Abstract
Heat-acclimation or salicylic acid (SA) treatments were previously shown to induce thermotolerance in mustard (Sinapis alba L.) seedlings from 1.5 to 4 h after treatment. In the present study we investigated changes in endogenous SA and antioxidants in relation to induced thermotolerance. Thirty minutes into a 1-h heat-acclimation treatment glucosylated SA had increased 5.5-fold and then declined during the next 6 h. Increases in free SA were smaller (2-fold) but significant. Changes in antioxidants showed the following similarities after either heat-acclimation or SA treatment. The reduced-to-oxidized ascorbate ratio was 5-fold lower than the controls 1 h after treatment but recovered by 2 h. The glutathione pool became slightly more oxidized from 2 h after treatment. Glutathione reductase activity was more than 50% higher during the first 2 h. Activities of dehydroascorbate reductase and monodehydroascorbate reductase decreased by at least 25% during the first 2 h but were 20% to 60% higher than the control levels after 3 to 6 h. One hour after heat acclimation ascorbate peroxidase activity was increased by 30%. Young leaves appeared to be better protected by antioxidant enzymes following heat acclimation than the cotyledons or stem. Changes in endogenous SA and antioxidants may be involved in heat acclimation.
Factors affecting plant adaptation to thermal extremes have recently been under scrutiny in relation to stress signaling (Foyer et al., 1997; Gong et al., 1998). Increases in AOS are typical of plant responses to biotic and abiotic stress (Foyer et al., 1997), and O2− and H2O2 levels have been shown to increase during HS in plant tissues (Doke et al., 1994; Foyer et al., 1997; Dat et al., 1998). Also, high temperature can alter the integrated system of enzymic and nonenzymic antioxidants involved in detoxification of AOS (Paolacci et al., 1997). However, antioxidant mechanisms and their complex interactions between tissues are only just now being elucidated (Doulis et al., 1997; May et al., 1998; Noctor et al., 1998).
We recently showed that thermotolerance of mustard (Sinapis alba L.) seedlings could be obtained by SA treatment and by heat acclimation (Dat et al., 1998). Either treatment induced a transient increase in H2O2 levels within 5 min, and then during the period of induced thermotolerance (1.5–4 h after treatment), H2O2 levels and catalase activity declined. We have also found that thermotolerance can be induced in potato microplant tissues by treatment with acetylsalicylic acid or H2O2 (Lopez-Delgado et al., 1998). These observations suggest that SA could be involved in heat acclimation and that its action may be linked to oxidative stress.
In the present paper we explore further the possible involvement of SA in heat-stress physiology using the mustard seedling system characterized by Dat et al. (1998), in which exogenous SA can induce a period of thermotolerance similar to that of conventional heat acclimation. If endogenous SA has a function during heat acclimation, changes in SA levels would be expected. Many studies have shown that resistance responses to infection are mediated by endogenous SA (Mur et al., 1997). Ozone and UV light also induce SA accumulation (Yalpani et al., 1994; Sharma et al., 1996), as does high-light-induced H2O2 accumulation in catalase-deficient transgenic tobacco (Chamnongpol et al., 1998).
There is also evidence that SA can alter the antioxidant capacity in plants (Chen et al., 1997; Fodor et al., 1997; Rao et al., 1997). Therefore, we compared changes in the antioxidant system during thermotolerance induced by either SA treatment or heat acclimation to explore whether SA might be acting through this system.
MATERIALS AND METHODS
Growth and Treatment of Plants
Mustard (Sinapis alba L.) seedlings (Kings Seeds, Essex, UK) were grown for 8 d, as described by Dat et al. (1998). For acclimation treatments, plants were exposed to a nonlethal high temperature (air temperature, 45°C) for 1 h in the dark. Changes in fresh weight during heat treatment were <10%. For SA treatments, plants were sprayed with a 100 μm solution of SA (Sigma) adjusted to pH 7.0 with KOH. Water used in the control sprays was also adjusted to pH 7.0 with KOH.
Measurement of SA
Endogenous free SA levels were measured in 1.2 g of shoot tissue by GC-MS with a [2H3]SA internal standard, according to the method of Scott and Yamamoto (1994). The shoot tissue consisted of the apical region of the shoot, including the cotyledons. Total SA (free and glucosylated) was also determined in aliquots of each sample by modification of the method of Malamy et al. (1992): Unfractionated aqueous extract (0.5 mL) containing the [2H3]SA internal standard was added to 0.5 mL of β-glucosidase (4 units; Sigma) in 0.2 m sodium acetate buffer (pH 4.5/acetic acid), incubated overnight at 37°C, and then analyzed as for free SA. Glucosylated SA was calculated as the difference between total SA and free SA determined for the two parts of each extract with reference to the same added internal standard.
Measurement of Ascorbate and Glutathione
Antioxidant metabolite content was determined in 0.5 g of shoot tissue. The shoot tissue (as defined above) was ground to a fine powder in liquid N2 and 1 mL of ice-cold 2.5 m HClO4 was added. The crude extracts were centrifuged (1 pulse, 16,000g), and the supernatant was collected and separated into two samples (400 μL each) kept on ice. The pellets were resuspended in 80% acetone (all chlorophyll was converted to pheophytin during acid extraction). Pheophytin content was determined following the method of Vernon (1960) and used as an estimate of total chlorophyll content (Doulis et al., 1997). AA and DHA were assayed by following the change in A265 after the addition of ascorbate oxidase, according to the method of Foyer et al. (1983). GSH and GSSG were assayed following the change in A412 after the addition of DTNB and GR, respectively, according to the method of Griffiths (1980).
Measurement of Antioxidant Enzymes
Antioxidant enzyme activity was determined in 0.5 g of shoot tissue (as defined above) or in 0.15 to 0.5 g of leaf, cotyledon, or stem tissue, which was finely ground in liquid N2. APX was measured spectrophotometrically by monitoring the change in A290, according to the method of Nakano and Asada (1987). GR was measured by following the change in A340, according to the method of Foyer and Halliwell (1976). MDHAR was assayed by following the change in A340 after the addition of ascorbate oxidase. DHAR was assayed by monitoring the change in A265, as described by Miyake and Asada (1992). Chlorophyll content was measured using 50 μL of extract in 80% acetone, according to the method of Arnon (1949).
Statistical Analysis
Data were analyzed by analysis of variance and Student's t test. Significance tests were performed on three experiments (n = 6–9; each replicate was an average of 3 seedlings) for measurements of antioxidant enzymes and metabolites and on three to four experiments (n = 3–10, each replicate was an average of 10–15 seedlings) for SA measurements.
RESULTS
Effect of Heat Acclimation on Endogenous SA
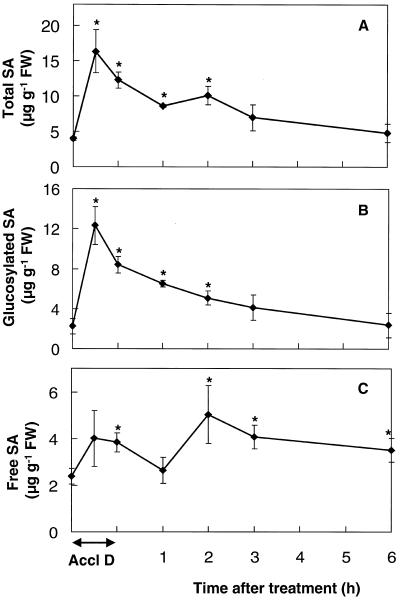
Endogenous SA levels were determined in shoots of mustard seedlings during and following heat acclimation (Fig. 1). Total SA (free and glucosylated) was rapidly and significantly increased to more than 400% of control levels by 30 min after the start of the acclimation treatment (Fig. 1A). Following this abrupt increase, total SA declined toward control levels during the next 6 h. The increase and subsequent decline in total SA were mostly due to changes in glucosylated SA (Fig. 1B), but changes in free SA were significant (Fig. 1C). Glucosylated SA levels rapidly increased to 550% of the controls during the first 30 min after acclimation and then declined during the next 6 h (Fig. 1B). Free SA increased 60% above control levels during acclimation and was more than 200% of control levels 2 h after acclimation (Fig. 1C). Levels of free SA remained significantly higher than the controls after 6 h (Fig. 1C).
Figure 1.
Endogenous levels of total SA (A), glucosylated SA (B), and free SA (C) in mustard seedling shoots during and following a 1-h temperature-acclimation treatment (45°C) in the dark (Accl D). Bars represent se of at least three samples (n = 3–10), each consisting of 10 to 15 seedlings. Control measurements taken during the course of the experiment showed no significant variation. Levels of free and glucosylated SA in controls kept in the dark for 1 h at 24°C were not significantly different from the controls kept in the light at 24°C, 1 and 2 h after dark treatment (data not shown). Asterisks indicate significant differences from controls (P < 0.05). FW, Fresh weight.
Effects of SA and Heat Acclimation on Antioxidant Metabolites
Levels of DHA and AA and GSSG and GSH were determined in shoots of mustard seedlings during the 6-h period following either spraying with SA or heat acclimation. This time was chosen because increased thermotolerance was maximal between 1.5 and 4 h following either treatment (Dat et al., 1998).
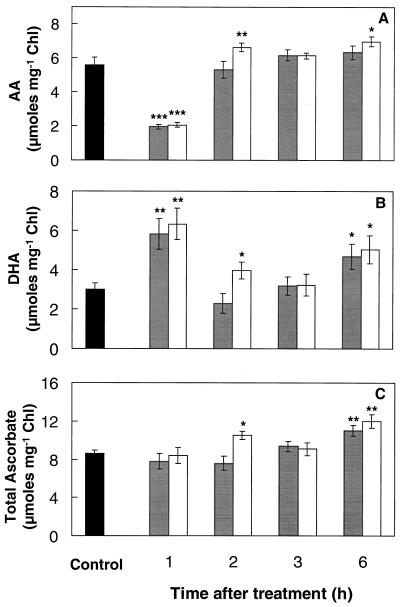
One hour after SA treatment or heat acclimation, levels of AA were significantly reduced (65%), whereas those of DHA were increased by more than 90% (Fig. 2, A and B). This resulted in a drastic decrease in the AA-to-DHA ratio from 1.8 to 0.33 at the 1-h point, although total ascorbate content remained unaffected by either treatment (Fig. 2C). By 2 h, however, the AA-to-DHA ratio had regained control levels, because of increased AA and decreased DHA. By 6 h, DHA and total ascorbate were significantly increased by at least 55% and 28%, respectively, compared with control levels following either treatment (Fig. 2, B and C).
Figure 2.
AA (A), DHA (B), and total ascorbate (C) contents of mustard seedling shoots during the thermoprotection period following either spraying with 100 μm SA solution (gray bars) or a 1-h temperature-acclimation treatment (45°C) in the dark (white bars). Controls (black bars) were kept at 24°C without spraying. Controls sprayed with water were not significantly different from nonsprayed controls. Control measurements taken during the course of the experiment showed no significant variation. Bars represent se (n = 6–10). Asterisks indicate significant differences from controls (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Chl, Chlorophyll.
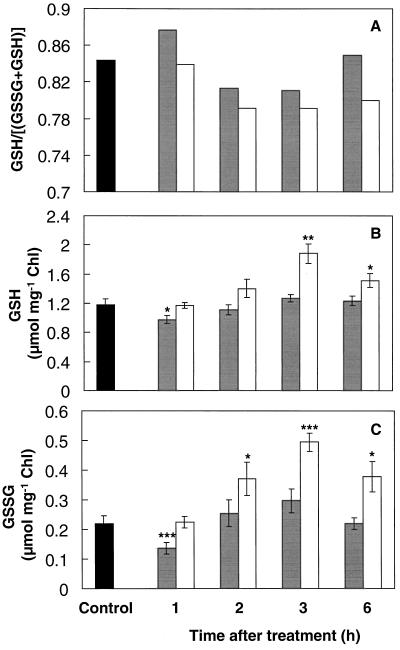
There was a small decline in the glutathione redox ratio ([GSH]/[GSH + GSSG]) in the shoot from 2 h after SA treatment or heat acclimation (Fig. 3A). This decline in the glutathione redox ratio was caused by a greater increase in GSSG than in GSH between the 1st and 3rd h after either treatment (Fig. 3, B and C). Both GSH and GSSG increased between the 1st and 3rd h by at least 30% following either treatment, but both were declining toward control levels by 6 h. However, GSH and GSSH levels were significantly reduced by at least 18% 1 h after spraying with SA (Fig. 3, B and C).
Figure 3.
Glutathione redox ratio (A), GSH (B), and GSSG (C) contents of mustard seedling shoots during the thermoprotection period following either spraying with 100 μm SA solution (gray bars) or a 1-h temperature-acclimation treatment (45°C) in the dark (white bars). Controls (black bars) were kept at 24°C without spraying. Controls sprayed with water were not significantly different from nonsprayed controls. Control measurements taken during the course of the experiment showed no significant variation. Bars represent se (n = 6–10). Asterisks are as in the Figure 2 legend. Chl, Chlorophyll.
Effects of SA and Heat Acclimation on Antioxidant Enzymes
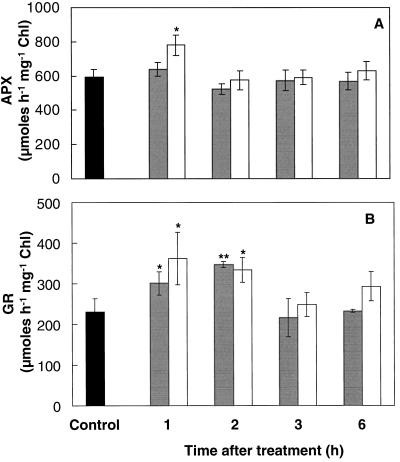
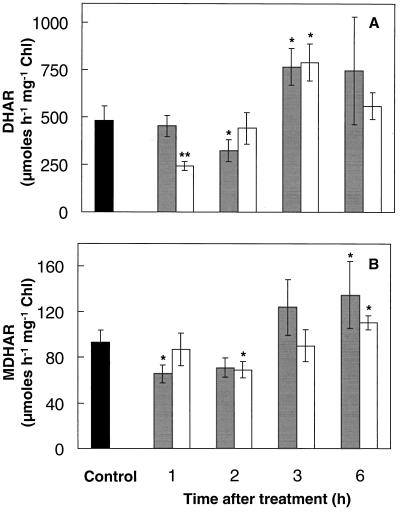
Antioxidant enzyme activities were determined in shoots of mustard seedlings during the period of induced thermotolerance following SA or heat-acclimation treatment. Sequential changes in APX, GR, DHAR, and MDHAR activities were observed following either treatment (Figs. 4 and 5). APX activity remained relatively stable following either treatment, although APX activity was increased by 30% 1 h following heat acclimation (Fig. 4A). GR activity was increased by at least 50% following either treatment (Fig. 4B). The increased GR activity remained high until the 2nd h, after which it declined toward control values (Fig. 4B). Activities of DHAR and MDHAR decreased by at least 25% during the first 2 h following either treatment (Fig. 5). However, DHAR activity was enhanced 60% by 3 h (Fig. 5A), and MDHAR activity was enhanced at least 20% by 6 h (Fig. 5B).
Figure 4.
APX (A) and GR (B) activity of mustard seedling shoots during the thermoprotection period following either spraying with 100 μm SA solution (gray bars) or a 1-h temperature-acclimation treatment (45°C) in the dark (white bars). Controls (black bars) were kept at 24°C without spraying. Controls sprayed with water were not significantly different from nonsprayed controls. Control measurements taken during the course of the experiment showed no significant variation. Bars represent se (n = 6–10). Asterisks are as in the Figure 2 legend. Chl, Chlorophyll.
Figure 5.
DHAR (A) and MDHAR (B) activity of mustard seedling shoots during the thermoprotection period following either spraying with 100 μm SA solution (gray bars) or a 1-h temperature-acclimation treatment (45°C) in the dark (white bars). Controls (black bars) were kept at 24°C without spraying. Controls sprayed with water were not significantly different from nonsprayed controls. Control measurements taken during the course of the experiment showed no significant variation. Bars represent se (n = 6–10). Asterisks are as in the Figure 2 legend. Chl, Chlorophyll.
Seedling death following HS (1.5 h at 55°C in the dark) was characterized by stem collapse 1 to 2 cm below the apex (Dat et al., 1998), and the visual signs of damage following HS were more pronounced on cotyledons than on young leaves. Cotyledons represented approximately 60%, stems about 15%, and young leaves approximately 25% of total shoot tissue sampled in Figures 1–5. Effects of heat acclimation on antioxidant enzymes in young leaves, cotyledons, and stems were therefore compared (Table I). The results confirmed the changes found with shoot measurements (Figs. 4 and 5) but showed that the antioxidant enzyme response to heat acclimation varied between shoot parts. APX activity was significantly (P < 0.05) increased (by 70%) in young leaves 1 h after acclimation but declined afterward. In cotyledon and stem tissue, however, APX activity did not increase significantly. GR activity was significantly (P < 0.05) increased in young leaves (by up to 106%) 1 and 3 h after acclimation but did not increase in the cotyledons until 3 h, whereas changes in the stem were not significant. As in the shoot measurements, DHAR and MDHAR activities showed more complex patterns, tending to decline and in some cases to increase later. Only in young leaves did both enzymes remain at least as high as the controls 1 h after acclimation.
Table I.
Activities of the main antioxidant enzymes in young leaves, cotyledons, and stems of mustard seedlings following heat acclimation in the dark (1 h at 45°C)
| Plant Part and Enzyme | Control | Time after Acclimation (h)
|
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| μmol h−1 mg−1 chlorophyll | ||||
| Young leaves | ||||
| APX | 330 ± 48 | 562 ± 101 | 255 ± 63 | 280 ± 52 |
| GR | 70 ± 7 | 145 ± 25 | 86 ± 10 | 92 ± 17 |
| DHAR | 512 ± 96 | 523 ± 91 | 371 ± 85 | 326 ± 54 |
| MDHAR | 42 ± 7 | 60 ± 9 | 26 ± 6 | 18 ± 7 |
| Cotyledons | ||||
| APX | 210 ± 31 | 253 ± 20 | 215 ± 3 | 164 ± 21 |
| GR | 42 ± 5 | 44 ± 6 | 44 ± 5 | 93 ± 9 |
| DHAR | 410 ± 50 | 159 ± 22 | 204 ± 20 | 262 ± 19 |
| MDHAR | 17 ± 3 | 16 ± 3 | 14 ± 1 | 25 ± 4 |
| Stems | ||||
| APX | 615 ± 69 | 622 ± 133 | 635 ± 117 | 643 ± 70 |
| GR | 592 ± 73 | 718 ± 183 | 511 ± 26 | 585 ± 79 |
| DHAR | 980 ± 208 | 1001 ± 237 | 1024 ± 139 | 1226 ± 110 |
| MDHAR | 129 ± 19 | 87 ± 20 | 63 ± 9 | 170 ± 27 |
Values are ±se (n ≥ 4).
DISCUSSION
This is the first report, to our knowledge, of increased SA levels during heat acclimation (Fig. 1). Conjugated SA accounted for most of the increase, as in other stresses (Yalpani et al., 1994; Sharma et al., 1996; Chamnongpol et al., 1998). The magnitude of the increase in total SA during heat acclimation was similar to other short-term increases (Sharma et al., 1996; Chamnongpol et al., 1998). The short-lived nature of the heat-induced glucosylated SA suggests that this metabolite is not a storage form but could fit the model of Seo et al. (1995), in which glucosylated SA was shown to be a less toxic and more water-soluble transport form of SA in the intercellular spaces. Glucosylated SA can be converted back to SA (Seo et al., 1995), and this mechanism may explain the increase in free SA 2 and 3 h after acclimation (Fig. 1C), because glucosylated SA was declining during the same period.
It is now apparent that changes in SA may play a role not only in pathogenesis (Mur et al., 1997) but also in UV, ozone (Yalpani et al., 1994; Sharma et al., 1996), and heat stresses. The fact that pathogenesis-related proteins may appear in all of these stresses implies some cross-talk between their signaling pathways (Margis-Pinheiro et al., 1993, 1994; Yalpani et al., 1994; Sharma et al., 1996). Exposure to UV can also induce HS proteins (Nedunchezhian et al., 1992). A program common to UV and heat stress responses was proposed following the characterization of the uvh6 mutant of Arabidopsis, which fails to grow at elevated temperatures (Jenkins et al., 1997).
Heat inhibition of SA accumulation at 32°C has been used to characterize the signaling pathway during tobacco mosaic virus infection of tobacco plants (Malamy et al., 1992). In the present study SA levels in mustard increased at the higher temperature of 45°C, indicating that SA accumulation per se is not inhibited by heat treatment (Fig. 1). This implies that the thermosensitive point in N-gene-mediated elicitation of the hypersensitive response (Mur et al., 1997) occurs upstream of SA induction.
Previously, we reported that exogenous SA between 10 and 500 μm could induce thermotolerance in mustard seedlings (Dat et al., 1998). If we assume an even distribution of SA in the tissue, the endogenous SA levels are about 15 to 120 μm, which is within the range of concentrations used to induce thermotolerance. This situation is different from most studies of the induction of systemic acquired resistance by SA, in which treatments with 500 μm to 2 mm are commonly needed (Shirasu et al., 1997). However, 10 to 50 μm SA potentiates the response of soybean cells to an avirulent Pseudomonas syringae pv glycinea strain (Shirasu et al., 1997).
SA levels in young mustard seedlings were of the same order as those reported for soybean and barley (Raskin et al., 1990), and less than those reported for rice (Raskin et al., 1990; Scott and Yamamoto, 1994; Chen et al., 1997) or poplar (Wilbert et al., 1998), although higher than for the mature leaves of tobacco or Arabidopsis used in pathogenesis studies (Yalpani et al., 1994; Mur et al., 1997). Further studies of SA levels in tissues of different ages and species are needed.
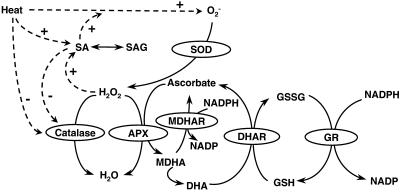
Figure 6 shows possible interactions during heat acclimation among SA, AOS, and antioxidants. Heat stress induces O2− and its product, H2O2, in plant tissues (Doke et al., 1994; Foyer et al., 1997; Dat et al., 1998). Elevation of H2O2 levels can stimulate SA accumulation (Chamnongpol et al., 1998); therefore, the link between heat and SA might be mediated by an increase in H2O2. In turn, SA can increase H2O2 (Rao et al., 1997; Shirasu et al., 1997; Dat et al., 1998). Reduction of catalase activity by SA or heat (Conrath et al., 1995; Dat et al., 1998; Lopez-Delgado et al., 1998) might enhance H2O2 accumulation, as seen in catalase-deficient plants under high light (Chamnongpol et al., 1998). H2O2 may be removed by catalase or by APX of the ascorbate-glutathione antioxidant cycle (Foyer et al., 1997).
Figure 6.
Hypothetical model representing possible effects of heat acclimation on AOS, SA, and the antioxidant system. Continuous arrows show metabolic conversions and dotted arrows show possible regulatory interactions, based on references (see text) relating to various plant species. SOD, Superoxide dismutase.
The period of induced thermotolerance in mustard seedlings was maximal between 1.5 and 4 h after either heat acclimation or SA treatment; during this period, H2O2 levels and catalase activity declined (Dat et al., 1998). The present study revealed further changes in antioxidants with sufficient parallels following either heat or SA treatment, suggesting that common mechanisms might be involved.
Reduced (AA) and oxidized (DHA) forms of ascorbate responded dramatically to SA treatment or heat acclimation, causing a substantial decrease in the AA-to-DHA ratio detected 1 h after treatment (Fig. 2). Similar changes in the AA-to-DHA ratio have been reported for seedlings grown at supraoptimal temperatures (Paolacci et al., 1997). AA functions as the reductant for APX (Fig. 6); therefore, the decline in AA may be linked to the previously reported decline in H2O2 levels following either treatment (Dat et al., 1998). A 30% increase in shoot APX activity was detected 1 h after heat acclimation (Fig. 4A), when analysis of shoot parts revealed a 70% increase in the young leaves (Table I). The increased APX activity, however, had disappeared by 2 h after acclimation. Other stresses causing APX activity to increase include ozone exposure (Kubo et al., 1995), chilling (O'Kane et al., 1996), and high light (Karpinski et al., 1997). In contrast, APX activity decreased in wheat following a 2.5-h exposure to 50°C (Kraus and Fletcher, 1994). In pea, heat increased APX gene transcript levels, but changes in APX activity were less marked (Mittler and Zilinskas, 1992). Several isoforms of APX are found in plants (Karpinski et al., 1997) and changes in total activity will reflect overall trends but not variations in specific isoforms.
DHAR reduces DHA back to AA using GSH as an electron donor, whereas MDHAR reduces monodehydroascorbate directly back to AA using NADPH as a donor (Fig. 6). DHAR and MDHAR in the shoots declined during the first 2 h after either treatment but then recovered above control levels (Fig. 5). Activities of both enzymes also fluctuated in the various plant parts following heat acclimation (Table I).
Despite an initial decrease after SA treatment, both GSH and GSSG increased from the 1st to the 3rd h following either heat or SA treatment (Fig. 3, B and C). Accumulation of GSH during stress has been reported during HS of maize roots (Nieto-Sotelo and Ho, 1986) and during chilling stress in zucchini (Wang, 1995), maize (Kocsy et al., 1996), and Arabidopsis (O'Kane et al., 1996). The increases in GSH and GSSG occurred during the period of induced thermoprotection, when catalase activity declined (Dat et al., 1998). Accumulation of total glutathione under conditions of reduced catalase activity has also been found (Smith, 1985).
The glutathione redox ratio decreased slightly from the 2nd h following either treatment (Fig. 3A). A decreased redox state of the glutathione pool was also observed following a temperature shift of sorghum from 37°C to 27°C (Badiani et al., 1997), growing seedlings at supraoptimal temperatures (Paolacci et al., 1997), and other abiotic stresses (Fadzilla et al., 1997; Karpinski et al., 1997). Such changes in the redox state of the glutathione pool may be involved in acclimatory stress signaling (Foyer et al., 1997; May et al., 1998).
High GR activity maintains the pool of glutathione in the reduced state, allowing GSH to be used by DHAR to reduce DHA to AA (Fig. 6; Noctor et al., 1998). Increased GR activity was detected 1 to 2 h following SA treatment or heat acclimation in shoots (Fig. 4B) and for 3 h following heat acclimation in young leaves (Table I). Increases in GR have been reported for other species during heat stress (Kraus and Fletcher, 1994) and low-temperature acclimation (Wang, 1995). Higher constitutive levels of GR have been linked to chilling tolerance in various species (Walker and McKersie, 1993; Kocsy et al., 1996), and increased expression of GR can enhance tolerance to oxidative stress (Noctor et al., 1998). Multiple forms of GR may be expressed differentially during various stresses, however, and total GR activity may be less significant than changes in individual isoenzymes (Edwards et al., 1990).
The AA-to-DHA ratio may function as a cellular regulatory signal in addition to the glutathione redox state (May et al., 1998). The states of these two redox cycles each fluctuated at different stages following SA or heat treatment; therefore, the potential exists for different combinations of these putative redox signals. The fact that changes in the antioxidant system were induced by an environmental stress and also by a putative signal molecule, SA, is consistent with a signaling role for redox-state changes and with an involvement of heat-induced SA accumulation in such signals.
Death of mustard seedlings following HS (1.5 h at 55°C) involved stem collapse 1 to 2 cm below the apex (Dat et al., 1998). Surviving seedlings often showed signs of damage and chlorophyll bleaching on the cotyledons, but young leaves remained relatively undamaged. The analysis of antioxidant enzymes in the various shoot parts following heat acclimation (Table I) would be consistent with the young leaves being the best-protected parts of the shoot. As discussed above, the enzymes measured showed greater or earlier increases or slower declines in the young leaves than in the more vulnerable cotyledons or stem.
In conclusion, this study showed that high temperature increased total endogenous SA rapidly, whereas SA treatment and heat acclimation induced comparable sequences of changes in the ascorbate and glutathione pools and antioxidant enzymes. Consequently, we propose that increases in endogenous SA and changes in antioxidants may be involved in heat acclimation in mustard.
ACKNOWLEDGMENTS
We are grateful to J.K. Heald for operation of the GC-MS and to H.B.J. Vanacker for technical advice. We thank Dr. H. Lopez-Delgado for valuable discussions and Dr. G. Noctor for critical reading of the manuscript.
Abbreviations:
- AA
reduced form of ascorbate
- AOS
active oxygen species
- APX
ascorbate peroxidase
- DHA
dehydroascorbate
- DHAR
dehydroascorbate reductase
- GR
glutathione reductase
- GSSG
oxidized glutathione
- HS
heat shock
- MDHAR
monodehydroascorbate reductase
- SA
salicylic acid
LITERATURE CITED
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani M, Paolacci AR, Fusari A, D'Ovidio R, Scandalios JG, Porceddu E, Sermanni GG. Non-optimal growth temperatures and antioxidants in the leaves of Sorghum bicolor (L.) Moench. II. Short-term acclimation. J Plant Physiol. 1997;151:409–421. [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, Van Montagu M, Inzé D, Van Camp W. Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA. 1998;95:5818–5823. doi: 10.1073/pnas.95.10.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Iyer S, Caplan A, Klessig DF, Fan B. Differential accumulation of salicylic acid and salicylic acid-sensitive catalase in different rice tissues. Plant Physiol. 1997;114:193–201. doi: 10.1104/pp.114.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath U, Chen Z, Ricigliano JR, Klessig DF. Two inducers of plant defence responses, 2,6-dichloroisonicotinic acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA. 1995;92:7143–7147. doi: 10.1073/pnas.92.16.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dat JF, Lopez-Delgado H, Foyer CH, Scott IM. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998;116:1351–1357. doi: 10.1104/pp.116.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doke N, Miura Y, Leandro MS, Kawakita K. Involvement of superoxide in signal transduction: responses to attack by pathogens, physical and chemical shocks, and UV irradiation. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defence Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 177–197. [Google Scholar]
- Doulis AG, Debian N, Kingston-Smith AH, Foyer CH. Differential localization of antioxidants in maize leaves. Plant Physiol. 1997;114:1031–1037. doi: 10.1104/pp.114.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EA, Rawsthorne S, Mullineaux PM. Subcellular distribution of multiple forms of glutathione reductase in leaves of pea (Pisum sativum L.) Planta. 1990;180:278–284. doi: 10.1007/BF00194008. [DOI] [PubMed] [Google Scholar]
- Fadzilla NM, Finch RP, Burdon RH. Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. J Exp Bot. 1997;48:325–331. [Google Scholar]
- Fodor J, Gullner G, Adam AL, Barna B, Komives T, Kiraly Z. Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid in tobacco. Plant Physiol. 1997;114:1443–1451. doi: 10.1104/pp.114.4.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant. 1997;100:241–254. [Google Scholar]
- Foyer CH, Rowell J, Walker D. Measurements of the ascorbate content of spinach leaf protoplasts and chloroplasts during illumination. Planta. 1983;157:239–244. doi: 10.1007/BF00405188. [DOI] [PubMed] [Google Scholar]
- Gong M, Van der Luit AH, Knight MR, Trewavas AJ. Heat-shock-induced changes in intracellular Ca2+ level in tobacco seedlings in relation to thermotolerance. Plant Physiol. 1998;116:429–437. [Google Scholar]
- Griffiths OW. Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Hennig J, Malamy J, Grynkrawicz G, Indulski J, Klessig DF. Interconversion of the salicylic acid signal and its glucoside in tobacco. Plant J. 1993;4:593–600. doi: 10.1046/j.1365-313x.1993.04040593.x. [DOI] [PubMed] [Google Scholar]
- Jenkins ME, Suzuki TC, Mount DW. Evidence that heat and ultraviolet radiation activate a common stress-response program in plants that is altered in the uvh6 mutant of Arabidopsis thaliana. Plant Physiol. 1997;115:1351–1358. doi: 10.1104/pp.115.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell. 1997;9:627–640. doi: 10.1105/tpc.9.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsy G, Brunner M, Ruegsegger A, Stamp P, Brunold C. Glutathione synthesis in maize genotypes with different sensitivities to chilling. Planta. 1996;198:365–370. [Google Scholar]
- Kraus TE, Fletcher A. Paclobutrazol protects wheat seedlings from heat and paraquat injury. Is detoxification of active oxygen species involved? Plant Cell Physiol. 1994;35:45–52. [Google Scholar]
- Kubo A, Saji H, Tanaka K, Kondo N. Expression of Arabidopsis cytosolic ascorbate peroxidase gene in response to ozone or sulphur dioxide. Plant Mol Biol. 1995;29:479–489. doi: 10.1007/BF00020979. [DOI] [PubMed] [Google Scholar]
- Lopez-Delgado H, Dat JF, Foyer CH, Scott IM. Induction of thermotolerance in potato microplants by acetylsalicylic acid and H2O2. J Exp Bot. 1998;49:713–720. [Google Scholar]
- Malamy J, Hennig J, Klessig DF. Temperature-dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell. 1992;4:359–366. doi: 10.1105/tpc.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margis-Pinheiro M, Marivet J, Burkard G. Bean class IV chitinase gene: structure, developmental expression and induction by heat stress. Plant Sci. 1994;98:163–173. [Google Scholar]
- Margis-Pinheiro M, Martin C, Didierjean L, Burkard G. Differential expression of bean chitinase genes by virus infection, chemical treatment and UV irradiation. Plant Mol Biol. 1993;22:659–668. doi: 10.1007/BF00047406. [DOI] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot. 1998;49:649–667. [Google Scholar]
- Mittler R, Zilinskas BA. Molecular cloning and characterization of a gene encoding pea cytosolic ascorbate peroxidase. J Biol Chem. 1992;262:21802–21807. [PubMed] [Google Scholar]
- Miyake C, Asada K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product of monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 1992;33:541–553. [Google Scholar]
- Mur LAJ, Bi Y-M, Darby RM, Firek S, Draper J. Compromising early salicylic acid accumulation delays the hypersensitive response and increases viral dispersion during lesion establishment in TMV-infected tobacco. Plant J. 1997;12:1113–1126. doi: 10.1046/j.1365-313x.1997.12051113.x. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28:131–140. [Google Scholar]
- Nedunchezhian N, Annamalainathan K, Kulandaivelu G. Induction of heat shock-like proteins in Vigna sinensis seedlings growing under ultraviolet-B (280–320 nm) enhanced radiation. Physiol Plant. 1992;85:503–506. [Google Scholar]
- Nieto-Sotelo J, Ho T-HD. Effect of heat shock on the metabolism of glutathione in maize roots. Plant Physiol. 1986;82:1031–1035. doi: 10.1104/pp.82.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer CH. Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transgenic plants. J Exp Bot. 1998;49:623–647. [Google Scholar]
- O'Kane D, Gill V, Boyd P, Burdon R. Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana callus. Planta. 1996;198:371–377. doi: 10.1007/BF00620053. [DOI] [PubMed] [Google Scholar]
- Paolacci AR, Badiani M, D'Annibale A, Fusari A, Matteucci G. Antioxidants and photosynthesis in the leaves of Triticum durum Desf. seedlings acclimated to non-stressing high temperatures. J Plant Physiol. 1997;150:381–387. [Google Scholar]
- Rao MV, Paliyath G, Ormrod P, Murr DP, Watkins CB. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Plant Physiol. 1997;115:137–149. doi: 10.1104/pp.115.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I, Skubatz H, Tang W, Meeuse BJD. Salicylic acid in thermogenic and non-thermogenic plants. Ann Bot. 1990;66:369–373. [Google Scholar]
- Scott IM, Yamamoto H. Mass spectrometric quantification of salicylic acid in plant tissues. Phytochemistry. 1994;37:335–336. [Google Scholar]
- Seo S, Ishizuka K, Ohashi Y. Induction of salicylic acid β-glucosidase in tobacco leaves by exogenous salicylic acid. Plant Cell Physiol. 1995;36:447–453. [Google Scholar]
- Sharma YK, León J, Raskin I, Davis KR. Ozone-induced responses in Arabidopsis thaliana—the role of salicylic acid in the accumulation of defence-related transcripts and induced resistance. Proc Natl Acad Sci USA. 1996;93:5099–5104. doi: 10.1073/pnas.93.10.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu K, Nakajima H, Rajasekhar VK, Dixon RA, Lamb C. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defence mechanisms. Plant Cell. 1997;9:261–270. doi: 10.1105/tpc.9.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IK. Stimulation of glutathione synthesis in photorespiring plants by catalase inhibitors. Plant Physiol. 1985;79:1044–1047. doi: 10.1104/pp.79.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon LP. Spectrophotometric determination of chlorophylls and pheophytins in plant extracts. Anal Chem. 1960;32:1140–1150. [Google Scholar]
- Walker MA, McKersie BD. Role of ascorbate-glutathione antioxidant system in chilling resistance in tomato. J Plant Physiol. 1993;141:234–239. [Google Scholar]
- Wang CY. Temperature preconditioning affects glutathione content and glutathione reductase activity in chilled zucchini squash. J Plant Physiol. 1995;145:148–152. [Google Scholar]
- Wilbert SM, Ericsson LH, Gordon MP. Quantification of jasmonic acid, methyl jasmonate, and salicylic acid in plants by capillary liquid chromatography electrospray tandem mass spectrometry. Anal Biochem. 1998;257:186–194. doi: 10.1006/abio.1997.2555. [DOI] [PubMed] [Google Scholar]
- Yalpani N, Enyedi AJ, León J, Raskin I. Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta. 1994;193:372–376. [Google Scholar]