Background: Cryptococcus neoformans traverses across the BBB to penetrate into the brain.
Results: C. neoformans activates RhoGTPases and subsequently PKCα, focal adhesion kinase, and ezrin in HBMEC.
Conclusion: Activation of host signaling proteins promotes Cryptococcus crossing the BBB.
Significance: Elucidation of the host signaling events that contributes to cryptococcal BBB traversal is important for a better understanding of its pathogenesis.
Keywords: Brain, Endothelial Cell, Fungi, Microbial Pathogenesis, Protein Phosphorylation, Rho GTPases, Cryptococcus Neoformans, Blood-Brain Barrier
Abstract
Cryptococcus neoformans is an opportunistic fungal pathogen that causes meningoencephalitis. Previous studies have demonstrated that Cryptococcus binding and invasion of human brain microvascular endothelial cells (HBMEC) is a prerequisite for transmigration across the blood-brain barrier. However, the molecular mechanism involved in the cryptococcal blood-brain barrier traversal is poorly understood. In this study we examined the signaling events in HBMEC during interaction with C. neoformans. Analysis with inhibitors revealed that cryptococcal association, invasion, and transmigration require host actin cytoskeleton rearrangement. Rho pulldown assays revealed that Cryptococcus induces activation of three members of RhoGTPases, e.g. RhoA, Rac1, and Cdc42, and their activations are required for cryptococcal transmigration across the HBMEC monolayer. Western blot analysis showed that Cryptococcus also induces phosphorylation of focal adhesion kinase (FAK), ezrin, and protein kinase C α (PKCα), all of which are involved in the rearrangement of host actin cytoskeleton. Down-regulation of FAK, ezrin, or PKCα by shRNA knockdown, dominant-negative transfection, or inhibitors significantly reduces cryptococcal ability to traverse the HBMEC monolayer, indicating their positive role in cryptococcal transmigration. In addition, activation of RhoGTPases is the upstream event for phosphorylation of FAK, ezrin, and PKCα during C. neoformans-HBMEC interaction. Taken together, our findings demonstrate that C. neoformans activates RhoGTPases and subsequently FAK, ezrin, and PKCα to promote their traversal across the HBMEC monolayer, which is the critical step for cryptococcal brain infection and development of meningitis.
Introduction
Infections caused by Cryptococcus neoformans, an encapsulated opportunistic fungal pathogen, have been increasing steadily since the advent of AIDS epidemic as well as the expanded use of immunosuppressive drugs (1–7). C. neoformans causes an estimated 1 million cases of meningoencephalitis globally per year in patients with AIDS, leading to ∼625,000 deaths (4). Inhaled C. neoformans cells can disseminate hematogenously from the lung to various organs including the brain and cause fatal meningoencephalitis unless treated. It is believed that C. neoformans penetrates into the central nervous system (CNS) by crossing the blood-brain barrier, but the mechanism by which yeast cells cross the blood-brain barrier (BBB)2 has not been fully understood.
The BBB is a structural and functional barrier that has a unique role in protecting the brain from toxic substances in the blood and filters harmful compounds from the brain back to the bloodstream. The BBB is mainly composed of brain microvascular endothelial cells that are influenced by brain resident cell types such as astrocytes, microglial cells, and pericytes (8). A unique property of the BBB is the presence of endothelial junction complexes such as adherens junctions and tight junctions between brain microvascular endothelial cells, which confer high transendothelial electrical resistance and low paracellular permeability. Those junction complexes enable the BBB to restrict the passage of circulating microorganisms from the capillaries of the CNS into the brain (8). However, bacterial and fungal pathogens causing CNS infection are capable of disrupting this physiologically impermeable BBB and penetrate into the CNS (9, 10).
Previous in vitro studies with human brain microvascular endothelial cells (HBMEC) have clearly shown that C. neoformans traverses the BBB to gain access into the CNS, which is the most critical process in the development of cryptococcal meningoencephalitis (11, 12). Although the molecular mechanism is not clear, C. neoformans invasion and traversal of the BBB induces significant morphological alterations of the HBMEC. As has been demonstrated by scanning electron microscopy, invading C. neoformans is associated with microvilli-like membrane protrusions on the surface of HBMEC before fungal entry (11, 12). CD44, the hyaluronic acid receptor, in lipid rafts has been identified as a host receptor, and its binding to Cryptococcus is involved in the activation of protein kinase Cα (PKCα), which is required for fungal invasion and transmigration (13–15). These findings strongly indicate the role of actin cytoskeleton reorganization during Cryptococcus-HBMEC interaction. It also suggests that the interaction with C. neoformans cells activates multiple signaling proteins in HBMEC to mediate fungal invasion and transmigration across the BBB. Therefore, we have focused on the host signaling events relevant to actin cytoskeleton remodeling during cryptococcal invasion and transmigration of the HBMEC monolayer.
In this study we have examined the host signal transduction pathway involved in C. neoformans traversal across the BBB using an in vitro human BBB model. Our results demonstrate that C. neoformans induces activation of RhoGTPases followed by phosphorylation of FAK, PKCα, and ezrin of HBMEC, all of which lead to fungal transmigration across the BBB. This is the first report demonstrating the role of host RhoGTPases and other signaling proteins related to actin cytoskeleton rearrangements in the traversal of C. neoformans across the BBB, which is the critical step in disease development.
EXPERIMENTAL PROCEDURES
HBMEC
HBMEC were obtained from Dr. Monique Stins (Johns Hopkins University, Baltimore MD) and cultured as previously described (16). Briefly, HBMEC were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 10% NuSerum, 2 mm glutamine, 1 mm sodium pyruvate, penicillin (100 units/ml), streptomycin (100 μg/ml), essential amino acids, and vitamins at 37 °C in a humid atmosphere of 5% CO2. The medium of confluent HBMEC culture was replaced with experiment medium containing Ham's F-12/M199 medium (1:1, v/v) and 5% heat-inactivated fetal bovine serum before each experiment.
C. neoformans Strains
C. neoformans B3501 and GFP-expressing B3501 strains were used in this study. The GFP-expressing strain was constructed under the control of histone 4 gene and used Agrobacterium to deliver the construct into B3501. Yeast cells were grown aerobically at 30 °C in YPD broth containing 1% yeast extract, 2% peptone, and 2% dextrose (12). Fungal cells were washed with phosphate-buffered saline (PBS) and resuspended in experiment medium before addition. The number of Cryptococcus cells was determined by a hemocytometer count.
Antibodies and Reagents
Monoclonal antibodies to RhoA, Cdc42, β-actin, c-myc (9E10), and polyclonal antibodies to phospho-ezrin and phospho-PKCα were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies to Rac1, FAK, and phospho-FAK were purchased from Millipore (Billerica, MA). The HRP-conjugated anti-mouse or anti-rabbit secondary antibodies were obtained from Invitrogen, whereas Super Signal chemiluminescence reagent was from Pierce. Inhibitors, e.g. cytochalasin D, genistein, Bapta/AM, and Y27632, were purchased from Calbiochem. CellTracker CMTMR Orange and Uvitex-2B were obtained from Invitrogen and Polysciences Inc. (Warrington, PA), respectively.
Western Blot Analysis
Confluent cell cultures of 6-well plates were incubated with 1 × 107 C. neoformans for the indicated times at 37 °C. The cells were then rinsed with ice-cold PBS and lysed in radioimmune precipitation assay buffer (Millipore) supplemented with proteinase inhibitor mixture (Pierce). The lysates were clarified by centrifugation for 20 min at 10,000 × g to remove cell debris. The supernatants were collected, and ∼30 μg of total protein of cell lysates was fractionated by electrophoresis on 4–20% gradient SDS-polyacrylamide precast gels (Invitrogen). Proteins were then transferred onto PDVF membranes using a Bio-Rad semidry transfer apparatus. The blot was blocked, incubated with the primary and horseradish peroxidase-conjugated secondary antibodies, and visualized with chemiluminescence substrate (Pierce). The same blot was stripped and re-probed with additional antibodies. The films were scanned, and a densitometric analysis of protein bands of interest was performed using ImageJ software, Version 1.44p (Research Services Branch of National Institute of Mental Health, NIH).
C. neoformans Transmigration Assay
An in vitro human BBB model was generated and used for fungal transmigration assays as previously described (11–14). HBMEC were seeded on Transwell inserts with a pore diameter of 8.0 μm (Corning Costar) and cultured until their transendothelial electrical resistance reached >350 ohms/cm2, as determined by an Endohm volt/ohm meter in conjunction with an Endohm chamber (World Precision Instruments, Sarasota, FL). The RPMI medium was replaced with experiment medium before each experiment. 105 Cryptococcus cells were added to the top compartment and then incubated at 37 °C. At 3, 6, and 9 h, the total medium in the bottom compartment was collected and immediately replaced with fresh experiment medium. The collected medium at each time point was plated on the blood agar plates, and the colonies were counted after 72 h incubation at 30 °C to determine the number of transmigrated viable fungal cells. Results were presented as the total number of colonies. Each set was performed in triplicate and repeated three times independently. For the inhibitor blocking experiments, each inhibitor was added and preincubated with HBMEC for 1 h. The cells were then washed with experiment medium to remove an inhibitor before incubation with Cryptococcus cells.
C. neoformans Association Assay
Total yeast cells associated with HBMEC were determined as previously described (11–14). Briefly, HBMEC were grown in 24-well tissue culture plates until confluence. Cryptococcus cells (106) were added and then incubated for 3 h at 37 °C. Free unbound yeast cells were removed by washing three times with PBS. HBMEC were lysed with sterile distilled water, and the lysates were diluted and plated onto blood agar plates. The colonies were counted, and results were presented as the number of associated yeast cells. Each set was in performed in triplicate and repeated three times independently. For the inhibitor blocking experiments, each inhibitor was added and preincubated with HBMEC for 1 h. The cells were then washed with experiment medium before incubation with Cryptococcus cells.
Cryptococcal Internalization Assay with Flow Cytometry
For the preparation of flow cytometry, the HBMEC were grown in 6-well tissue culture plates until confluence. HBMEC were labeled with CellTracker CMTMR Orange (10 μm, red) for 10 min at room temperature, washed 3 times with PBS, and resuspended in experiment medium. GFP-expressing C. neoformans cells (green) grown overnight in yeast extract/peptone/dextrose broth were washed and resuspended in experiment medium. The labeled HBMEC were incubated with 107 Cryptococcus at 37 °C. After 3 h of incubation, HBMEC were washed 3 times with PBS to remove unbound yeast cells and then incubated with warmed trypsin/EDTA for 5 min to lift cells and dissociate bound yeasts from HBMEC. To distinguish HBMEC with internalized yeasts from those with surface-bound as well as free yeasts, the harvested HBMEC were incubated with Uvitex-2B (blue) to preferentially stain yeast cell walls. After washing three times with PBS to remove excess Univex-2B, the mixture was analyzed by an LSRII flow cytometry with FACSDiva software (BD Biosciences). GFP-expressing fungal cells were detected with a GFP filter, whereas CMTMR Orange and Uvitex-2B were detected with filters for Orange Tracker and DAPI, respectively. The subpopulation showing Orange Tracker-positive (red), GFP-positive (green), and Uvitex-negative was considered as HBMEC with internalized yeast cells. The experiments were performed in the absence or presence of inhibitors and repeated twice independently. Inhibitors were pretreated with the HBMEC for 1 h and washed out before incubation with Cryptococcus cells.
Transduction of HBMEC
For dominant-negative RhoGTPases expressions, HBMEC were transduced with adenoviruses encoding c-myc tagged N19RhoA, N17Rac1, or N17Cdc42 as previously described (17). For FAK knockdown, HBMEC were transduced with lentiviruses containing human FAK or scrambled short hairpin RNA (shRNA) as described (18). Briefly, HEK 293 cells were transfected with plasmids; pLentilox 3.7 containing FAK or Scrambled shRNA, CMV-VSVG envelope vector pMD.G, RSV-Rev, and packaging vector pMDL g/p RRE in the presence of Lipofectamine. These constructs were kindly provided by Dr. D. Schlaepfer at the University of California in San Diego, CA. The culture medium containing lentivirus was harvested after 48 h incubation, filtered, and used to transduce HBMEC. The transductions of RhoGTPases or FAK shRNA were confirmed by Western blotting with anti-myc or anti-FAK antibody, respectively. For ezrin transfection, HBMEC was transfected with pCB6 plasmids harboring the wild type or T567A mutant ezrin in the presence of Lipofectamine 2000 according to the manufacturer's instruction (Invitrogen). Transfected cells were selected with G418 (800 μg/ml), and the expression of ezrin was verified by Western blotting with VSVG antibody. Those ezrin plasmids were a kind gift from Dr. Monique Arpin (Institut Curie, Centre de Recherche, Paris, France) and have been used in many studies (19).
Pulldown Assay for Rho, Rac, and Cdc42 Activation
The activation of RhoGTPases was determined as previously described (17, 20). Confluent culture of HBMEC was treated with C. neoformans for the indicated times and lysed with radioimmune precipitation assay buffer (Millipore). The lysates were collected and incubated with agarose beads conjugated with RBD of rhotekin or PBD of p21-associated kinase (PAK) to pull down active, GTP-bound RhoA or Cdc42/Rac1, respectively. Beads were washed and bound RhoGTPases were resolved by 4–20% SDS-PAGE. The amounts of active RhoA, Cdc42 and Rac1 were determined by Western blot analysis with corresponding antibodies.
Statistical Analysis
The statistical analysis of the data from our in vitro studies was done with a two-tailed Student t test. Statistical significance was determined at p < 0.001.
RESULTS
C. neoformans Association and Transmigration Require Host Signaling Events Involved in Actin Cytoskeleton Rearrangement
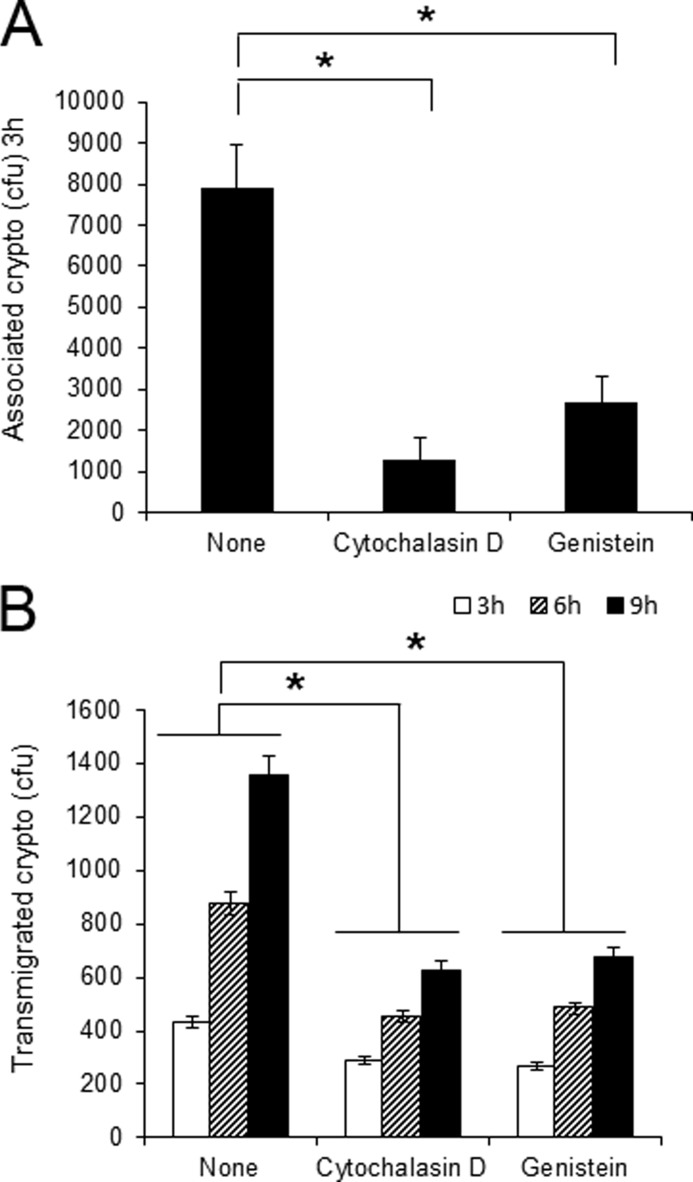
Previous studies have demonstrated that Cryptococcus-HBMEC interaction induces host actin cytoskeleton rearrangements, resulting in fungal invasion and transmigration across the BBB (11, 12). To further investigate the role of actin remodeling in HBMEC during interaction with C. neoformans, association and transmigration assays were performed in the presence of specific pharmaceutical reagents regulating the actin cytoskeleton. Cytochalasin D is known to block actin polymerization and genistein inhibits tyrosine protein kinase activity. Both reagents have been previously demonstrated to reduce meningitis-causing Escherichia coli K1 invasion of HBMEC by affecting the actin cytoskeleton (10). As shown in Fig. 1A, those inhibitors reduce the level of C. neoformans association with HBMEC monolayers compared with that of the untreated control. After 3 h of incubation, the number of associated Cryptococcus in HBMEC pretreated with cytochalasin D exhibited ∼20% of the untreated control, whereas genistein treatment reduced fungal association by 60%. Cryptococcal transmigration assay was also done with an in vitro human BBB model system in the presence of aforementioned inhibitors as described under “Experimental Procedures” (Fig. 1B). Both inhibitors reduced the rate of C. neoformans crossing of the BBB in a time-dependent manner. The numbers of transmigrated Cryptococcus cells with treatment of either cytochalasin D or genistein were less than those of the untreated control, exhibiting a 50% reduction after 9 h incubation. Taken together, our results indicate that association of C. neoformans with HBMEC monolayers and its transmigration across the BBB depends on actin polymerization and phosphorylation of tyrosine kinases in HBMEC, both of which involve in the regulation of host actin cytoskeleton.
FIGURE 1.
Association and transmigration of C. neoformans require actin cytoskeleton rearrangement in HBMEC. Cryptococcal association (A) and transmigration assay (B) were performed in the presence of either cytochalasin D or genistein. HBMEC were grown in 24-well plates or Transwells for association or transmigration assays, respectively. Confluent culture of HBMEC was preincubated with reagents for 1 h before the addition of C. neoformans. The numbers (colony forming units (cfu)) of associated or transmigrated Cryptococcus were determined after 3 h (Association assay) or 3, 6, and 9 h of incubation (Transmigration assay). Values are the means ± S.D. of three independent experiments done in triplicate. *, p < 0.001.
C. neoformans Internalization into HBMEC Also Requires Host Signaling Events Involved in Actin Cytoskeleton Rearrangements
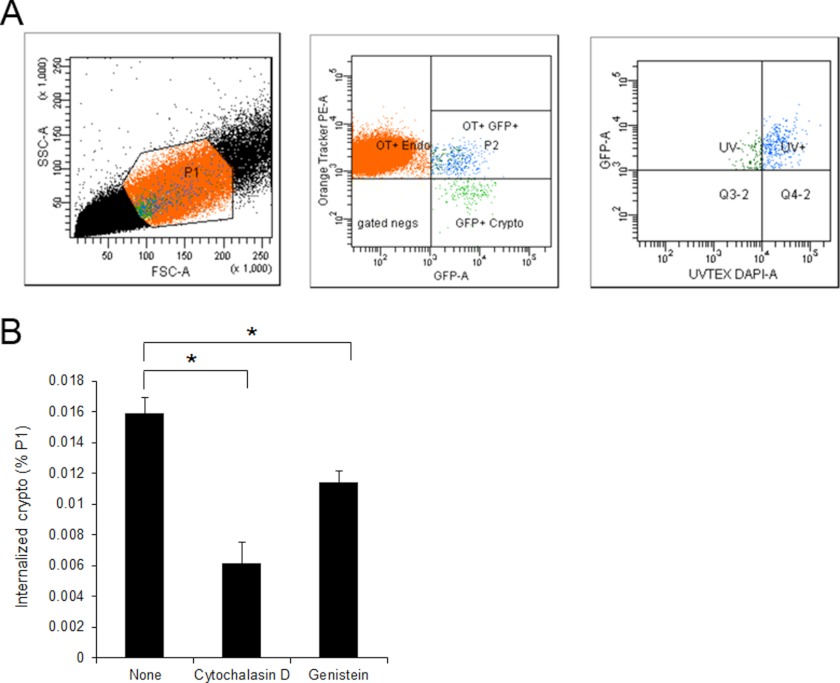
C. neoformans has been shown to adhere to and invade the HBMEC. However, determination of fungal invasion rate has been challenging. In the case of bacterial pathogens interacting with HBMEC, gentamicin-protection assays have been widely utilized to determine their invasion abilities. However, the same approach is not applicable to Cryptococcus due to the unavailability of anti-fungal agents, which function like gentamicin. Cryptococcal internalization was previously determined by either direct counting of fungi colocalized with a host cellular protein or flow cytometry analysis with labeling of host cells and GFP-expressing cryptococci (14, 21). Both approaches were able to distinguish the cell-associated fungi from the free ones. However, there is a limitation in the determination of numbers of internalized versus surface-bound fungi with them. Hence, we have applied a triple labeling approach using flow cytometry to monitor the internalization of C. neoformans into the HBMEC as described under “Experimental Procedures” (Fig. 2). HBMEC were labeled with CellTracker Orange and C. neoformans was labeled with GFP. Uvitex-2B was utilized to distinguish the free or surface-bound fungal cells from the internalized ones because it is membrane-impermeable and preferentially stains fungal cell wall. It is noteworthy that Uvitex-2B also stained HBMEC, so higher cutoff (104) was used to separate fungal cells from HBMEC. Among the HBMEC associated with fungal cells (the population displaying red-positive and green-positive), the population with blue-negative was determined as the HBMEC with internalized fungal cells, whereas the blue-positive was determined as those with bound ones (Fig. 2A). When repeated in the presence of the aforementioned reagents inhibiting fungal association and transmigration, the level of internalization was also significantly reduced, indicating that actin polymerization and protein tyrosine phosphorylation are also involved in the cryptococcal invasion of the HBMEC (Fig. 2B).
FIGURE 2.
C. neoformans internalization into HBMEC involves host actin cytoskeleton rearrangement. Cryptococcal internalization was determined using flow cytometry with GFP-expressing Cryptococcus cells. HBMEC monolayers were labeled with CellTracker CMTMR Orange (red) and incubated with GFP-expressing cryptococci (crypto, green) for 3 h. The HBMEC were washed with PBS to remove unbound fungi followed by incubation with trypsin/EDTA to dissociate bound fungi from HBMEC as well as to collect HBMEC. The cell mixture was subsequently stained with Uvitex-2B (blue). A, the mixed population of HBMEC and fungal cells was analyzed by flow cytometry using triple filter sets. P1 region (left) of total population was analyzed with red (Orange Tracker) and green (GFP) filters, and then the population showing double-positive (P2 region, middle) was further analyzed with blue (DAPI) filter. The population exhibiting Green+/Red+/Blue− was determined as the HBMEC with internalized fungal cells (right). B, a cryptococcal internalization assay was repeated in the presence of inhibitors. The percentages of HBMEC with internalized fungal cells among the P1 region were plotted. These experiments were repeated twice independently. *, p < 0.001.
C. neoformans Incubation Induces Activation of RhoGTPases in HBMEC
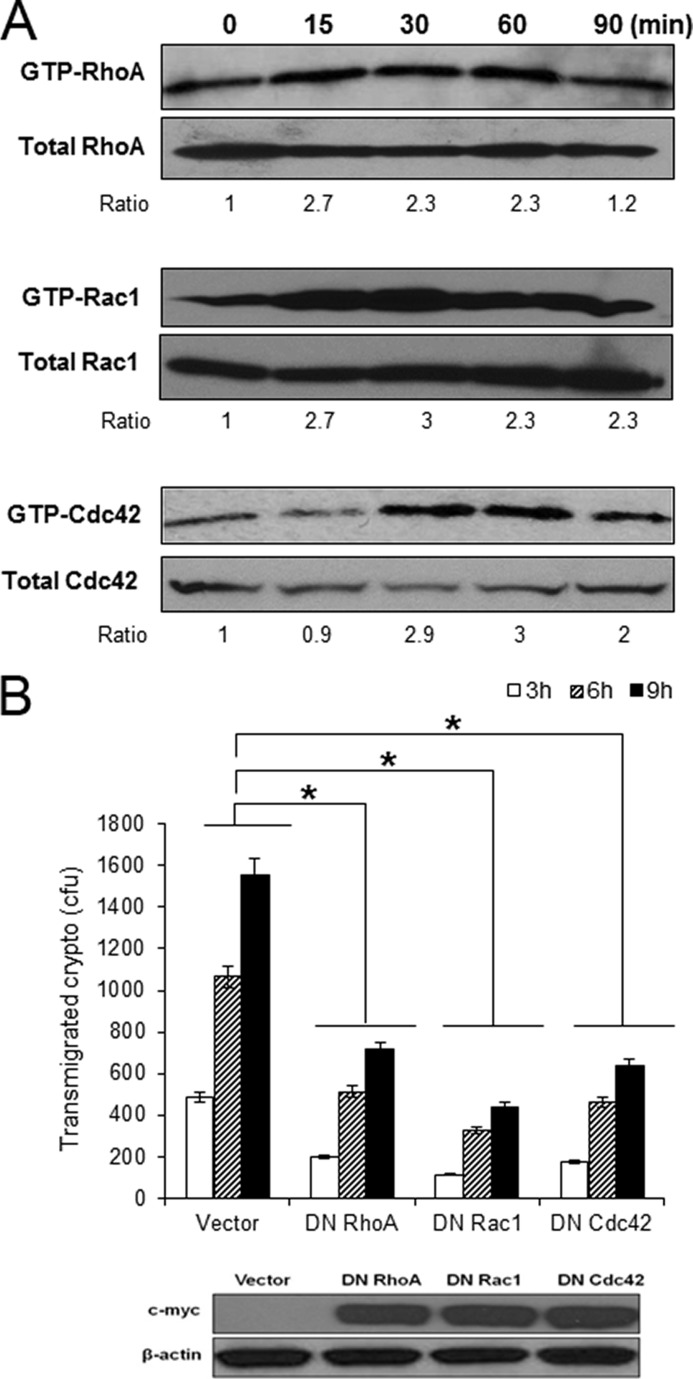
It is well established that the Rho family of small GTPases regulates the reorganization of actin cytoskeleton in mammalian cells (22–24). Previous studies with bacterial pathogens crossing the BBB, e.g. E. coli K1, group B Streptococcus, and Neisseria meningitides, have shown that RhoGTPases are key signaling proteins promoting host actin cytoskeleton rearrangement required for their invasion of the HBMEC (25–27). Therefore, we examined whether RhoGTPases of the HBMEC were also activated in response to C. neoformans. A Rho pulldown assay demonstrated that C. neoformans incubation induces activation of RhoA, Rac1, and Cdc42 (Fig. 3A). Densitometry analysis showed that the levels of GTP-bound active RhoA and Rac1 had increased by ∼2.7-fold after 15 min and was maintained until 60 min. The level of GTP-RhoA returned to that of the control in a 90-min incubation, whereas Rac1 remained activated continuously. In contrast, Cdc42 activation was not shown in 15 min but became evident after 30 min of incubation, displaying a 2.9-fold increase (Fig. 3A). Similar to Rac1, the amount of active Cdc42 was still 2-fold greater even after 90 min of incubation. These findings indicate that C. neoformans is able to activate all three members of RhoGTPases, RhoA, Rac1, and Cdc42, during interaction with HBMEC.
FIGURE 3.
C. neoformans induces activation of RhoGTPases in HBMEC required for their transmigration. A, confluent HBMEC monolayers were incubated with C. neoformans (1 × 106) for the indicated times, and Rho pulldown assays were performed to determine activation of RhoA, Rac1, and Cdc42. The level of activation was determined by densitometry analysis (measuring the ratios of GTP-Rho/total Rho). B, HBMEC was transduced with adenoviral constructs of DN RhoGTPases mutants (N19RhoA, N17Rac1 or N17Cdc42). Expression of DN RhoGTPases in the transduced HBMEC was verified by Western blotting with anti-myc antibody (bottom). Transmigration assay with HBMEC expressing DN RhoGTPases were carried out. These experiments were repeated three times independently. *, p < 0.001.
Next, we examined the role of RhoA, Rac1, or Cdc42 activation in the Cryptococcus-HBMEC interaction. HBMEC was transduced with dominant-negative (DN) adenoviral constructs of each RhoGTPase, and the expression of DN RhoGTPases was confirmed by Western blot analysis with anti-myc antibody (Fig. 3B, bottom). C. neoformans transmigration assays with HBMEC expressing DN RhoGTPases showed that the ability of C. neoformans to traverse the HBMEC monolayer was decreased in all three DN RhoGTPase-transduced cells. After 3 h of incubation, the number of transmigrated Cryptococcus in the DN RhoA-transduced cells was attenuated by 50% compared with the vector control, and the reduced transmigration was maintained during 6 and 9 h of incubation (Fig. 3B). Both DN Rac1 and DN Cdc42 exhibited a similar inhibition effect, indicating that three members of RhoGTPases, RhoA, Rac1, and Cdc42, play a positive role in the cryptococcal traversal across the HBMEC monolayer.
C. neoformans Induces Activation of PKCα, FAK, and Ezrin in HBMEC
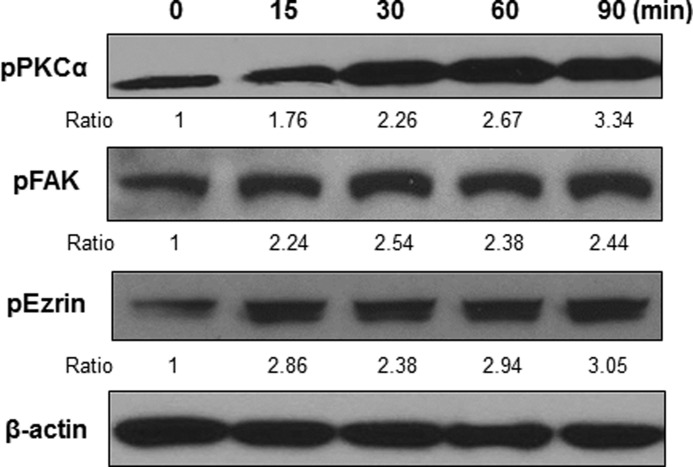
Previous studies showed that Cryptococcus induces activation of PKCα, which contributes to fungal invasion and transmigration (14). Therefore, we further examined the phosphorylation status of host signaling proteins known to regulate actin cytoskeleton such as FAK and ezrin during the interaction with C. neoformans. Activation of those proteins was determined by Western blot analysis with phospho-specific antibodies for FAK (Tyr-397) and ezrin (Thr-567) (Fig. 4). Upon incubation with C. neoformans, the phosphorylation of PKCα was detected at 15 min, and the level of phosphorylated PKCα increased 3.34-fold after 90 min of incubation, which is consistent with the previous finding (14). Phosphorylations of FAK and ezrin were also induced by C. neoformans and exhibited 2.24- and 2.85-fold higher amounts compared with the untreated control, respectively (Fig. 4). Thus, our results demonstrate that C. neoformans is able to activate multiple host proteins involved in the rearrangement of host actin cytoskeleton.
FIGURE 4.
C. neoformans induces phosphorylation of PKCα, FAK, and ezrin in HBMEC. Confluent cultures of HBMEC monolayer were incubated with C. neoformans for the indicated times, and the lysates were analyzed by Western blotting with phospho-specific antibodies of PKCα, FAK, and ezrin. β-Actin was detected as an internal loading control. The amount of phosphorylated proteins was determined by densitometric analysis by measuring the relative ratios of amount of each band against that of the untreated control. The representative image of three independent experiments was shown.
Activation of PKCα, FAK, and Ezrin Contributes to C. neoformans Transmigration
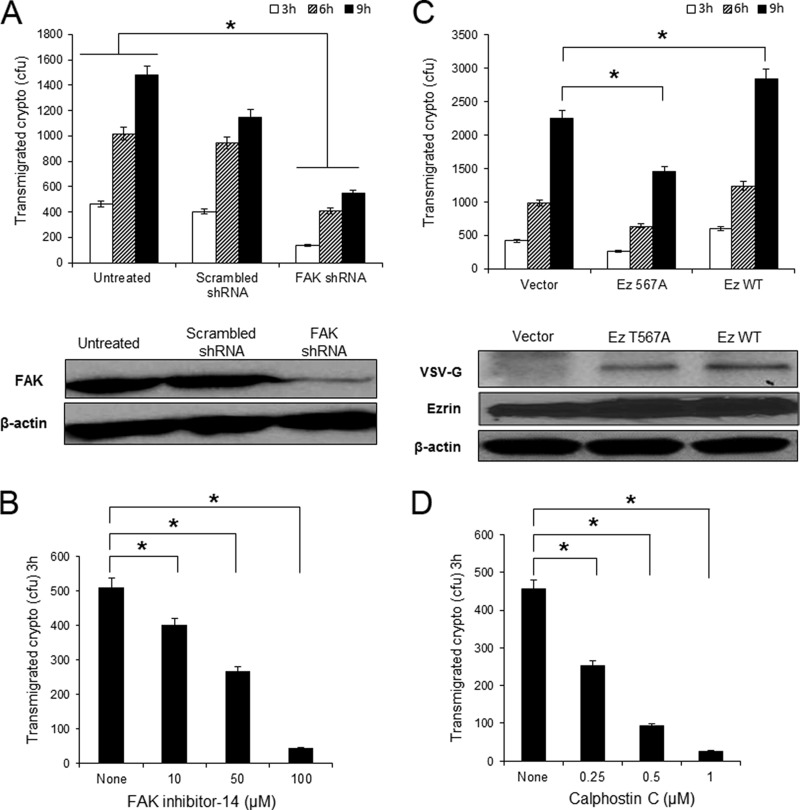
We further examined the role of FAK and ezrin in cryptococcal transmigration across the BBB. To determine FAK function, the HBMEC was transduced with lentiviral constructs containing either human FAK shRNA or scrambled shRNA. The FAK knockdown was confirmed by Western blot analysis with anti-FAK antibody. As shown in Fig. 5A, bottom, the amount of FAK was significantly reduced by FAK shRNA after 2 days of incubation, whereas there was no change in FAK amount in the scrambled shRNA cells. Subsequently transmigration assays were performed with FAK knockdown HBMEC. FAK knockdown significantly reduced fungal transmigration by 70% after 3 h of incubation compared with that with the normal HBMEC (Fig. 5A). The reduced transmigration was maintained until 9 h of incubation. In contrast, the scrambled shRNA transduction did not affect fungal transmigration ability until 6 h incubation, exhibiting comparable number of transmigrated fungal cells (Fig. 5A). These results indicate a positive role of FAK in cryptococcal transmigration. Moreover, transmigration assays in the presence of FAK inhibitor-14, an inhibitor preventing FAK phosphorylation at tyrosine 397, also blocked fungal transmigration in a dose-dependent manner (Fig. 5B). This finding further supports the requirement of FAK activation in cryptococcal traversal across the HBMEC monolayer.
FIGURE 5.
FAK, ezrin, and PKCα are required for cryptococcal transmigration of HBMEC monolayer. A, HBMEC was transduced with FAK shRNA to knock down FAK expression. Western blotting with anti-FAK antibody showed the reduction of FAK in the cells with FAK shRNA but not in the scrambled shRNA cells. The transmigration assay was performed with the FAK knockdown cells. B, transmigration assay was done with normal HBMEC in the presence of FAK inhibitor-14. The number of transmigrated Cryptococcus cells was determined by plating the medium collected from the bottom compartment after 3 h of incubation. The result was normalized to show the relative transmigration rate compared with the untreated control. C, HBMEC was transfected with the wild type or DN T567A ezrin mutant construct. Transfection was verified by Western blotting with anti-VSVG antibody (bottom). The transmigration assay was performed with the ezrin transfected cells. D, a transmigration assay carried out with normal HBMEC in the presence of calphostin C, an inhibitor for PKC. *, p < 0.001.
Next, the role of ezrin was studied with the ezrin-transfected HBMEC. Because ezrin is known to be activated through phosphorylation of a threonine residue at 567, overexpression of T567A mutant ezrin behaves like a dominant-negative, whereas the wild type ezrin behaves like a dominant-positive (28). Ezrin transfections were confirmed by the presence of a VSVG tag in either wild type or T567A mutant ezrin but not in vector control (Fig. 5C). Transmigration assays with the transfected HBMEC revealed that the level of cryptococcal transmigration was elevated in the wild type ezrin-transfected cells but reduced in the T567A ezrin transfection compared with the vector control (Fig. 5C). This result suggests that Cryptococcus-induced ezrin phosphorylation contributes to fungal transmigration of the BBB. Of interest, the effect of ezrin transfection in transmigration became evident after 9 h of incubation even though the same trend was detected during 3 and 6 h of incubation.
In addition, our results have shown that C. neoformans induces phosphorylation of PKCα in the HBMEC in a time-dependent manner. To verify whether PKCα activation plays a role in the C. neoformans transmigration, transmigration assay was performed in the presence of a PKC-specific inhibitor, calphostin C (29). As shown in Fig. 5D, calphostin C was effective in blocking the transmigration of C. neoformans in a dose-dependent manner, further supporting the role of PKCα in fungal transmigration. Taken together, our results demonstrate that activations of FAK, ezrin, and PKCα by C. neoformans contribute to fungal transmigration across the HBMEC monolayer.
Cryptococcus-induced RhoGTPases Activation Leads to Phosphorylation of FAK, Ezrin, and PKCα in HBMEC
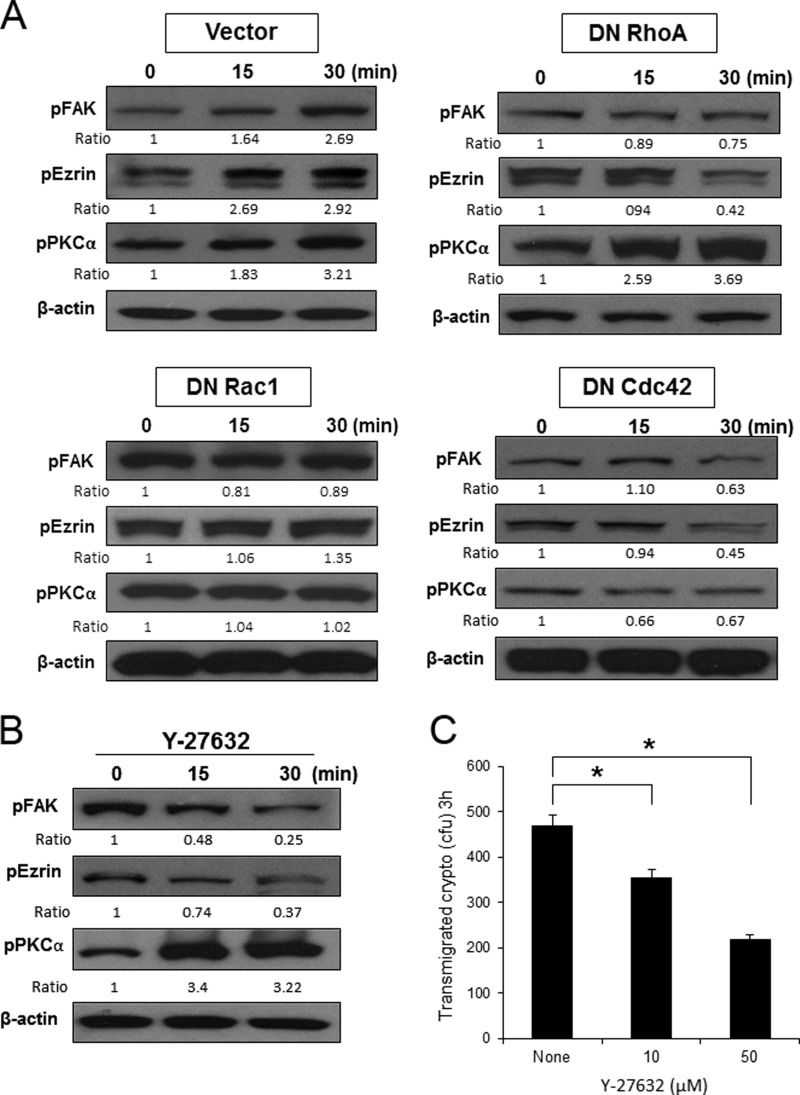
We have shown that C. neoformans activates host signaling proteins such as RhoGTPases, PKCα, FAK, and ezrin, all of which are involved in fungal BBB traversal. To define the host signal transduction pathway triggered by cryptococci, Western blot analysis was carried out with HBMEC expressing DN RhoA, DN Rac1, or DN Cdc42. C. neoformans incubation induced phosphorylation of FAK, ezrin, and PKCα in the HBMEC with vector control, which is consistent with the previous result with normal HBMEC (Fig. 6A, Vector). However, DN RhoA inhibited phosphorylation of FAK and ezrin, but the amount of phosphorylated PKCα still increased during incubation with Cryptococcus cells (Fig. 6A, DN RhoA). In contrast, expression of DN Rac1 or DN Cdc42 abolished phosphorylation of FAK, ezrin, and PKCα (Fig. 6A, DN Rac1 and DN Cdc42). These findings indicate that phosphorylation of FAK, ezrin, and PKCα is a downstream event of Cryptococcus-induced RhoGTPases activation. In addition, phosphorylation of PKCα depends on activities of Rac1 and Cdc42 rather than RhoA, whereas FAK and ezrin activations require all three members of RhoGTPases.
FIGURE 6.
RhoGTPases activation contributes to phosphorylation of PKCα, FAK, and ezrin in HBMEC. A, C. neoformans cells (1 × 106) were incubated with the HBMEC-expressing DN mutant of RhoGTPases (N19RhoA, N17Rac1, and N17Cdc42) for 15 and 30 min. B, normal HBMEC was incubated with C. neoformans cells (1 × 106) in the presence of Y27632, an inhibitor of Rho kinase, for 15 and 30 min. The cell lysates was analyzed by Western blotting with phospho-specific antibodies of PKCα, FAK, and ezrin. β-Actin was detected as an internal loading control. The amounts of phosphorylated proteins were determined by densitometry analysis. C, cryptococcal transmigration assay was done in the presence of Y-27632. *, p < 0.001.
Next, Western blot analysis was carried out in the presence of Y27632, an inhibitor of Rho kinase that is a downstream effector of active RhoA. Pretreatment of Y27632 prevented the activation of either ezrin or FAK but not PKCα during cryptococcal incubation (Fig. 6B). This finding is consistent with our result obtained from DN RhoA expression, supporting that RhoA activation by C. neoformans is involved in phosphorylation of ezrin and FAK but not PKCα. Cryptococcal transmigration assay in the presence of Y27632 also showed a decrease in the number of transmigrated Cryptococcus in a dose-dependent manner compared with the untreated control (Fig. 6C). Our results, thus, reveal that Cryptococcus cells induce activation of RhoGTPases and subsequently FAK, ezrin, and PKCα in the HBMEC, leading to fungal transmigration across the HBMEC monolayer.
DISCUSSION
C. neoformans has a predilection for central nervous system-causing devastating meningoencephalitis, which is one of the most serious fungal diseases globally. Penetration of C. neoformans across the BBB is a crucial step leading to brain infection and disease development. However, the mechanism underlying Cryptococcus traversal of BBB remains unclear. We, thus, investigated host signal proteins involved in host actin cytoskeleton rearrangement and found that host RhoGTPases, FAK, PKCα, and ezrin play a major role in cryptococcal crossing of the HBMEC monolayer.
Previous studies have demonstrated that C. neoformans adhesion and invasion of HBMEC is a prerequisite for its traversal across the BBB through a transcellular mechanism (11–14). Transcellular traversal of BBB has also been demonstrated in a majority of meningitis-causing pathogens (10). Bacterial pathogens infecting the CNS include E. coli, Group B Streptococcus, Listeria monocytogenes, Mycobacterium tuberculosis, Citrobacter freundii, Haemophilus influenza, Streptococcus pneumonia, and N. meningitidis (26, 27, 30–35). In addition to C. neoformans, another fungal pathogen, Candida albicans, has been shown to cross the BBB in vitro without affecting the integrity of the BBB (36). Although the molecular mechanism by which those pathogens transmigrate across the BBB is unclear, their invasion of HBMEC and traversal of BBB is commonly found to require actin cytoskeleton rearrangements of the HBMEC through an activation of host signaling proteins regulating actin dynamics. In this study we have shown that host actin cytoskeleton rearrangement is important for cryptococcal interaction with HBMEC. Cytochalasin D inhibits Cryptococcus association, internalization, and transmigration of HBMEC monolayer. These findings are consistent with previous reports showing that inhibition of actin polymerization blocks adherence and invasion of the HBMEC by pathogens that cause the CNS infection (11, 34, 37, 38). Genistein also blocks interaction of C. neoformans with HBMEC including association, invasion, and transmigration, suggesting the involvement of phosphorylation of host signaling proteins regulating host actin cytoskeleton. Taken together, these results suggest that Cryptococcus-host interaction triggers signaling events, leading to host actin cytoskeleton rearrangements and cryptococcal binding, invasion, and traversal across the HBMEC monolayer.
To determine Cryptococcus internalization, we have utilized a three-color labeling approach using flow cytometry and GFP-expressing C. neoformans. The advantage of this approach is to distinguish internalized fungal cells from the surface-bound or free fungal cells. Our flow cytometry approach in the presence of inhibitors revealed that the internalization of Cryptococcus cells requires the same host signaling events that are involved in fungal transmigration, suggesting that activation of the host proteins triggered by Cryptococcus contributes to fungal invasion of HBMEC as well as their transmigration across the BBB. It is noteworthy that there is a drawback of this approach. Due to the inability to distinguish HBMEC with one internalized fungal cell from those with multiple fungal cells, this flow cytometry approach underestimates the number of internalized Cryptococcus cells.
Small GTP binding Rho family proteins, e.g. RhoA, Rac1, and Cdc42, are key regulators of the actin cytoskeleton in mammalian cells (22, 23). RhoGTPases have been implicated in several processes including cell motility, phagocytosis, pinocytosis, organization of intercellular junctions, gene expression, and cell cycle entry (22–24). Many pathogens have been shown to modulate RhoGTPases to alter actin polymerization required for their invasion of host cells. Intracellular bacteria such as Salmonella and Shigella modulate Cdc42 and Rac1 activity, whereas Brucella, Chlamydia, Pseudomonas, and Bartonella act on the Rho family of GTPases (27). The effectors and the delivery systems used by pathogens to regulate activity of RhoGTPases have been well characterized in several intracellular pathogens (15, 40). Dominant-negative forms of Rho proteins have been particularly useful in analyzing their functions because they inhibit the activity of their respective endogenous RhoGTPases by competitive binding to exchange factors and downstream effectors. In this study we have revealed that C. neoformans activates all three members of the RhoGTPases, RhoA, Rac1, and Cdc42. Further studies showed that activations of all members of RhoGTPases by cryptococcal cells are required for cryptococcal traversal across the HBMEC monolayer. Although it is not known how Cryptococcus cells induce activation of RhoGTPases, the most recent study of Maruvada et al. (41) demonstrates that C. neoformans induces activation of Rac1 in HBMEC, whose activation requires cryptococcal phospholipase 1. They also showed that Rac1 activation promotes cryptococcal transmigration across the HBMEC monolayers as well as their brain infection in an animal model (41). Our results are consistent with their findings. Further studies are required to characterize the molecular mechanisms underlying the Cryptococcus-mediated activation of RhoGTPases in HBMEC.
Recent studies have demonstrated that cryptococci invade HBMEC via lipid rafts endocytic pathway (42). CD44, hyaluronic acid receptor, present in the lipid rafts plays a role as a host receptor mediating cryptococcal binding and invasion of HBMEC. However, it is not known how cryptococcal binding to the host GPI-anchored CD44 triggers intracellular signal transduction pathways required for fungal invasion and transmigration. Our results revealed that C. neoformans incubation triggers activation of RhoGTPases, RhoA, Rac1, and Cdc42, all of which are required for cryptococcal traversal of HBMEC monolayer. Thus, it would be interesting to examine how the Cryptococcus-CD44 interaction is able to induce activation of RhoGTPases. In addition, activation of RhoGTPases is an upstream event leading to phosphorylation of PKCα, FAK, and ezrin of the HBMEC, all of which are involved in the host actin regulation. Considering the results with inhibitors, it is conceivable that activation of the host signal cascade triggered by Cryptococcus interaction induces morphological changes through the rearrangement of host actin cytoskeleton, which leads to fungal invasion of the HBMEC and subsequently transmigration across the HBMEC monolayer. For example, activation of FAK by phosphorylation at tyrosine 397 has previously been demonstrated during invasion of HBMEC by meningitis-causing bacteria such as E. coli K1, L. monocytogenes, and group B Streptococcus (9, 10). These bacterial pathogens activate FAK phosphorylation to induce host actin cytoskeleton rearrangements, which is a prerequisite for their invasion into HBMEC and then traversal across the BBB. Either FAK knockdown or a FAK inhibitor pretreatment resulted in a significant decrease in the number of transmigrated Cryptococcus cells, suggesting the involvement of FAK in C. neoformans invasion and transmigration of the HBMEC monolayer.
Ezrin is a member of the ERM (ezrin, radixin, moesin) family, and upon activation through phosphorylation at threonine 567, it cross-links F-actin to membrane proteins such as CD44 to promote formation of membrane protrusions (43). Moreover, ezrin has been reported to participate in the invasion of mammalian cells by several pathogens such as E. coli and N. meningitides (30, 44). In this study we have found that C. neoformans induces phosphorylation of ezrin in Rho-dependent pathways and ezrin activation is required for cryptococcal traversal across the HBMEC monolayer. Therefore, it is possible that ezrin, upon activation by phosphorylation, could bind to CD44 and F-actin through its N and C termini, respectively, resulting in formation of microvilli-like membrane protrusions associated with invading Cryptococcus cells, as shown by scanning electron microscopic observation (12).
In addition, we have also shown the role of PKCα activation during the interaction of HBMEC with Cryptococcus. PKC is known as a downstream effecter of intracellular Ca2+ mobilization and plays a significant role in the signaling cascades leading to actin cytoskeleton rearrangements, involved in cell-cell junction modulation (45) and host cell invasion of E. coli, Neisseria gonorrhea, and Legionella pneumophila (10, 46). Our results derived from transmigration assays in the presence of calphostin C further support the previous report that the activation of PKCα by C. neoformans contributes to the fungal transmigration across the HBMEC monolayer. Furthermore, pretreatment of HBMEC with Bapta/AM, an intracellular calcium chelator, reduces PKCα activation as well as cryptococcal invasion and transmigration rates.3 Further studies on the role of each signaling protein are needed to understand the molecular mechanisms underlying cryptococcal invasion and transmigration processes.
Previous studies suggest that C. neoformans might engage multiple entry mechanisms during brain infection. Studies with an in vitro BBB model have demonstrated that C. neoformans adheres to the confluent HBMEC monolayer followed by internalization and escape from the host cells as live cells. Although this transcytosis process is considered to be the major mechanism for fungal penetration of BBB, mouse infection model studies have also suggested that C. neoformans crosses the BBB between brain endothelial cells (paracellular penetration) and/or transmigration with infected leukocytes (Trojan horse mechanism) accompanied by modulation of tight junctions in the BBB (11, 47, 48). Recent imaging studies of real time imaging of traversal further support transcellular transmigration even though it is not clear whether C. neoformans uses either transcytosis or a paracellular mechanism (49). In this study we have investigated host signaling events triggered by C. neoformans that are involved in the fungal association, internalization, and transmigration of the HBMEC monolayer, supporting the transcytosis mechanism. However, our findings do not rule out other alternative penetration mechanisms. Because the host signaling proteins activated by Cryptococcus, i.e. RhoGTPases, PKCα, FAK, and ezrin, are able to modulate tight junctions of the BBB (8, 50, 51), fungal penetration by paracellular and/or Trojan horse mechanisms can occur via modulation of the tight junctions. This possibility is supported by tight junction disruption of HBMEC monolayer during interaction with C. neoformans.3 Further studies are warranted to determine all routes of BBB traversal by C. neoformans for brain infection.
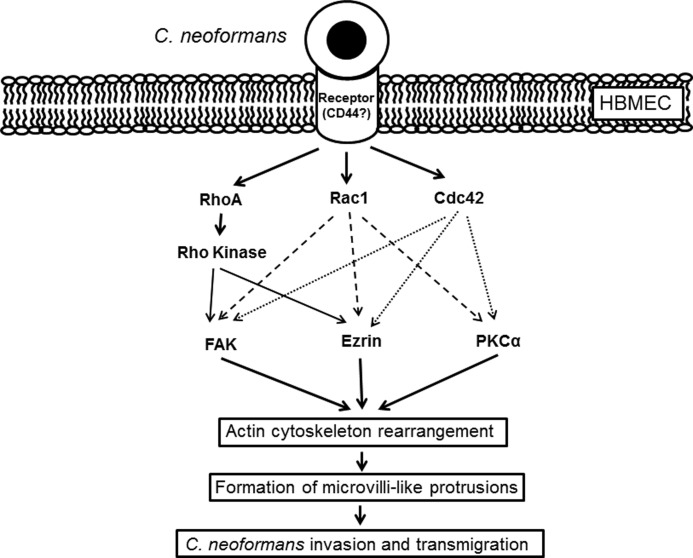
In summary, we have investigated the host intracellular signal transduction pathway required for cryptococcal transmigration of BBB. C. neoformans-induced activation of RhoGTPases (RhoA, Rac1, and Cdc42) and subsequent signaling proteins (FAK, ezrin, and PKCα) led to host actin cytoskeleton rearrangements and promote fungal association and internalization followed by their traversal across the BBB (Fig. 7). Further studies are necessary to identify the fungal ligand(s) and host receptor(s) responsible for the activation of intracellular signaling pathway to elucidate molecular mechanisms of cryptococcal penetration across the BBB, which is the key step for brain infection and development of meningitis.
FIGURE 7.
Current model of the host signaling events involved in cryptococcal traversal of the BBB. Cryptococcus binding to the host receptor(s) on HBMEC triggers activation of RhoGTPases such as RhoA, Rac1, and Cdc42. Subsequently, active RhoGTPases induces phosphorylation of other host signaling proteins regulating actin cytoskeleton. Both Rac1 and Cdc42 are involved in phosphorylation of FAK, ezrin, and PKCα, whereas RhoA leads to phosphorylation of FAK and ezrin but not PKCα. Activation of those host proteins results in the host actin cytoskeleton rearrangement and formation of microvilli-like membrane protrusions associated with invading Cryptococcus cells followed by the cryptococcal invasion and transmigration across the HBMEC monolayer. CD44 has shown to play a role as a host cellular receptor for hyaluronic acid of Cryptococcus. However, it is not fully understood how Cryptococcus-CD44 interaction triggers activation of host proteins yet.
This work was supported, in whole or in part, by National Institutes of Health Grants P20RR016443 and R01DA027729 (to K. J. K.) and NIAID intramural grants (to Y. C. C. and K. J. K.-C.).
J.-C. Kim, H.-J. Kwon, and K. J. Kim, unpublished data.
- BBB
- blood-brain barrier
- HBMEC
- human brain microvascular endothelial cell(s)
- DN
- dominant-negative
- FAK
- focal adhesion kinase
- VSVG
- vesicular stomatitis virus glycoprotein.
REFERENCES
- 1. Alspaugh J. A., Perfect J. R., Heitman J. (1997) Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 11, 3206–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casadevall A., Perfect J. R. (1998) Cryptococcus neoformans, American Society for Microbiology, Washington, D. C. [Google Scholar]
- 3. Chayakulkeeree M., Perfect J. R. (2006) Cryptococcosis. Infect. Dis. Clin. North Am. 20, 507–544 [DOI] [PubMed] [Google Scholar]
- 4. Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., Chiller T. M. (2009) Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530 [DOI] [PubMed] [Google Scholar]
- 5. Pukkila-Worley R., Mylonakis E. (2008) Epidemiology and management of cryptococcal meningitis. Developments and challenges. Expert Opin. Pharmacother. 9, 551–560 [DOI] [PubMed] [Google Scholar]
- 6. Kwon-Chung K. J., Sorrell T. C., Dromer F., Fung E., Levitz S. M. (2000) Cryptococcosis. Clinical and biological aspects. Med. Mycol. 38, 205–213 [PubMed] [Google Scholar]
- 7. Mitchell T. G., Perfect J. R. (1995) Cryptococcosis in the era of AIDS. 100 years after the discovery of Cryptococcus neoformans. Clin. Microbiol. Rev. 8, 515–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rubin L. L., Staddon J. M. (1999) The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 22, 11–28 [DOI] [PubMed] [Google Scholar]
- 9. Kim K. S. (2003) Neurological diseases. Pathogenesis of bacterial meningitis. From bacteremia to neuronal injury. Nat. Rev. Neurosci. 4, 376–385 [DOI] [PubMed] [Google Scholar]
- 10. Kim K. S. (2008) Mechanisms of microbial traversal of the blood-brain barrier. Nat. Rev. Microbiol. 6, 625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen S. H., Stins M. F., Huang S. H., Chen Y. H., Kwon-Chung K. J., Chang Y., Kim K. S., Suzuki K., Jong A. Y. (2003) Cryptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J. Med. Microbiol. 52, 961–970 [DOI] [PubMed] [Google Scholar]
- 12. Chang Y. C., Stins M. F., McCaffery M. J., Miller G. F., Pare D. R., Dam T., Paul-Satyaseela M., Kim K. S., Kwon-Chung K. J. (2004) Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 72, 4985–4995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jong A., Wu C. H., Shackleford G. M., Kwon-Chung K. J., Chang Y. C., Chen H. M., Ouyang Y., Huang S. H. (2008a) Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell. Microbiol. 10, 1313–1326 [DOI] [PubMed] [Google Scholar]
- 14. Jong A., Wu C. H., Prasadarao N. V., Kwon-Chung K. J., Chang Y. C., Ouyang Y., Shackleford G. M., Huang S. H. (2008b) Invasion of C. neoformans into human brain microvascular endothelial cells requires protein kinase C-α activation. Cell. Microbiol. 10, 1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bishop A. L., Hall A. (2000) Rho GTPases and their effector proteins. Biochem. J. 348, 241–255 [PMC free article] [PubMed] [Google Scholar]
- 16. Kim K. J., Chung J. W., Kim K. S. (2005) The 67-kDa laminin receptor promotes CNF1-expressing Escherichia coli K1 internalization into human brain microvascular endothelial cells. J. Biol. Chem. 280, 1360–1368 [DOI] [PubMed] [Google Scholar]
- 17. Shin S., Lu G., Cai M., Kim K. S. (2005) Escherichia coli outer membrane protein A adheres to human brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 330, 1199–1204 [DOI] [PubMed] [Google Scholar]
- 18. Wu L., Bernard-Trifilo J. A., Lim Y., Lim S. T., Mitra S. K., Uryu S., Chen M., Pallen C. J., Cheung N. K., Mikolon D., Mielgo A., Stupack D. G., Schlaepfer D. D. (2008) Distinct FAK-Src activation events promote α5β1 and α4β1 integrin-stimulated neuroblastoma cell motility. Oncogene 27, 1439–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Algrain M., Turunen O., Vaheri A., Louvard D., Arpin M. (1993) Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J. Cell Biol. 120, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chung J. W., Hong S. J., Kim K. J., Goti D., Stins M. F., Shin S., Dawson V. L., Dawson T. M., Kim K. S. (2003) Identification of the receptor for cytotoxic necrotizing factor-1 of Escherichia coli K1. J. Biol. Chem. 278, 16857–16862 [DOI] [PubMed] [Google Scholar]
- 21. Voelz K., Johnston S. A., Rutherford J. C., May R. C. (2010) Automated analysis of cryptococcal macrophage parasitism using GFP-tagged cryptococci. PLoS One 5, e15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Aelst L., D'Souza-Schorey C. (1997) Rho GTPases and signaling networks. Genes Dev. 11, 2295–2322 [DOI] [PubMed] [Google Scholar]
- 23. Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science 279, 509–514 [DOI] [PubMed] [Google Scholar]
- 24. Ridley A. J. (2001) Rho family proteins. Coordinating cell responses. Trends Cell Biol. 11, 471–477 [DOI] [PubMed] [Google Scholar]
- 25. Khan N. A., Wang Y., Kim K. J., Chung J. W., Wass C. A., Kim K. S. (2002) Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 277, 15607–15612 [DOI] [PubMed] [Google Scholar]
- 26. Masuda M., Betancourt L., Matsuzawa T., Kashimoto T., Takao T., Shimonishi Y., Horiguchi Y. (2000) Activation of rho through a cross-link with polyamines catalyzed by Bordetella dermonecrotizing toxin. EMBO J. 19, 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Finlay B. B. (2005) Bacterial virulence strategies that utilize RhoGTPases. Curr. Top. Microbiol. Immunol. 291, 1–10 [DOI] [PubMed] [Google Scholar]
- 28. Arpin M., Chirivino D., Naba A., Zwaenepoel I. (2011) Emerging role for ERM proteins in cell adhesion and migration. Cell Adh. Migr. 5, 199–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jarvis W. D., Grant S. (1999) Protein kinase C targeting in antineoplastic treatment strategies. Invest. New Drugs 17, 227–240 [DOI] [PubMed] [Google Scholar]
- 30. Xie Y., Kim K. J., Kim K. S. (2004) Current concepts on Escherichia coli K1 translocation of the blood-brain barrier. FEMS Immunol. Med. Microbiol. 42, 271–279 [DOI] [PubMed] [Google Scholar]
- 31. Nizet V., Kim K. S., Stins M., Jonas M., Chi E. Y., Nguyen D., Rubens C. E. (1997) Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65, 5074–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson S. L., Drevets D. A. (1998) Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. J. infect. Dis. 178, 1658–1666 [DOI] [PubMed] [Google Scholar]
- 33. Jain S. K., Paul-Satyaseela M., Lamichhane G., Kim K. S., Bishai W. R. (2006) Mycobacterium tuberculosis invasion and traversal across an in vitro human blood-brain barrier as a pathogenic mechanism for central nervous system tuberculosis. J. Infect. Dis. 193, 1287–1295 [DOI] [PubMed] [Google Scholar]
- 34. Badger J. L., Stins M. F., Kim K. S. (1999) Citrobacter freundii invades and replicates in human brain microvascular endothelial cells. Infect. Immun. 67, 4208–4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orihuela C. J., Mahdavi J., Thornton J., Mann B., Wooldridge K. G., Abouseada N., Oldfield N. J., Self T., Ala'Aldeen D. A., Tuomanen E. I. (2009) Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J. Clin. Invest. 119, 1638–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jong A. Y., Stins M. F., Huang S. H., Chen S. H., Kim K. S. (2001) Traversal of Candida albicans across human blood-brain barrier in vitro. Infect. Immun. 69, 4536–4544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prasadarao N. V., Wass C. A., Stins M. F., Shimada H., Kim K. S. (1999) Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect Immun. 67, 5775–5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sheen T. R., Ebrahimi C. M., Hiemstra I. H., Barlow S. B., Peschel A., Doran K. S. (2010) Penetration of the blood-brain barrier by Staphylococcus aureus. Contribution of membrane-anchored lipoteichoic acid. J. Mol. Med. 88, 633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deleted in proof
- 40. Fiorentini C., Falzano L., Travaglione S., Fabbri A. (2003) Hijacking RhoGTPases by protein toxins and apoptosis. Molecular strategies of pathogenic bacteria. Cell Death Differ. 10, 147–152 [DOI] [PubMed] [Google Scholar]
- 41. Maruvada R., Zhu L., Pearce D., Zheng Y., Perfect J., Kwon-Chung K. J., Kim K. S. (2012) Cryptococcus neoformans phospholipase B1 activates host cell Rac1 for traversal across the blood-brain barrier. Cell. Microbiol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang S. H., Long M., Wu C. H., Kwon-Chung K. J., Chang Y. C., Chi F., Lee S., Jong A. (2011) Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3). J. Biol. Chem. 286, 34761–34769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bourguignon L. Y., Peyrollier K., Gilad E., Brightman A. (2007) Hyaluronan-CD44 interaction with neural Wiskott-Aldrich syndrome protein (N-WASP) promotes actin polymerization and ErB2 activation leading to β-catenin nuclear translocation, transcriptional up-regulation, and cell migration in ovarian tumor cells. J. Biol. Chem. 282, 1265–1280 [DOI] [PubMed] [Google Scholar]
- 44. Eugène E., Hoffmann I., Pujol C., Couraud P. O., Bourdoulous S., Nassif X. (2002) Microvilli-like structure are associated with the internalization of virulent capsulated Neisseria meningitides into vascular endothelial cells. J. Cell Sci. 115, 1231–1241 [DOI] [PubMed] [Google Scholar]
- 45. Keenan C., Kelleher D. (1998) Protein kinase C and the cytoskeleton. Cell. Signal. 10, 225–232 [DOI] [PubMed] [Google Scholar]
- 46. Sukumaran S. K., Prasadarao N. V. (2002) Regulation of protein kinase C in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J. Biol. Chem. 277, 12253–12262 [DOI] [PubMed] [Google Scholar]
- 47. Charlier C., Nielsen K., Daou S., Brigitte M., Chretien F., Dromer F. (2009) Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 77, 120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Casadevall A. (2010) Cryptococci at the brain gate. Break and enter or use a Trojan horse? J. Clin. Invest. 120, 1389–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shi M., Li S. S., Zheng C., Jones G. J., Kim K. S., Zhou H., Kubes P., Mody C. H. (2010) Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J. Clin. Invest. 120, 1683–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sandoval K. E., Witt K. A. (2008) Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol. Dis. 32, 200–219 [DOI] [PubMed] [Google Scholar]
- 51. Vandenbroucke E., Mehta D., Minshall R., Malik A. B. (2008) Regulation of endothelial junctional permeability. Ann. N.Y. Acad. Sci. 1123, 134–145 [DOI] [PubMed] [Google Scholar]