at least in this in vitro system.
p38 MAPK is not Required for PKG-dependent SERT Phosphorylation
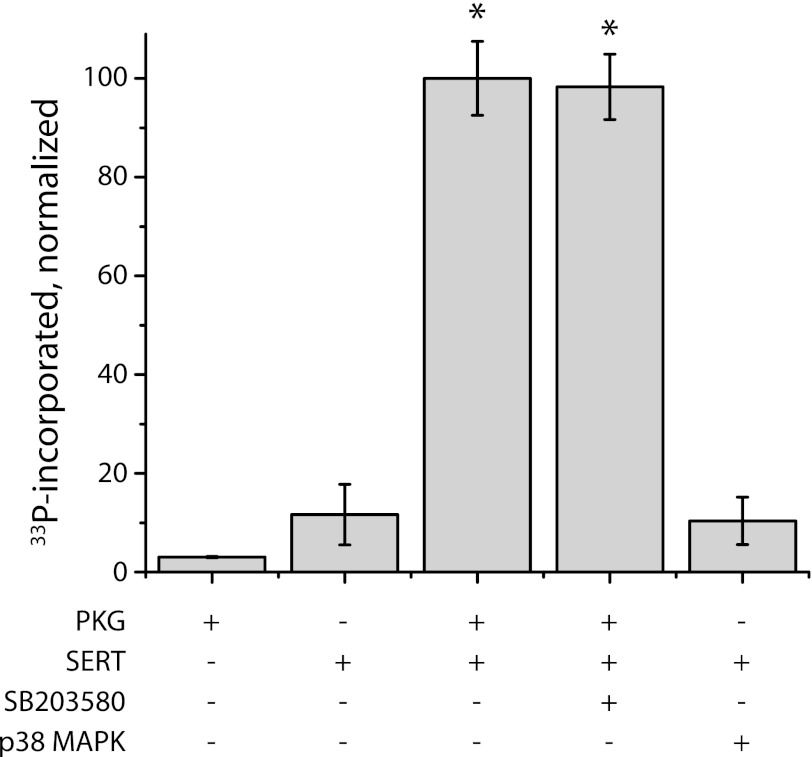
Several reports suggest that p38 MAPK is involved in the activation of SERT-mediated 5-HT transport and may lead to SERT phosphorylation (8, 9, 22). Accordingly, we tested the effect of the p38 MAPK inhibitor SB203580 on PKG-dependent SERT phosphorylation in this in vitro system. To ensure we could adequately inhibit p38 activity with SB203580, we first tested the effectiveness of the compound in blocking phosphorylation of myelin basic protein by purified, activated p38 MAPK. Phosphorylation of myelin basic protein by p38 MAPK was inhibited 95% at 1 μm SB203580 and >99% at 10 μm (data not shown). Fig. 6 demonstrates that addition of 10 μm SB203580 had no significant effect on the PKG-dependent phosphorylation of SERT. Moreover, addition of activated p38 MAPK to membranes from SERT-expressing HeLa cells could not substitute for PKG in stimulating SERT phosphorylation.
FIGURE 6.
p38 MAPK is not required for PKG-dependent SERT phosphorylation. Membranes from SERT-expressing HeLa cells were treated, as indicated, with purified PKG Iα (2.4 pmol) or p38 MAPK (1.6 pmol), and phosphate incorporation from γ[33P]ATP was analyzed as in Fig. 4B. Where indicated, the specific p38 MAPK inhibitor SB203580 was added at 10 μm. The results represent data from three independent experiments (means ± S.E.). Asterisks indicate statistically significant increases in phosphorylation (p < 0.05).
DISCUSSION
Many protein kinases have been mutated to extend their substrate range to ATP analogs with bulky substituents (23). The modification of a gatekeeper residue in the active site that accommodates a substituted adenine ring allows the mutant kinase to be specifically targeted by inhibitors that have negligible affinity for WT kinases (13). These mutants have also allowed identification of protein targets for kinases by using ATP analogs that are not substrates for WT kinases (24–26). The results in Figs. 1 and 2 demonstrate that mutation of cGMP-dependent protein kinase Iα rendered it sensitive to inhibition by N6-benzyl-ATP and capable of using this ATP analog as a phosphate donor. To our knowledge, this is the first example of an analog-sensitive mutant of PKG. Of the two PKG Iα mutants generated, M438G was more sensitive to inhibition by N6-benzyl-ATP than M438A, presumably because the absence of a side chain at that position allows the N6-benzyl substituent to fit better into the ATP site. Although the M438G mutant did not utilize ATP as well as WT PKG Iα, it was much more active with N6-benzyl-ATP as a substrate. This preference for the bulky ATP analog resulted in a 2-fold increase in Vmax and a 3-fold decrease in Km relative to ATP (Table 1). Although the decreased Km might reflect a higher affinity for N6-benzyl-ATP relative to ATP, we cannot exclude an increased rate of phosphate transfer that allowed saturation of the rate-determining nucleotide diphosphate dissociation step at a lower substrate concentration when the bulky analog was used.
Using an in vitro system in which phosphorylation of SERT was absolutely dependent on addition of purified PKG Iα, the M438G mutant was unable to phosphorylate SERT directly using N6-benzyl[γ-33P]ATP as a substrate (Fig. 5B). This result suggests that the direct target of PKG in this system was another protein, possibly the kinase that directly phosphorylated SERT. The PKG Iα M438G mutant was clearly capable of stimulating phosphorylation and activation of SERT in both intact cells (Fig. 5A) and in vitro (Fig. 5C) when ATP was the phosphate donor. Both catalytic parameters (kcat and Km) of PKG Iα M438G using N6-benzyl-ATP as a substrate are more favorable than WT PKG Iα using unmodified ATP as a substrate, indicating that its inability to directly phosphorylate SERT in vitro was not due to impaired kinase function. Because the yet unidentified kinase that was activated by PKG Iα could not utilize N6-benzyl[γ-33P]ATP, no SERT phosphorylation occurred unless ATP was present. As a control for possible 33P incorporation into other proteins in the immunoprecipitate, we mutated the proposed SERT phosphate acceptor residue, Thr-276 to alanine. This mutation blocked ATP-dependent SERT phosphorylation by both WT PKG Iα and the M438G mutant (Fig. 5C). Finally, PKG Iα M438G used N6-benzyl-[γ-33P]ATP to phosphorylate a known PKG substrate, VASP, as efficiently as WT PKG Iα with [γ-33P]ATP (Fig. 4C).
Stimulation of SERT activity in vivo has been proposed to occur by two mechanisms, with one requiring PKG Iα that leads to increased cell surface expression and the other requiring p38 MAPK that increases catalytic activity (8, 9). The results in Fig. 6 apparently rule out the possibility that PKG-dependent SERT phosphorylation requires participation of p38 MAPK. The inability of the p38 MAPK inhibitor SB203580 to affect PKG-dependent SERT phosphorylation or of p38 MAPK to replace PKG (Fig. 6) agree with our previous observation that p38 MAPK inhibition did not affect SERT stimulation by 8-Br-cGMP in intact cells (5). In light of these results, it is unlikely that p38 MAPK is involved in PKG-dependent SERT phosphorylation, but we cannot exclude an independent pathway of SERT regulation through activation of p38 MAPK.
The inability of PKG to directly phosphorylate SERT is consistent with the deviation of the proposed SERT phosphorylation sequence from the PKG consensus sequence. The SERT phosphoacceptor residue was identified as a threonine from the observation that SERT phosphothreonine increased in response to PKG activation (4). Moreover, mutation of Thr-276, but no other threonine residue on the cytoplasmic face of SERT, prevented SERT stimulation and phosphorylation in response to 8-Br-cGMP (Fig. 5B) (4, 5). Analysis of all mapped sites of PKG phosphorylation suggests and our peptide library screens confirm that arginine and lysine are strongly favored in the P-2 and P-3 positions. As basic residues are found at neither position in the sequence surrounding Thr-276 in SERT, it would seem unlikely for this site to be directly phosphorylated by PKG. We note that there are numerous reports of kinases phosphorylating sites within true substrates that diverge from their consensus sequences, and there are other reported PKG substrates that lack basic residues at the most critical positions (27). In these cases, kinase substrate-docking interactions or perhaps scaffold proteins presumably compensate for reduced phosphorylation efficiency due to a poor phosphorylation site sequence.
Although the results presented here argue strongly that PKG does not directly phosphorylate SERT and that p38 MAPK is not involved in PKG-dependent SERT phosphorylation in vitro, it is possible that factors present in vivo mediate direct SERT phosphorylation by PKG or couple PKG activation of p38 MAPK to SERT phosphorylation. If this is true, those factors would presumably be removed by preparation of the membranes used in our in vitro system or not present in the HeLa cells from which those membranes were isolated. However, SERT remains associated with several components of the PKG signaling pathway even in detergent extracts. Studies have shown association of A3 adenosine receptor, NOS1, PKG and PP2A with SERT, suggesting formation of a stable regulatory complex that is not disrupted simply by membrane isolation (5–7, 28–30).
The results presented here raise two immediate questions: what protein target of PKG initiates SERT phosphorylation and what kinase phosphorylates SERT? The two activities may even be properties of the same protein, but a more complex pathway is also possible. It is difficult to speculate as to the identity of the kinase that directly phosphorylates SERT. Other than PKG, only CaMKII has been shown to be present in complex with SERT (31), although extensive proteomic analyses of SERT-associated proteins have not been published, and it is not possible to rule out kinases by their lack of association with the membrane preparation used here as a source for SERT because a kinase with low affinity to membranes could still be associated with the preparation by protein-protein interactions. Furthermore, the sequence surrounding Thr-276 of SERT does not conform well to known consensus motifs for other protein kinases (32). Experiments to identify the proteins linking PKG with SERT are now underway.
Acknowledgments
We thank Kevan Shokat for helpful suggestions and Anthony Koleske and Scott Boyle for their generous gift of reagents.
This work was supported by National Institutes of Health Grants R01 DA008213 (to G. R.) and R01 GM079498 (to B. E. T.) from the USPHS.
- SERT
- serotonin transporter
- PKG
- cGMP-dependent protein kinase
- 5-HT
- 5-hydroxytryptamine (serotonin)
- VASP
- vasodilator-stimulated phosphoprotein.
REFERENCES
- 1. Kilic F., Murphy D. L., Rudnick G. (2003) A human serotonin transporter mutation causes constitutive activation of transport activity. Mol. Pharmacol. 64, 440–446 [DOI] [PubMed] [Google Scholar]
- 2. Prasad H. C., Zhu C. B., McCauley J. L., Samuvel D. J., Ramamoorthy S., Shelton R. C., Hewlett W. A., Sutcliffe J. S., Blakely R. D. (2005) Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 102, 11545–11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller K. J., Hoffman B. J. (1994) Adenosine A3 receptors regulate serotonin transport via nitric oxide and cGMP. J. Biol. Chem. 269, 27351–27356 [PubMed] [Google Scholar]
- 4. Ramamoorthy S., Samuvel D. J., Buck E. R., Rudnick G., Jayanthi L. D. (2007) Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J. Biol. Chem. 282, 11639–11647 [DOI] [PubMed] [Google Scholar]
- 5. Zhang Y. W., Gesmonde J., Ramamoorthy S., Rudnick G. (2007) Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive compulsive disorder. J. Neurosci. 27, 10878–10886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y. W., Rudnick G. (2011) Myristoylation of cGMP-dependent protein kinase dictates isoform specificity for serotonin transporter regulation. J. Biol. Chem. 286, 2461–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steiner J. A., Carneiro A. M., Wright J., Matthies H. J., Prasad H. C., Nicki C. K., Dostmann W. R., Buchanan C. C., Corbin J. D., Francis S. H., Blakely R. D. (2009) cGMP-dependent protein kinase Iα associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake. Mol. Brain 2, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu C. B., Hewlett W. A., Feoktistov I., Biaggioni I., Blakely R. D. (2004) Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol. Pharmacol. 65, 1462–1474 [DOI] [PubMed] [Google Scholar]
- 9. Zhu C. B., Carneiro A. M., Dostmann W. R., Hewlett W. A., Blakely R. D. (2005) p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 280, 15649–15658 [DOI] [PubMed] [Google Scholar]
- 10. Mitchell R. D., Glass D. B., Wong C. W., Angelos K. L., Walsh D. A. (1995) Heat-stable inhibitor protein derived peptide substrate analogs: phosphorylation by cAMP-dependent and cGMP-dependent protein kinases. Biochemistry 34, 528–534 [DOI] [PubMed] [Google Scholar]
- 11. Tegge W., Frank R., Hofmann F., Dostmann W. R. (1995) Determination of cyclic nucleotide-dependent protein kinase substrate specificity by the use of peptide libraries on cellulose paper. Biochemistry 34, 10569–10577 [DOI] [PubMed] [Google Scholar]
- 12. Goldsmith E. J., Akella R., Min X., Zhou T., Humphreys J. M. (2007) Substrate and docking interactions in serine/threonine protein kinases. Chem Rev 107, 5065–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bishop A. C., Ubersax J. A., Petsch D. T., Matheos D. P., Gray N. S., Blethrow J., Shimizu E., Tsien J. Z., Schultz P. G., Rose M. D., Wood J. L., Morgan D. O., Shokat K. M. (2000) A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature 407, 395–401 [DOI] [PubMed] [Google Scholar]
- 14. Shah K., Liu Y., Deirmengian C., Shokat K. M. (1997) Engineering unnatural nucleotide specificity for Rous sarcoma virus tyrosine kinase to uniquely label its direct substrates. Proc. Natl. Acad. Sci. U.S.A. 94, 3565–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyle S. N., Koleske A. J. (2007) Use of a chemical genetic technique to identify myosin IIb as a substrate of the Abl-related gene (Arg) tyrosine kinase. Biochemistry 46, 11614–11620 [DOI] [PubMed] [Google Scholar]
- 16. Blethrow J., Zhang C., Shokat K. M., Weiss E. L. (2004) Curr. Protoc. Mol. Biol. Chapter 18, Unit 18.11 [DOI] [PubMed] [Google Scholar]
- 17. Sheridan D. L., Kong Y., Parker S. A., Dalby K. N., Turk B. E. (2008) Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. J. Biol. Chem. 283, 19511–19520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mok J., Kim P. M., Lam H. Y., Piccirillo S., Zhou X., Jeschke G. R., Sheridan D. L., Parker S. A., Desai V., Jwa M., Cameroni E., Niu H., Good M., Remenyi A., Ma J. L., Sheu Y. J., Sassi H. E., Sopko R., Chan C. S., De Virgilio C., Hollingsworth N. M., Lim W. A., Stern D. F., Stillman B., Andrews B. J., Gerstein M. B., Snyder M., Turk B. E. (2010) Deciphering protein kinase specificity through large-scale analysis of yeast phosphorylation site motifs. Sci. Signal. 3, ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 20. Smolenski A., Bachmann C., Reinhard K., Hönig-Liedl P., Jarchau T., Hoschuetzky H., Walter U. (1998) Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J. Biol. Chem. 273, 20029–20035 [DOI] [PubMed] [Google Scholar]
- 21. Lohmann S. M., Walter U. (2005) Tracking functions of cGMP-dependent protein kinases (cGK). Front Biosci. 10, 1313–1328 [DOI] [PubMed] [Google Scholar]
- 22. Samuvel D. J., Jayanthi L. D., Bhat N. R., Ramamoorthy S. (2005) A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J. Neurosci. 25, 29–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knight Z. A., Shokat K. M. (2005) Features of selective kinase inhibitors. Chem. Biol. 12, 621–637 [DOI] [PubMed] [Google Scholar]
- 24. Ubersax J. A., Woodbury E. L., Quang P. N., Paraz M., Blethrow J. D., Shah K., Shokat K. M., Morgan D. O. (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425, 859–864 [DOI] [PubMed] [Google Scholar]
- 25. Carlson S. M., Chouinard C. R., Labadorf A., Lam C. J., Schmelzle K., Fraenkel E., White F. M. (2011) Large-scale discovery of ERK2 substrates identifies ERK-mediated transcriptional regulation by ETV3. Sci. Signal. 4, rs11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banko M. R., Allen J. J., Schaffer B. E., Wilker E. W., Tsou P., White J. L., Villén J., Wang B., Kim S. R., Sakamoto K., Gygi S. P., Cantley L. C., Yaffe M. B., Shokat K. M., Brunet A. (2011) Chemical genetic screen for AMPKα2 substrates uncovers a network of proteins involved in mitosis. Mol. Cell 44, 878–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hornbeck P. V., Chabra I., Kornhauser J. M., Skrzypek E., Zhang B. (2004) PhosphoSite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics 4, 1551–1561 [DOI] [PubMed] [Google Scholar]
- 28. Zhu C. B., Lindler K. M., Campbell N. G., Sutcliffe J. S., Hewlett W. A., Blakely R. D. (2011) Colocalization and regulated physical association of presynaptic serotonin transporters with A adenosine receptors. Mol. Pharmacol. 80, 458–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chanrion B., Mannoury la Cour C., Bertaso F., Lerner-Natoli M., Freissmuth M., Millan M. J., Bockaert J., Marin P. (2007) Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc. Natl. Acad. Sci. U.S.A. 104, 8119–8124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bauman A. L., Apparsundaram S., Ramamoorthy S., Wadzinski B. E., Vaughan R. A., Blakely R. D. (2000) Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J. Neurosci. 20, 7571–7578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ciccone M. A., Timmons M., Phillips A., Quick M. W. (2008) Calcium/calmodulin-dependent kinase II regulates the interaction between the serotonin transporter and syntaxin 1A. Neuropharmacology 55, 763–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller M. L., Jensen L. J., Diella F., Jørgensen C., Tinti M., Li L., Hsiung M., Parker S. A., Bordeaux J., Sicheritz-Ponten T., Olhovsky M., Pasculescu A., Alexander J., Knapp S., Blom N., Bork P., Li S., Cesareni G., Pawson T., Turk B. E., Yaffe M. B., Brunak S., Linding R. (2008) Linear motif atlas for phosphorylation-dependent signaling. Sci. Signal. 1, ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crooks G. E., Hon G., Chandonia J. M., Brenner S. E. (2004) WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]