Abstract
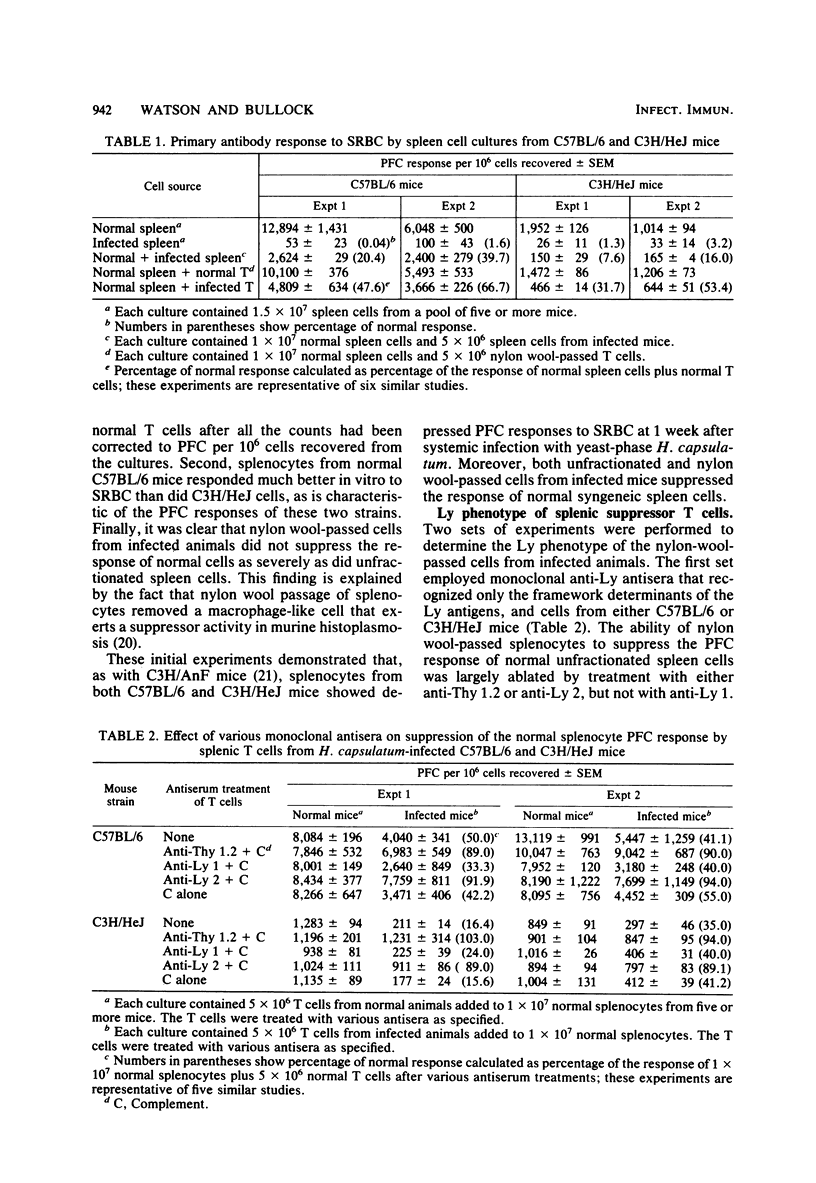
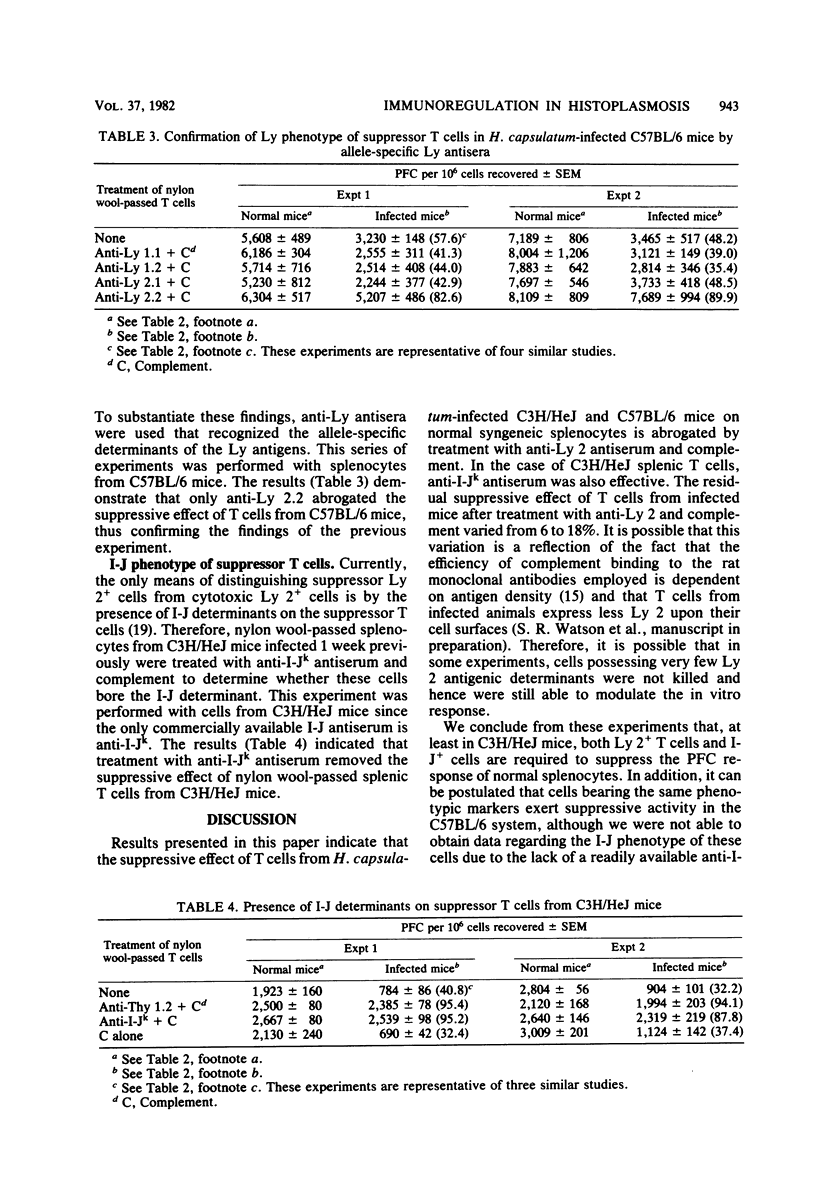
C57BL/6 and C3H/HeJ mice were infected intravenously with 6 X 10(5) yeast-phase Histoplasma capsulatum organisms. After 1 week, splenocytes from both mouse strains showed diminished antibody responses to sheep erythrocytes in vitro; these cells also were able to suppress the response of normal syngeneic cells. Passage of splenocytes from infected mice through a nylon wool column yielded a population enriched for T cells that exerted suppressor activity, although to a smaller extent than did unfractionated cells. Treatment of the T-cell-enriched population from both strains of mice with either anti-Thy 1.2 or anti-Ly 2 and complement resulted in a loss of this immunosuppressive ability. In addition, anti-I-Jk antiserum was effective in ablating the suppressive effect of C3H/HeJ nylon wool-passed spleen cells. The conclusion drawn from these experiments is that the T cells from H. capsulatum-infected animals which are capable of modulating the in vitro plaque-forming cell response to sheep erythrocytes bear Ly 2 and I-J determinants on their surfaces.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: depression of T-cell-dependent and T-effectory responses by activation of splenic suppressor cells. Infect Immun. 1979 Mar;23(3):893–902. doi: 10.1128/iai.23.3.893-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: lymphoid organ histopathology and serological studies. Infect Immun. 1979 Mar;23(3):884–892. doi: 10.1128/iai.23.3.884-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Gershon R. K. Immunological circuits: cellular composition. Fed Proc. 1979 Jun;38(7):2058–2064. [PubMed] [Google Scholar]
- Cantor H., Weissman I. Development and function of subpopulations of thymocytes and T lymphocytes. Prog Allergy. 1976;20:1–64. [PubMed] [Google Scholar]
- Chensue S. W., Boros D. L. Modulation of granulomatous hypersensitivity. I. Characterization of T lymphocytes involved in the adoptive suppression of granuloma formation in Schistosoma mansoni-infected mice. J Immunol. 1979 Sep;123(3):1409–1414. [PubMed] [Google Scholar]
- Chensue S. W., Wellhausen S. R., Boros D. L. Modulation of granulomatous hypersensitivity. II. Participation of Ly 1+ and Ly 2+ T lymphocytes in the suppression of granuloma formation and lymphokine production in Schistosoma mansoni-infected mice. J Immunol. 1981 Jul;127(1):363–367. [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Eardley D. D., Hugenberger J., McVay-Boudreau L., Shen F. W., Gershon R. K., Cantor H. Immunoregulatory circuits among T-cell sets. I. T-helper cells induce other T-cell sets to exert feedback inhibition. J Exp Med. 1978 Apr 1;147(4):1106–1115. doi: 10.1084/jem.147.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Benacerraf B. Helper and suppressor T cell factors. Springer Semin Immunopathol. 1980 May;3(1):93–127. doi: 10.1007/BF00199927. [DOI] [PubMed] [Google Scholar]
- Green D. R., Eardley D. D., Kimura A., Murphy D. B., Yamauchi K., Gershon R. K. Immunoregulatory circuits which modulate responsiveness to suppressor cell signals: characterization of an effector cell in the contrasuppressor circuit. Eur J Immunol. 1981 Dec;11(12):973–980. doi: 10.1002/eji.1830111205. [DOI] [PubMed] [Google Scholar]
- Green W. F., Colley D. G. Modulation of Schistosoma mansoni egg-induced granuloma formation: I-J restriction of T cell-mediated suppression in a chronic parasitic infection. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1152–1156. doi: 10.1073/pnas.78.2.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Herzenberg L. A., Okumura K., Herzenberg L. A., McDevitt H. O. A new I subregion (I-J) marked by a locus (Ia-4) controlling surface determinants on suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):699–712. doi: 10.1084/jem.144.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson D. A., Havens R. A., Bullock W. E. Immunoregulation in disseminated histoplasmosis: characterization of splenic suppressor cell populations. Cell Immunol. 1981 May 15;60(2):287–297. doi: 10.1016/0008-8749(81)90270-7. [DOI] [PubMed] [Google Scholar]
- Warr G. W., Lee J. C., Marchalonis J. J. Null cells in the mouse possess membrane immunoglobulins. J Immunol. 1978 Nov;121(5):1767–1772. [PubMed] [Google Scholar]



