Abstract
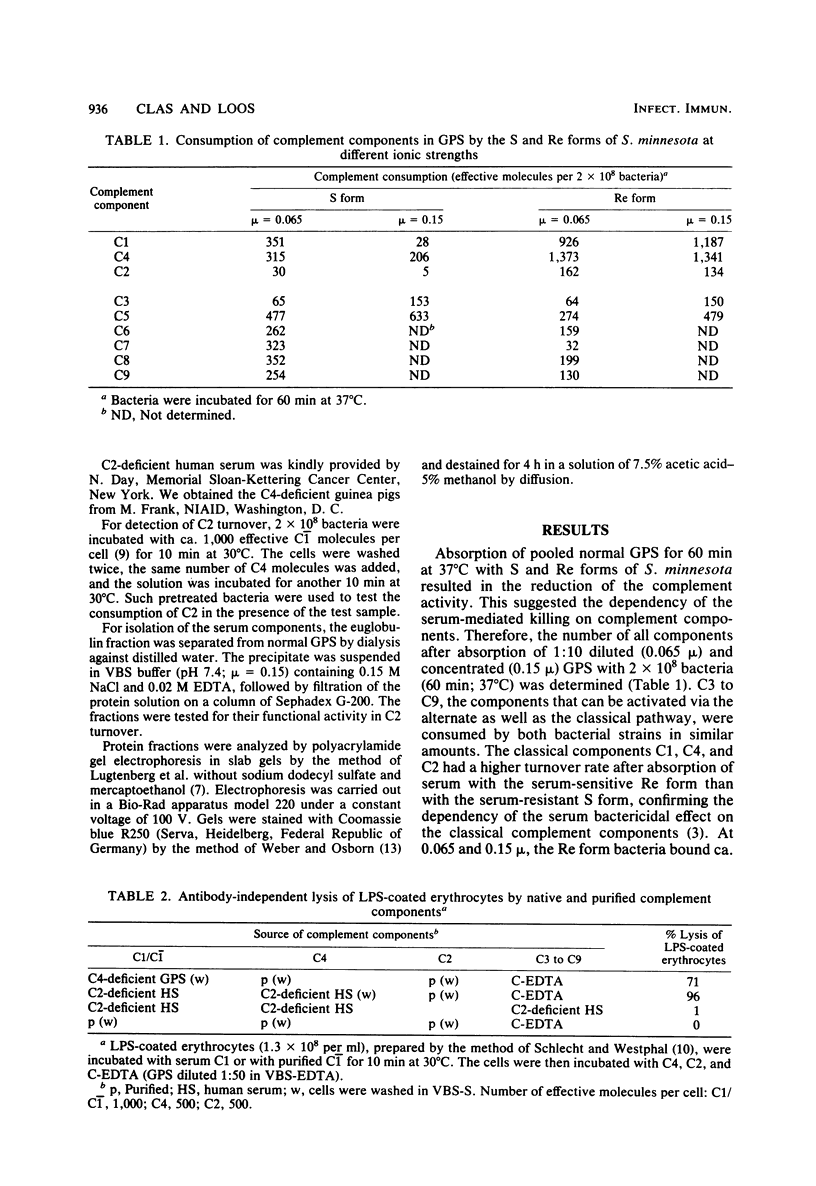
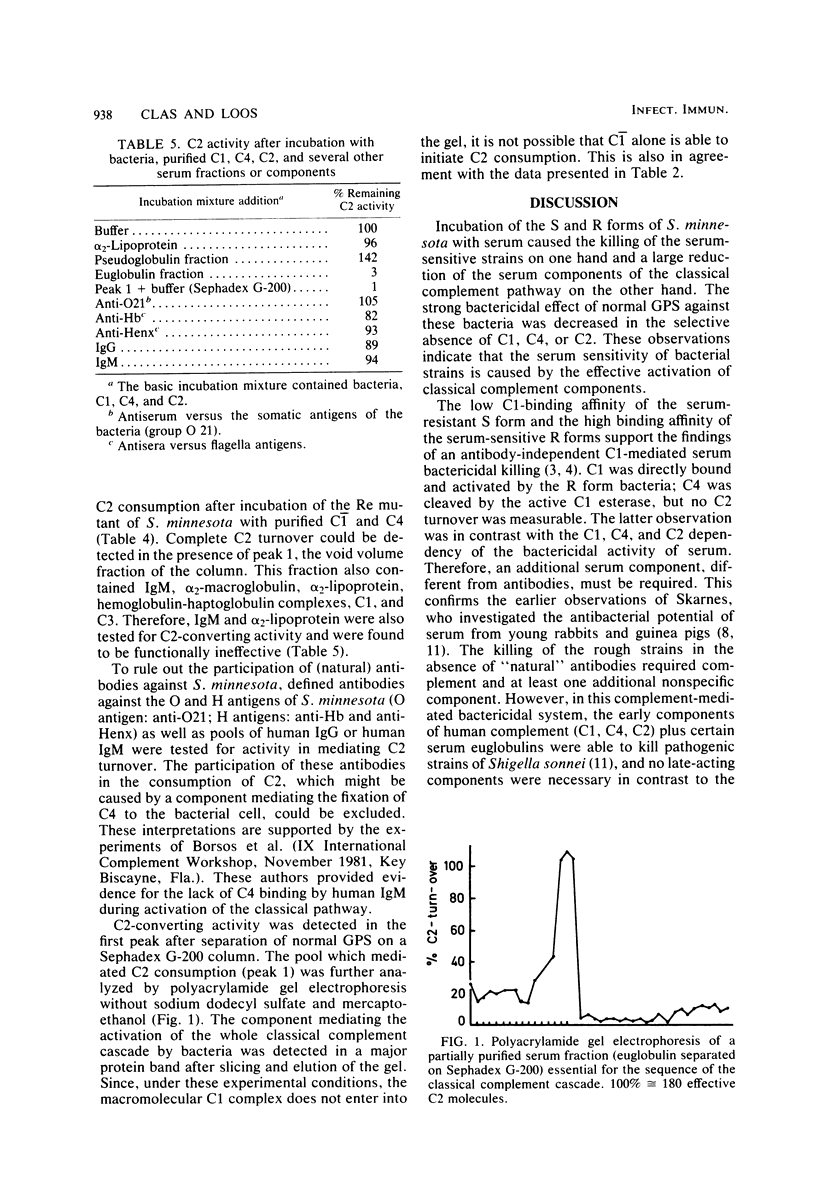
Killing of Salmonella minnesota and Salmonella typhimurium S and R strains in serum of nonimmune humans and guinea pigs was drastically reduced in the selective absence of C1q, C1r, Ca2+, C4, or C2, the components of the classical complement pathway. Binding of C1 and C1q to the S form and six different core-deficient R mutant strains became stronger the shorter the lipopolysaccharide molecule. C1 and C1q had, under physiological conditions, no affinity to the serum-resistant S forms, whereas these components were bound by the serum-sensitive R forms with high affinity. However, a mixture of the individual complement components C1-C9, which rapidly lysed sensitized erythrocytes, did not kill the serum-sensitive bacteria. Isolated C1 bound to these bacteria cleaved fluid-phase C4 but did not convert C2. C2 turnover could be detected only when serum was used as a source of C1 or C4, indicating that an additional serum component is necessary for the antibody-independent bactericidal effect. Functional tests indicated that this factor is a euglobulin which mediates binding of C4 to the bacteria even in the absence of C1 or after treatment with EDTA. Binding of C4 followed by the generation of C4b sites as acceptors for C2 was a prerequisite for the killing of the bacteria. The factor could not be replaced by immunoglobulin G or immunoglobulin M, nor was it blocked by preincubation with anti-immunoglobulin G or anti-immunoglobulin M.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew R. M., Esser A. F. Differences in activation of human and guinea pig complement by retroviruses. J Immunol. 1978 Nov;121(5):1748–1751. [PubMed] [Google Scholar]
- Bredt W., Wellek B., Brunner H., Loos M. Interactions between mycoplasma pneumoniae and the first components of complement. Infect Immun. 1977 Jan;15(1):7–12. doi: 10.1128/iai.15.1.7-12.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clas F., Loos M. Antibody-independent binding of the first component of complement (C1) and its subcomponent C1q to the S and R forms of Salmonella minnesota. Infect Immun. 1981 Mar;31(3):1138–1144. doi: 10.1128/iai.31.3.1138-1144.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clas F., Loos M. Killing of the S and Re forms of Salmonella minnesota via the classical pathway of complement activation in guinea-pig and human sera. Immunology. 1980 Aug;40(4):547–556. [PMC free article] [PubMed] [Google Scholar]
- Loos M., Wellek B., Thesen R., Opferkuch W. Antibody-independent interaction of the first component of complement with Gram-negative bacteria. Infect Immun. 1978 Oct;22(1):5–9. doi: 10.1128/iai.22.1.5-9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Moreau S. C., Skarnes R. C. Complement-mediated bactericidal system: evidence for a new pathway of complement action. Science. 1975 Oct 17;190(4211):278–280. doi: 10.1126/science.1101380. [DOI] [PubMed] [Google Scholar]
- Skarnes R. C. Humoral bactericidal systems: antibacterial potential of serum from young animals. Infect Immun. 1978 Feb;19(2):510–514. doi: 10.1128/iai.19.2.510-514.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson A. B., Prichard-Thomas S., Lachmann P. J., Coombs R. R. Receptors on guinea-pig erythrocytes specific for cell-bound fourth component of human complement (C4). Immunology. 1980 Feb;39(2):195–202. [PMC free article] [PubMed] [Google Scholar]


