Background: The pathogenic mechanism of Serratia marcescens is poorly understood.
Results: S. marcescens kills immune cells via an lipopolysaccharide- and flagella-dependent mechanism.
Conclusion: S. marcescens suppresses innate immunity by killing immune cells.
Significance: This is the first evidence to suggest that S. marcescens evades the immune system.
Keywords: Apoptosis, Innate Immunity, Insect Immunity, Lipopolysaccharide (LPS), Macrophages
Abstract
Injection of Serratia marcescens into the blood (hemolymph) of the silkworm, Bombyx mori, induced the activation of c-Jun NH2-terminal kinase (JNK), followed by caspase activation and apoptosis of blood cells (hemocytes). This process impaired the innate immune response in which pathogen cell wall components, such as glucan, stimulate hemocytes, leading to the activation of insect cytokine paralytic peptide. S. marcescens induced apoptotic cell death of silkworm hemocytes and mouse peritoneal macrophages in vitro. We searched for S. marcescens transposon mutants with attenuated ability to induce apoptosis of silkworm hemocytes. Among the genes identified, disruption mutants of wecA (a gene involved in lipopolysaccharide O-antigen synthesis), and flhD and fliR (essential genes in flagella synthesis) showed reduced motility and impaired induction of mouse macrophage cell death. These findings suggest that S. marcescens induces apoptosis of host immune cells via lipopolysaccharide- and flagella-dependent motility, leading to the suppression of host innate immunity.
Introduction
Living organisms are continuously in danger of infection by environmental pathogens, such as soil bacteria. Environmental pathogens cause infectious diseases that can be particularly dangerous to aged humans and patients with chronic disease. The Gram-negative bacterium Serratia marcescens is an environmental pathogen that infects a wide range of hosts, such as plants, invertebrates, and vertebrates (1). In compromised humans, S. marcescens causes respiratory infection, urinary tract infection, meningitis, and sepsis (2). Strains of S. marcescens that are resistant to various antibiotics, including β-lactam, aminoglycoside, and fluoroquinolone, have recently emerged (3). Although overcoming S. marcescens infection has gained clinical importance over the last 40 years, the underlying mechanism of the pathogenesis of S. marcescens remains poorly understood.
To investigate the virulence mechanisms of pathogens, infection experiments must be performed with the appropriate model animals. Invertebrates possessing simple biologic systems have recently gained attention as model hosts for studies of infectious diseases (4, 5). We previously postulated the usefulness of a bacterial infection model using the silkworm Bombyx mori due to their low cost and ease of handling (e.g. injection into either the bloodstream or the gut is possible, and each organ can be dissected for biochemical and pharmacologic experiments), and the absence of ethical problems associated with the use of mammalian models (6–8). Silkworms are killed by infection with human pathogens such as Staphylococcus aureus, and are cured by antibiotics clinically effective for humans (6, 7). Among mutant strains of S. aureus in which genes with unknown functions were disrupted, we previously identified novel virulence genes, cvfA, cvfB, and cvfC, by screening using the silkworm infection model (9, 10). Furthermore, the silkworm model is applicable for assessing the virulence of microorganisms isolated from environmental sources (11, 12). Therefore, we consider silkworms to be a suitable model host for examining the pathogenesis of S. marcescens.
Insects such as silkworms lack antibody-producing organs, but possess innate immune systems to combat infectious agents. Invertebrate innate immune systems are similar to those of mammals (13). In silkworms, immune reactions are categorized as either humoral or cellular. Humoral immunity is represented by the production of antimicrobial peptides (AMPs)2 (14, 15), and cellular immunity includes phagocytosis of microorganisms by blood cells (hemocytes) (16). Our recent studies have focused on an insect cytokine named paralytic peptide (PP) that regulates both humoral and cellular immunity in silkworms (17, 18). Nakahara et al. (19) originally reported that PP has biologic activity such as paralysis accompanied by muscle contraction, and induces morphologic changes in silkworm hemocytes. Injection of bacterial cell wall components in the silkworm blood (hemolymph) induces the conversion of PP from an inactive precursor to the active form (17). Moreover, treatment with a cytotoxic reagent inhibits the in vitro PP activation triggered by microbial factors, suggesting that live hemocytes are required in the process (17). The active form of PP induces both the expression of AMP genes in the fat body and the promotion of hemocyte phagocytosis of bacteria (18). Thus, similar to mammalian cytokines, insect cytokine PP seems to be involved in global regulation of multiple immune responses. The common features of the innate immune systems between silkworms and mammals make the silkworm a suitable model for investigating S. marcescens infection. Here we describe that S. marcescens suppresses innate immune reactions by killing immune cells of silkworms and mice.
EXPERIMENTAL PROCEDURES
Animals, Bacteria, and Reagents
Silkworm eggs (Hu·Yo × Tukuba·Ne) were purchased from Ehime Sanshu (Ehime, Japan). Silkworm larvae were reared on an antibiotic-free artificial diet at 27 °C. C57BL/6J mice were purchased from CLEA Japan. MyD88 knock-out mice were provided by Dr. Shizuo Akira (University of Osaka), Dr. Kaori Denda-Nagai, Dr. Nobuaki Higashi, and Dr. Tatsuo Irimura (University of Tokyo). S. marcescens 2170 strain and a methicillin-susceptible S. aureus strain (MSSA-1) were harvested from Brain Heart Infusion broth (BD Biosciences) after overnight culture at 30 °C. Glucan from bakers' yeast was purchased from Oriental Yeast Co., Ltd. The glucan was suspended in saline and sonicated before use. The active form of PP was chemically synthesized, as described previously (19). SP600125, ML3403, and wortmannin, pharmacologic inhibitors of JNK, p38, and PI3K, respectively, were purchased from Calbiochem, and dissolved in DMSO. Ac-DEVD-CHO and Z-VAD-fmk, caspase inhibitors, were purchased from Sigma and BIOMOL, respectively.
Infection of Silkworm Larvae
Fifth instar larvae of day 2 were injected with 50 μl of bacterial cells suspended in saline. The supernatant of the bacterial culture was prepared by centrifugation at 6000 × g for 5 min followed by filtration through Millex-GV 0.22-μm Durapore membrane filters (Millipore). Heat-killed bacteria were obtained by autoclaving the bacteria at 121 °C for 20 min. Larvae were injected with various bacterial samples and kept at 27 °C without feeding, and survival rates were determined. Survival curves plotted using the Kaplan-Meier method were tested for significance using the log rank test.
Measurement of Hemocyte Viability
Bacterial suspension (50 μl) and pharmacologic JNK inhibitor (1 mm, 50 μl) were injected into the hemolymph through the dorsal surface of the silkworm. Larval legs were cut with scissors and the hemolymph was collected in ice-cold tubes. Approximately 0.2 ml of hemolymph was obtained per larva (day 2 of 5th instar). Ten microliters of each hemolymph sample was mixed with an equal volume of 0.1% trypan blue and immediately observed under a microscope. The numbers of trypan blue-negative and -positive cells were counted using a cytometer.
Detection of the Phosphorylated Form of JNK
Twenty larvae (day 2 of 5th instar) were injected with saline or live S. marcescens cells and incubated at 27 °C for 30 min. Approximately 2 ml of hemolymph was collected in 8 ml of 1 mm benzamidine in phosphate-buffered saline (PBS). After centrifugation at 1000 × g for 5 min at 4 °C, the precipitated cells were lysed with sodium dodecyl sulfate sample buffer. Samples were subjected to SDS-PAGE, and the separated proteins were transferred onto Immobilon-P polyvinylidene fluoride membranes (Millipore). The membranes were sequentially soaked in 5% phosphoBLOCKERTM (Cell Biolabs) dissolved in Tris-buffered saline-Tween 20 (TBST; 20 mm Tris, 130 mm NaCl, 0.1% Tween 20, pH 7.6), anti-active JNK pAb (pTPpY) (Promega number V793A) 1/1000 diluted in the blocking solution, and horseradish peroxidase-linked anti-rabbit antibody (GE Healthcare; 1/5000 diluted in the blocking solution). Bands were detected using Western LightningTM Chemiluminescence Reagent Plus (PerkinElmer Life sciences) and Amersham Biosciences Hyperfilm ECL (GE Healthcare).
Detection of Caspase Activation in Hemocytes
Activation of caspase in hemocytes was detected with the NucViewTM 488 Caspase-3 Assay Kit for Live Cells (Biotium number 30029) according to the manufacturer's instructions with slight modifications. Fifteen larvae (day 2 of 5th instar) were injected with 50 μl of insect physiological saline (IPS; comprising 150 mm NaCl, 5 mm KCl, and 1 mm CaCl2) or an overnight culture of S. marcescens suspended in IPS, and after 1.5 h the hemolymph was collected in ice-cold tubes containing 5 ml of collection buffer (1 mm benzamidine dissolved in IPS). The hemolymph was centrifuged at 400 × g for 5 min, and precipitated cells were suspended in IPS containing 10 μm of the caspase inhibitor Ac-DEVD-CHO. The cells were transferred to a poly-l-lysine chamber slide and incubated at 27 °C for 15 min. Aliquots were removed and 400 μl of NucView 488 caspase-3 substrate diluted in IPS was added. After incubation at 27 °C for 30 min, the cells were fixed in PBS containing 10% formaldehyde for 10 min. Aliquots were then removed and samples were mounted with Prolong® Gold antifade reagent with DAPI (Invitrogen number P36935). Cells were observed under a fluorescence microscope (Leica DFC300 FX). Cells with NucView 488-stained nuclei were detected.
Detection of Apoptotic Hemocytes
Apoptotic hemocytes were detected with a GFP-CertifiedTM Apoptosis/Necrosis Detection Kit (Enzo Life Sciences number ENZ-51002–25) according to the manufacturer's instructions with slight modifications. Ten larvae (day 2 of 5th instar) were injected with 50 μl of IPS or an overnight culture of S. marcescens suspended in IPS, and after 1.5 h the hemolymph was collected in ice-cold tubes containing 5 ml of 1 mm benzamidine dissolved in IPS. The hemolymph was centrifuged at 400 × g for 5 min and washed again with the collection buffer. Precipitated hemocytes were suspended in 500 μl of detection solution (1× binding buffer, apoptosis detection reagent containing Annexin V-EnzoGold (enhanced Cyanine 3) conjugate, necrosis detection reagent containing 7-AAD, and 10 μm of a caspase inhibitor Ac-DEVD-CHO in IPS) and transferred to a poly-l-lysine-coated chamber slide (Iwaki number 4722-040). After 15 min at room temperature, aliquots were removed, and cells were fixed with 2% formaldehyde in PBS for 10 min. The cells were mounted and stained with DAPI as described above.
Muscle Contraction Assay
Measurement of muscle contraction activity in silkworm was described previously (17, 20). The intensity of the muscle contraction was expressed as the contraction value (C), calculated by measuring the maximum length of each specimen before (x cm) and after (y cm) the injection using the formula (x − y)/x (17). To study the effects of preinjection of bacterial cells on glucan- and PP-dependent muscle contraction, 50 μl of bacterial suspension was injected into the hemocoel of the larval specimen, and after 2 h, 100 μl of glucan (50 μg/ml) or 50 μl of active PP (4 μg/ml) was injected.
Measurement of Viability of Mouse Peritoneal Macrophages
Mice (C57BL/6J) were intraperitoneally injected with 100 μl of 2% Brewer thioglycollate medium (Kanto Chemical Co., Inc.), and peritoneal macrophages were collected 3 days later. Macrophages were suspended in PBS (approximately 3 × 106 cells/ml) and incubated in tissue culture-treated 96-well plates for 0.5 to 1 h at 37 °C, and then the supernatant was discarded to remove unattached cells. A bacterial suspension (approximately 2 × 1010 cells/ml) was added and incubated with macrophages for 2 h at 37 °C. The numbers of trypan blue-negative and -positive cells were counted using a cytometer.
Construction of S. marcescens Transposon-inserted and Gene-disrupted Mutants
Transposon-inserted mutants were constructed by conjugation of parent S. marcescens 2170 and Escherichia coli SM10 λpir harboring pUTmini-Tn5-Km1 plasmid as previously described (21, 22). Gene-disrupted mutants were constructed by homologous recombination using pir-dependent plasmid pFS100 or pFS200 (21, 23). See supplemental Methods for the PCR primers used in the construction of gene-disrupted mutants.
Analysis of LPS and Flagella Fractions of S. marcescens
LPS fraction of S. marcescens was prepared by ethanol precipitation as previously described (24). The LPS fraction was analyzed on a 15% SDS-polyacrylamide gel, and the gel was stained with Sil-best stain One (Nacalai Tesque). The flagella fraction of S. marcescens was prepared as previously described (25). The flagellin protein was separated on a 12.5% SDS-polyacrylamide gel, and the gel was stained with Coomassie Brilliant Blue.
Motility Assay of S. marcescens Mutants
Culture medium (10 g of Bacto-Trypton (BD Biosciences) and 5 g of NaCl (Wako Pure Chemical Industries, Ltd.) dissolved in 1 liter of reverse osmosis water) containing 0.4% agar (Nacalai Tesque) was autoclaved for 20 min at 121 °C. Agar medium was poured into a sterile Petri dish and dried for 20 min at room temperature without the lid. Overnight culture (1 μl) of each bacterial strain was spotted on the soft agar and dried for 10 min. The dish was then covered and incubated for 9 h at 30 °C.
Determination of LD50 of S. marcescens against Silkworm Larvae
Fifth instar larvae of day 2 were injected with 2-fold serially diluted suspension of S. marcescens and incubated at 27 °C. After 16 h, the number of viable larvae was counted. LD50 (the lethal dose for 50% of the larvae) values were determined using the Reed-Muench method.
RESULTS
Killing of Silkworms by S. marcescens
Various organisms pathogenic to humans killed silkworms when injected into the hemolymph (6, 12). Among the pathogenic bacteria previously tested in the silkworm infection model, S. marcescens had exceptionally high virulence in silkworms. Injection of 1 × 108 cells/larva of S. aureus was required to kill silkworms within 36 h (Fig. 1A). In contrast, 5 × 106 or 5 × 103 S. marcescens cells/larva killed silkworms within 12 or 36 h, respectively (Fig. 1B). Neither a 1/10-fold dilution of the filtrated culture supernatant nor heat-killed S. marcescens cells killed silkworms within 80 h (Fig. 1), indicating that the high virulence of S. marcescens in silkworms requires live bacterial cells.
FIGURE 1.
Killing of silkworms by S. aureus or S. marcescens. A, overnight culture of S. aureus (1 × 108 or 1 × 107 cfu/larva) was injected into the hemolymph of 10 larvae, and the survival rates were determined. B, overnight culture of S. marcescens (5 × 106 or 5 × 103 cfu/larva), autoclaved S. marcescens cells (5 × 108 cfu/larva), or a 10-fold dilution of the culture supernatant of S. marcescens was injected into the hemolymph of 10 larvae, and the survival rates were determined. The experiment was repeated three times with similar results.
Killing of Hemocytes in the Silkworm Hemolymph by S. marcescens
In general, the ability to attack host immunity is important for pathogens to exert full virulence. We hypothesized that S. marcescens cells effectively kills silkworms by impairing the host immune system. Insect immune responses are divided into two categories: humoral immunity, such as AMP production, and cellular immunity, including phagocytosis by hemocytes. The resistance of S. marcescens to insect AMPs is comparable with that of other pathogens (26). Therefore, we considered the possibility that S. marcescens evades cellular immune responses.
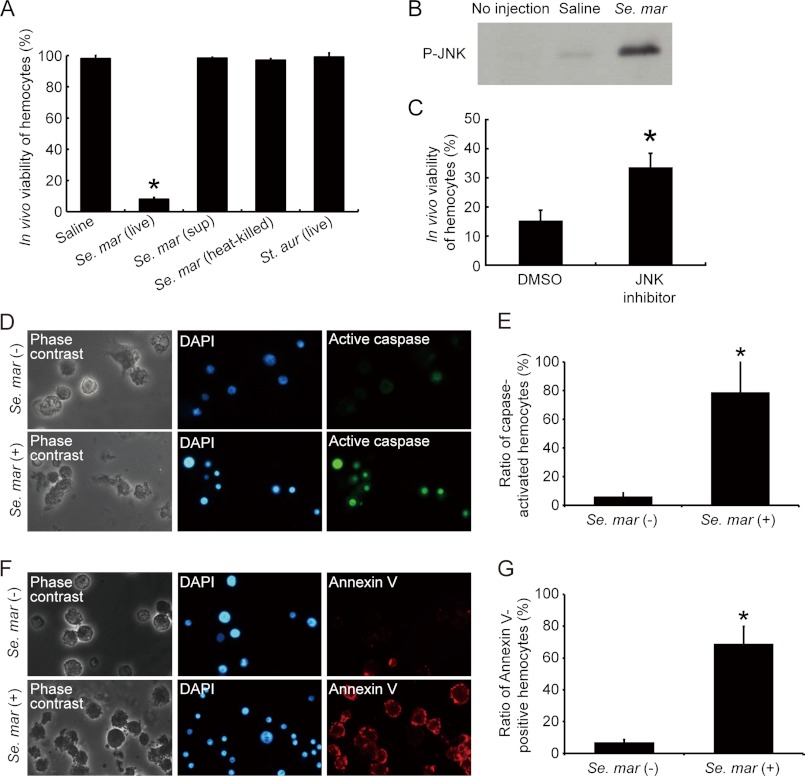
First, we tested the cytotoxicity of S. marcescens in hemocytes from silkworm hemolymph. Three hours after injecting the S. marcescens suspension, most of the isolated hemocytes were stained with trypan blue, an indicator of cell death (Fig. 2A). In contrast, injection of either the filtrated culture supernatant of S. marcescens, heat-killed S. marcescens, or a suspension of live S. aureus did not increase the ratio of trypan blue-positive cells in silkworm hemocytes (Fig. 2A). These results suggest that infection of silkworms with live S. marcescens kills hemocytes.
FIGURE 2.
Induction of hemocyte death in vivo by S. marcescens. A, killing of silkworm hemocytes in vivo by S. marcescens. Saline, a suspension of live S. marcescens (1 × 1010 cfu/ml), a culture supernatant of S. marcescens, a suspension of autoclaved S. marcescens, or a suspension of live S. aureus (2 × 1010 cfu/ml) was injected into the hemolymph of 3 larvae (50 μl/larva), and after 3 h the hemocytes were isolated and stained with trypan blue. The ratio of trypan blue-negative hemocytes was determined. Statistical significance was determined by Student's t test (*, p < 0.05). B, in vivo activation of JNK in silkworm hemocytes by S. marcescens. Saline or S. marcescens suspension (1 × 1010 cfu/ml) was injected into the hemolymph of 20 larvae (50 μl/larva), and the hemocytes were collected after 30 min. The phosphorylated form of JNK in the samples, extracted from equal numbers of hemocytes (6 × 106 cells/50 μl), was detected by Western blot analysis. The experiment was repeated three times and representative data are shown. C, effect of JNK inhibitor on in vivo hemocyte killing by S. marcescens. Fifty microliters of 10% DMSO (a solvent) or the JNK inhibitor SP600125 (1 mm) was preinjected into 3 larvae (50 μl/larva), and 30 min later either saline or S. marcescens suspension (2 × 1010 cfu/ml) was injected (50 μl/larva). After 3 h, the hemocytes were collected and cell viability was determined by trypan blue staining. Data represent mean ± S.D. of 3 larvae. Statistical significance was determined by Student's t test (*, p < 0.05). D and E, NucView 488 staining of caspase-activated hemocytes in S. marcescens-infected silkworms. IPS or S. marcescens suspension (1 × 1010 cfu/ml) was injected into the hemolymph of 15 larvae (50 μl/larva), and the hemocytes were collected after 1.5 h. Caspase-activated cells were detected by using NucView 488-caspase substrate conjugate. When the substrates are cleaved by active caspases, they release DNA-binding dyes that stain the cell nucleus, which produces a staining pattern similar to DAPI. F and G, Annexin V staining of apoptotic hemocytes in S. marcescens-infected silkworms. IPS or S. marcescens suspension (1 × 1010 cfu/ml) was injected into the hemolymph of 10 larvae (50 μl/larva), and the hemocytes were collected after 1.5 h. Cell nuclei were stained with DAPI. Cells undergoing apoptosis were detected using Annexin V-EnzoGold (enhanced Cyanine 3) conjugate, which binds to phosphatidylserine on the outer plasma membrane. Five to seven microscopic areas were observed and 300 to 400 cells were counted to measure the ratio of NucView 488- (E) or Annexin V- (G) positive cells. The statistical significance of differences was determined using Student's t test (*, p < 0.05).
The apoptosis signaling pathways are well conserved among species (27, 28). Factors such as c-Jun NH2-terminal kinase (JNK) and caspases are involved in apoptotic processes in Drosophila and mammals (29, 30). Therefore, we examined whether S. marcescens induces cell death in silkworm hemocytes via activation of these apoptotic factors. We obtained hemocytes from silkworms injected with either saline or S. marcescens, and prepared cell homogenates. Western blot analysis revealed that injection of S. marcescens induced the phosphorylation of JNK within 30 min (Fig. 2B). We then tested the effect of SP600125, a pharmacologic JNK inhibitor, on hemocyte viability after infection by S. marcescens. Preinjection of SP600125 attenuated hemocyte killing, as assessed by trypan blue staining (Fig. 2C). Furthermore, we examined whether caspase was activated and apoptosis occurred in hemocytes of S. marcescens-injected silkworms. Staining with NucViewTM 488-caspase substrate, a conjugate of a caspase substrate DEVD peptide and a DNA-binding dye that stains the nucleus when cleaved by active caspases, revealed that caspase-activated hemocytes were increased by injection of S. marcescens (Fig. 2, D and E). In addition, the surfaces of most hemocytes collected from S. marcescens-injected silkworms were stained with the Annexin V-Cyanine 3 fluorphore conjugate (Fig. 2, F and G). Annexin V is widely used to detect apoptosis because it has a high affinity to phosphatidylserine, which is translocated from the inner to the outer plasma membrane of apoptotic cells. S. marcescens-infected cells were also stained with 7-AAD, a dye that binds to DNA in membrane-permeabilized cells, suggesting that most of the cells were in the late phase of apoptosis (data not shown). Taken together, these findings suggest that S. marcescens infection induces the activation of apoptotic factors, followed by hemocyte death in silkworms.
Suppression of Cytokine Activation via Hemocyte Killing by S. marcescens
We previously reported that PP regulates multiple immune responses and contributes to host defense in silkworms (17, 18). Activation of PP is stimulated by pathogen cell wall components, such as glucan, injected in the hemolymph (17). In this process, hemocytes seem to be required for the recognition of pathogenic components and the subsequent production of reactive oxygen species resulting in PP activation (17). Active PP has a paralytic effect on silkworm larvae accompanied by muscle contraction (17). Thus, injection of glucan into the hemolymph of larval muscle specimens induces contraction via PP activation (17). To examine whether S. marcescens inhibits PP activation under conditions in which hemocytes in a larval specimen are killed by S. marcescens, we first injected a suspension of live S. marcescens cells, and then glucan, a representative PP-activation stimulant, and measured the muscle contraction strength in silkworm larval specimens. When the specimens were injected with saline and then with glucan (Fig. 3, column 1), they exhibited strong muscle contraction (contraction value (C) = 0.28, see “Experimental Procedures” for details regarding the C-value calculation), which seemed to result from PP activation in the hemolymph. Preinjection of live S. marcescens cells suppressed glucan-induced muscle contraction to C = 0.06 (Fig. 3, column 2), whereas heat-killed S. marcescens cells (Fig. 3, column 3) and live S. aureus cells (Fig. 3, column 4) did not. Thus, the effects of live S. marcescens cells, heat-killed S. marcescens cells, and live S. aureus cells on the suppression of glucan-induced muscle contraction (Fig. 3), an indicator of PP activation, were very consistent with their in vivo toxicity to hemocytes (Fig. 2A). On the other hand, when specimens pretreated with live S. marcescens were further injected with the purified active form of PP, the C values were still high (Fig. 3, column 5). Therefore, the responsiveness of muscle specimens to active PP seemed to be retained even under conditions in which hemocytes are killed by live S. marcescens. These results suggest that inhibition of glucan-dependent muscle contraction in silkworm specimens by live S. marcescens was due to the death of the hemocytes upstream of PP activation.
FIGURE 3.
Inhibitory effect of S. marcescens on glucan-induced contraction of silkworm larval muscle specimen. Suspensions of live S. marcescens (2 × 1010 CFU/ml), autoclaved S. marcescens, or live S. aureus (1 × 1010 cfu/ml) were preinjected into larval muscle specimens (50 μl/larva). After 2 h, 100 μl of glucan (50 μg/ml) (G) or 50 μl of active paralytic peptide (4 μg/ml) (PP) was injected, and the contraction value was measured. Data represent mean ± S.D. of 3 or 4 specimens. Statistical significance was determined by Student's t test (*, p < 0.05).
Induction of Apoptosis of Host Immune Cells in Vitro by S. marcescens
As described above, hemocytes in silkworms injected with S. marcescens were killed via JNK activation. We thus hypothesized that S. marcescens acts directly on hemocytes to induce apoptosis. To test this hypothesis, we analyzed the effect of S. marcescens on the viability of hemocytes isolated from the hemolymph of silkworms in vitro. When hemocytes were incubated with live S. marcescens, hemocyte viability decreased after 1.5 h (Fig. 4, A and B). Consistent with the in vivo experiments (Fig. 2A), heat-killed S. marcescens and live S. aureus did not affect hemocyte viability in vitro within 3 h (Fig. 4B). We then tested the effects of a JNK inhibitor on the cytotoxic effect of S. marcescens on hemocytes. Hemocytes isolated from silkworms were pretreated in vitro with SP600125 and incubated with S. marcescens for 3 h. Based on trypan blue staining, hemocytes treated with SP600125 were more viable than control hemocytes (Fig. 4C). Moreover, pretreatment of hemocytes with pharmacologic caspase inhibitors (Ac-DEVD-CHO and Z-VAD-fmk) suppressed hemocyte death induced by S. marcescens in vitro (Fig. 4D). In contrast, inhibitors of other cell-signaling factors such as p38 MAPK and PI3K were not effective against S. marcescens-induced hemocyte killing (supplemental Fig. S1). These findings suggest that S. marcescens acts directly on silkworm hemocytes and activates apoptotic factors such as JNK and caspase, which leads to hemocyte death.
FIGURE 4.
Induction of apoptosis of immune cells in vitro by S. marcescens. A, killing of silkworm hemocytes in vitro by S. marcescens. Hemocytes isolated from silkworm larvae were incubated with S. marcescens cells in saline, and after 3 h the cells were stained with trypan blue and observed under a microscope. B, time course of hemocyte killing by S. marcescens. Hemocytes (1 × 107 cells/ml) were incubated in saline with live cells of S. marcescens (1 × 109 cfu/ml), autoclaved cells of S. marcescens, or live cells of S. aureus (1 × 109 cfu/ml), and stained with trypan blue at the indicated time points. C, effect of a JNK inhibitor on hemocyte killing in vitro. Silkworm hemocytes suspended in PBS (3–4 × 106 cells/ml) were supplied with 100 or 400 μm SP600125, a pharmacologic JNK inhibitor. After 1 h, S. marcescens cells (1–2 × 108 cfu/ml) were added to the hemocytes and the cells were incubated for 3 h. Hemocyte viability was determined by trypan blue staining. Data represent mean ± S.D. of three experiments. Statistical significance was determined by Student's t test (*, p < 0.05). D, effect of caspase inhibitors on hemocyte killing in vitro. Hemocytes suspended in PBS (6–7 × 106 cells/ml) were supplied with Ac-DEVD-CHO (200 μm) or Z-VAD-fmk (400 μm), pharmacologic inhibitors of caspase. After 1 h, S. marcescens cells (1–2 × 108 cfu/ml) were added to the hemocytes and the cells were incubated for 3 h. Hemocyte viability was determined by trypan blue staining. Data represent mean ± S.D. of three experiments. Statistical significance was determined by Student's t test (*, p < 0.05). E, effects of inhibitors of apoptotic signaling factors (JNK and caspase) on killing of mouse macrophages in vitro by S. marcescens. Mouse peritoneal macrophages suspended in PBS (3–4 × 106 cells/ml) were supplied with SP600125 (100 μm), Ac-DEVD-CHO (200 μm), or Z-VAD-fmk (400 μm). After 1 h, S. marcescens cells (1–2 × 1010 cfu/ml) were added to the macrophages and the cells were incubated for 2 h. Macrophage viability was determined by trypan blue staining. Data represent mean ± S.D. of three experiments. Statistical significance was determined by Student's t test (*, p < 0.05).
We then tested whether S. marcescens induced cell death in mammalian immune cells. Similar to silkworm hemocytes, mouse peritoneal macrophages incubated with live S. marcescens were killed (Fig. 4E). Moreover, treatment with a JNK inhibitor (SP600125) or caspase inhibitors (Ac-DEVD-CHO or Z-VAD-fmk) prior to incubation with S. marcescens increased the viability of mouse macrophages (Fig. 4E). These results suggest that S. marcescens induces JNK- and caspase-dependent apoptosis in mouse macrophages as well as in silkworm hemocytes.
Transposon Mutagenesis Screening of S. marcescens Genes Involved in Hemocyte Apoptosis
To identify the virulence genes required to induce apoptosis in the host immune cells, we then screened S. marcescens transposon mutants. Among a total of 1049 transposon mutant strains, we identified 16 strains with attenuated in vitro cytotoxicity to silkworm hemocytes (supplemental Fig. S2). The killing effects on silkworms, based on by the LD50 were attenuated in these mutants; 4 strains (STM91, 447, 673, and 898) had a 30–110-fold increase in the LD50, and the remaining 12 strains had a 2–4-fold increase in the LD50 (data not shown). We determined the genome sequences near the insertion positions of transposons in these 16 strains. Fifteen ORFs in which the transposons were inserted either within or upstream are shown in Table 1 (see also supplemental Fig. S3). Because the genomic sequence was not obtained for the remaining strain, STM149, further analysis of this strain was suspended. Among the determined ORFs, we focused on wecA, which is required for O-glycosylation of LPS, and flhD or fliR, which are essential in flagella biosynthesis. We constructed gene-disrupted S. marcescens mutants of wecA, flhD, or fliR (ΔwecA, ΔflhD, or ΔfliR, respectively) and analyzed their LPS and flagella structures. As expected, the LPS O-antigen was abolished in ΔwecA, whereas that in ΔflhD and ΔfliR seemed to be intact (supplemental Fig. S4A). In addition, the wecA mutant lacked the 39-kDa flagellin protein (supplemental Fig. S4B), consistent with a previous report showing that LPS O-antigen ligase is required for flagella biosynthesis in the Gram-negative bacterium Proteus mirabilis (31). We next confirmed that ΔwecA, ΔflhD, and ΔfliR had impaired hemocyte killing ability compared with the parent strain (Fig. 5, A and B). Moreover, induction of apoptosis in mouse macrophages was impaired in these gene disruptants (Fig. 5, C and D). These results suggest that LPS and flagella were involved in S. marcescens-induced apoptosis of silkworm hemocytes and mouse macrophages.
TABLE 1.
Genome analysis of S. marcescens transposon-inserted mutants with attenuated virulence against silkworm hemocytes
| Strain no. | Gene with inserted transposon | Species with homologous gene | Identity | Gene product function |
|---|---|---|---|---|
| % | ||||
| STM91 | wecA | Serratia proteamaculans | 93 | LPS O-antigen synthesis |
| STM162 | PST family polysaccharide transporter | Serratia odorifera | 86 | Amino sugar metabolism |
| STM261 | citC | Escherichia coli | 94 | Citrate metabolism |
| STM314 | Phage integrase | γ- Proteobacterium | 89 | Lysogeny regulation |
| STM315 | Hypothetical protein PROSTU_00814 | Providencia stuartii | 61 | Unknown |
| STM316 | AraC/XylS family transcriptional activator | Xenorhabdus bovienii | 29 | Resistance to antibiotics and heavy metals |
| STM394 | Permease | S. odorifera | 97 | Membrane transport |
| STM396 | Hypothetical protein ESCAB7627_2151 | Escherichia albertii | 49 | Unknown |
| STM417 | Hypothetical protein Sden_2787 | Shewanella denitrificans | 57 | Unknown |
| STM447 | fliR | S. odorifera | 91 | Flagellar formation (flagellar biosynthetic protein) |
| STM639 | Hypothetical protein H16_A1750 | Ralstonia eutropha | 77 | Unknown |
| STM673 | flgH | S. odorifera | 95 | Flagellar formation (flagellar l-ring protein) |
| STM854 | bsmB | Serratia liquefaciens | 95 | Biofilm formation |
| STM855 | Protein kinase HdNI_03960 | γ-Proteobacterium | 89 | Unknown |
| STM898 | flhD | S. marcescens | 100 | Flagellar formation (flagellar transcriptional activator) |
FIGURE 5.
S. marcescens gene-disrupted mutants with impaired motility and attenuated virulence against silkworm hemocytes and mouse macrophages. A–D, immune cell killing by S. marcescens gene-disrupted mutants. Silkworm hemocytes (A and B) or mouse peritoneal macrophages (C and D) were suspended in PBS and incubated with either S. marcescens wild-type (WT), wecA disrupted mutant (ΔwecA), wecA complemented strain (ΔwecA/pMWwecA), flhD disrupted mutant (ΔflhD), or fliR disrupted mutant (ΔfliR). Data represent mean ± S.D. of three to four experiments. Statistical significance was determined by Student's t test (*, p < 0.05). E, motility of S. marcescens gene disrupted mutants on soft agar plates. Colony size of each strain was measured. Data represent mean ± S.D. of three experiments. Statistical significance was determined by Student's t test (*, p < 0.005).
We then examined whether the LPS or flagella of S. marcescens directly induce the cell death of silkworm hemocytes. The in vitro viability of hemocytes was not decreased by the injection of either an LPS or flagella fraction prepared from S. marcescens wild-type strain within 3 h (data not shown). Moreover, LPS and flagella extracted from wild-type S. marcescens showed neither direct cytotoxicity nor restoration of attenuated hemocyte-killing ability in ΔwecA mutants lacking both components (supplemental Fig. S5). To further examine whether S. marcescens induced cell death via direct toxicity of LPS and flagella, we prepared macrophages from myeloid differentiation factor 88 (MyD88) knock-out mice that lack the potential to respond to stimulation of most Toll-like receptors. S. marcescens still induced cell death in macrophages obtained from MyD88-knock-out mice (supplemental Fig. S6), suggesting that mechanisms independent of Toll-like receptor-MyD88 pathways are involved in the killing process. LPS O-antigen and flagella are required for bacterial motility (32–34). The mutants of wecA, flhD, or fliR constructed in our experiments were not motile on soft agar plates (Fig. 5E). These findings suggest that the motility of S. marcescens via LPS and flagella, and not the toxic effects of these components, is required for efficient killing of hemocytes in the host body or culture medium.
We further determined the LD50 values of ΔwecA, ΔflhD, and ΔfliR in a silkworm infection model. Although the LD50 of the parent strain (WT) was 8 ± 4 cfu/larva, the LD50 of ΔwecA was 8.7 × 103 ± 5.1 × 103 cfu/larva, an approximately 103-fold increase (Fig. 6, p < 0.01). In contrast, the strain complemented with a plasmid harboring the wec gene cluster (ΔwecA/pMWwecA) had an LD50 (6 ± 2 cfu/larva) similar to that of the WT (Fig. 6). The LD50 of flagella mutants ΔflhD and ΔfliR was 1.6 × 102 ± 0.5 × 102 and 2.0 × 102 ± 0.8 × 102 cfu/larva, respectively, a greater than 20-fold increase (Fig. 6, p < 0.01). We constructed LPS- and flagella-double mutants using ΔwecA as the parent strain, named ΔwecAΔflhD and ΔwecAΔfliR, and determined the LD50 values against silkworms. The LD50 values of ΔwecAΔflhD and ΔwecAΔfliR were 1.6 × 104 ± 0.6 × 104 and 1.3 × 104 ± 0.6 × 104 cfu/larva, respectively (Fig. 6). The LD50 of the double mutants was indistinguishable from that of the parent ΔwecA. Together with the above observation that motility was lost in the ΔwecA, ΔflhD, and ΔfliR single mutants (Fig. 5E), these results suggest that motility of S. marcescens has a critical role in silkworm killing.
FIGURE 6.
Determination of the LD50 of S. marcescens mutants on silkworm larvae. Silkworm larvae were injected with a 2-fold serial dilution of bacterial suspension of S. marcescens wild-type (WT), wecA disrupted mutant (ΔwecA), wecA complemented strain (ΔwecA/pMWwecA), flhD disrupted mutant (ΔflhD), fliR disrupted mutant (ΔfliR), wecA and flhD double-mutant (ΔwecA ΔflhD), or wecA and fliR double-mutant (ΔwecA ΔfliR). After 16 h, the silkworm survival rate was determined and LD50 values were calculated. Data represent mean ± S.D. of three to eight experiments. Statistical significance was determined by Student's t test (*, p < 0.01).
DISCUSSION
Mammals protect themselves against environmental pathogens using antibody-producing systems, called acquired immunity, and other systems categorized as innate immunity. Insects like silkworms rely solely on innate immunity for self-defense. Antibody production is impaired in most aged and immunocompromised human patients, and therefore opportunistic infection models using insects as host animals are considered to mimic the pathology of compromised humans. Here, we analyzed the virulence mechanism of a human opportunistic pathogen, S. marcescens, in a silkworm infection model. The data obtained suggest that the high virulence of S. marcescens against silkworm larvae is due to the death of host immune cells, thereby suppressing systemic immune responses.
Cell death pathways in higher organisms are required not only for morphologic formation during developmental stages, but also for immunologic reactions (35). Host cells that are damaged by invading pathogens actively undergo cell death to avoid the systemic spreading of infection (35). Conversely, at the same time, the loss of immune cells seems to slow down the elimination of pathogens in tissues and bloodstream, leading to serious problems such as excessive pathogen proliferation. Aeromonas hydrophila (36) and Group A Streptococcus (37), causative agents of diarrhea and sepsis, induce immune cell death, inhibit cytokine production, and promote bacterial growth in mammalian tissues. Although S. marcescens causes apoptosis-like cell death in cultured Chinese hamster ovary cells (38), there are no reports suggesting that S. marcescens escapes host immunity via the killing of immune cells. The present study is the first to demonstrate that S. marcescens has a strategy to disrupt the immune system to effectively kill the host.
There are few reports that insect cytokines possess immune-modulating function similar to mammalian cytokines. We previously demonstrated that PP, the multifunctional peptide in the hemolymph of silkworms, contributes to the activation of various immune responses and host protection against infections (17, 18). In addition, we reported that hemocytes stimulated by microbial infections are necessary for activating the serine proteases responsible for proteolytic cleavage of the inactive PP precursor to generate the active form of PP (17). The present results suggest that, in the process of S. marcescens infection, hemocytes required for PP activation are killed and therefore the onset of acute immune responses including AMP production and phagocytosis of bacteria are suppressed, leading to severe impairment of host resistance. In mammals, many types of immune cells, such as macrophages and natural killer cells, have critical roles in the early stages of infection by producing various cytokines such as tumor necrosis factor-α and interleukins. In our experiments, we found that S. marcescens induces JNK- and caspase-dependent apoptotic cell death in mouse peritoneal macrophages in vitro. Thus, we speculated that S. marcescens exerts virulence by a common mechanism in insects and mammals; killing host immune cells to impair cytokine production and other immune responses. Although it seems that most aspects of innate immunity are conserved among species, there are some differences between invertebrate and vertebrate immune systems. Therefore, carefully controlled experiments using alternative animal models should be performed to elucidate the pathogenesis of S. marcescens.
Factors involved in cell death reactions are highly conserved among species (27, 28). JNK (39) and caspases (40) have been identified in silkworms as well as in other organisms. Moreover, stress response pathways such as p38 and PI3K are also conserved and are involved in both immune responses (41) and apoptosis (42). Our experiments using pharmacologic inhibitors suggested that activation of JNK but not p38 or PI3K was required for host cell killing by S. marcescens; the possible involvement of other stress-signaling pathways, however, is not ruled out. Some pathogens exert virulence by overactivating stress factors in the host cells. For example, Francisella tularensis causes excess caspase-3 activation in mouse organs (43). Pseudomonas aeruginosa activates the JNK-dependent caspase pathway through a type III secretion system (44, 45). We recently reported that live and heat-killed Porphyromonus gingivalis, a human orthodontic pathogen, causes excessive activation of an immune reaction called melanization in the silkworm hemolymph, leading to the overproduction of reactive oxygen species and activation of caspases in larval tissues (46). In contrast, we did not observe extensive melanization in the hemolymph of silkworms injected with live S. marcescens. Thus, the underlying mechanism of apoptosis induction seems to differ between P. gingivalis and S. marcescens. Exotoxins (47) and cell wall components (48) of certain bacteria induce host cell death. In the present experiments, acute killing of hemocytes by the culture supernatant and heat-killed cells of S. marcescens was not observed. Therefore, we considered a novel mechanism in which S. marcescens induces hemocyte death by direct interaction. Although the type III secretion system is one such mechanism, we know of no reports to date that have examined its presence and function in Serratia. On the other hand, Serratia ShlB protein is known as a transporter that mediates two-partner secretion (49). Identification of the responsible factors on the surface of the bacteria is currently underway in our laboratory.
Kurz and colleagues (24) performed in vivo screening using Caenorhabditis elegans to search for S. marcescens virulence factors and found that gene mutations of enzymes involved in LPS production attenuated the virulence of S. marcescens in nematodes. Whether these factors contribute to cell death in immune cells, however, has yet to be determined. In this report, we screened for mutants of S. marcescens with decreased killing abilities against hemocytes and identified virulence genes required for the apoptosis induction of hemocytes. Among those, bsmB is required for adhesion to solid surfaces and biofilm formation in a closely related bacterium Serratia liquifaciens (50, 51), whereas its role in virulence against host cells and animals is not yet clear. Another gene, citC, encoding citrate lyase ligase, which is an enzyme involved in anaerobic citrate metabolism in some bacteria (52), might contribute to intracellular infection of Shigella flexneri to HeLa cells (53). We identified several other genes whose functions have not been fully characterized. Studies of the functions of those genes might help to clarify the cell death-inducing mechanism of S. marcescens and other microbes with homologous genes.
Mutants of the wecA gene (responsible for LPS synthesis) and the flhD and fliR genes (responsible for flagella synthesis) had severely impaired host cell-killing phenotypes. These genes contribute to motility and virulence processes in several different bacterial species (32, 54). Therefore, we concluded that S. marcescens motility, which is dependent on LPS and flagella, was critical to the high pathogenicity of this bacterium on silkworms. Other possibilities, however, could not be ruled out. LPS and flagella are both major bulky complexes on the bacterial surface that could affect extracellular secretion and adhesion (55, 56). Therefore, the mutants we obtained might have impaired secretion and adhesion, which could attenuate killing ability. In our experimental conditions, neither the LPS nor flagella fraction prepared from S. marcescens caused cell death, but it is still possible that the intact forms of these components on the bacterial surface possess cytotoxic activity and that this activity was lost during sample preparation. Moreover, LPS might show cytotoxic effects via mechanisms other than direct stimulation of Toll-like receptor-MyD88 pathways, because complex activation mechanisms of LPS-dependent apoptosis involving other receptors, such as scavenger receptors, have been reported in mouse macrophages (57). Further studies are required to identify the executive factor and downstream mechanisms of host cell killing.
The LD50 of the wecA gene disrupted mutant (ΔwecA) was more than 10-fold higher than that of the flhD or fliR mutants (ΔflhD or ΔfliR), whereas those mutants were completely immotile on soft agar plates (Fig. 5E). Although we concluded that the motility of S. marcescens is important for silkworm killing, these results suggest that wecA might be involved in processes other than motility. In contrast to the significantly different killing effects of these mutants on silkworms, the numbers of mutant bacteria required for hemocyte killing were comparable (supplemental Fig. S7). Thus, the above difference in LD50 against silkworms does not seem to be explained by differences in the hemocyte killing ability, but could be due to other factors (“host killing factors”), likely regulated by wecA-dependent O-glycosylation, that directly cause silkworm death. Injection of a higher concentration of S. marcescens culture supernatant than that shown in Fig. 1 killed silkworms (whereas a higher concentration of supernatant still did not induce hemocyte cell death, similar to that shown in Fig. 2A), and that its host killing activity was partially dependent on wecA.3 Identification of the wecA-dependent host-killing factor in the supernatant is now underway. Determining the functional difference between wecA and flagella synthetic genes (flhD and fliR) in regard to animal killing might lead to better understanding of the virulence mechanism of S. marcescens.
Acknowledgments
We are grateful to Dr. Shizuo Akira (Osaka University), and Dr. Kaori Denda-Nagai, Dr. Nobuaki Higashi, and Dr. Tatsuo Irimura (University of Tokyo) for providing the MyD88 knock-out mice.
This work was supported by a Grant-in-Aid for Young Scientists (B) (21790063) and a Grant-in-Aid for Japan Society for the Promotion of Science Fellows (21-10519) from JSPS. This study was supported in part by a Grant-in-Aid for Scientific Research (B) (20390021), a Grant-in-Aid for Scientific Research on Priority Areas (21022015), and grants from Genome Pharmaceuticals Co. Ltd.

This article contains supplemental Methods and Figs. S1–S7.
K. Ishii, H. Hamamoto, and K. Sekimizu, unpublished data.
- AMP
- antimicrobial peptide
- IPS
- insect physiological saline
- PP
- paralytic peptide
- Z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone.
REFERENCES
- 1. Hejazi A., Falkiner F. R. (1997) Serratia marcescens. J. Med. Microbiol. 46, 903–912 [DOI] [PubMed] [Google Scholar]
- 2. Villari P., Crispino M., Salvadori A., Scarcella A. (2001) Molecular epidemiology of an outbreak of Serratia marcescens in a neonatal intensive care unit. Infect. Control. Hosp. Epidemiol. 22, 630–634 [DOI] [PubMed] [Google Scholar]
- 3. Maragakis L. L., Winkler A., Tucker M. G., Cosgrove S. E., Ross T., Lawson E., Carroll K. C., Perl T. M. (2008) Outbreak of multidrug-resistant Serratia marcescens infection in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 29, 418–423 [DOI] [PubMed] [Google Scholar]
- 4. Sifri C. D., Begun J., Ausubel F. M. (2005) The worm has turned. Microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 13, 119–127 [DOI] [PubMed] [Google Scholar]
- 5. Scully L. R., Bidochka M. J. (2006) Developing insect models for the study of current and emerging human pathogens. FEMS Microbiol. Lett. 263, 1–9 [DOI] [PubMed] [Google Scholar]
- 6. Kaito C., Akimitsu N., Watanabe H., Sekimizu K. (2002) Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 32, 183–190 [DOI] [PubMed] [Google Scholar]
- 7. Hamamoto H., Kurokawa K., Kaito C., Kamura K., Manitra Razanajatovo I., Kusuhara H., Santa T., Sekimizu K. (2004) Quantitative evaluation of the therapeutic effects of antibiotics using silkworms infected with human pathogenic microorganisms. Antimicrob. Agents Chemother. 48, 774–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamamoto H., Sekimizu K. (2005) Evaluation of the therapeutic effects of antibiotics using silkworm as an animal model. Res. Adv. Antimicrob. Agents Chemother. 5, 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaito C., Kurokawa K., Matsumoto Y., Terao Y., Kawabata S., Hamada S., Sekimizu K. (2005) Silkworm pathogenic bacteria infection model for identification of novel virulence genes. Mol. Microbiol. 56, 934–944 [DOI] [PubMed] [Google Scholar]
- 10. Kaito C., Sekimizu K. (2007) A silkworm model of pathogenic bacterial infection. Drug Discov. Ther. 1, 89–93 [PubMed] [Google Scholar]
- 11. Usui K., Miyazaki S., Kaito C., Sekimizu K. (2009) Purification of a soil bacteria exotoxin using silkworm toxicity to measure specific activity. Microb. Pathog. 46, 59–62 [DOI] [PubMed] [Google Scholar]
- 12. Kaito C., Usui K., Kyuma T., Sekimizu K. (2011) Isolation of mammalian pathogenic bacteria using silkworms. Drug Discov. Ther. 5, 66–70 [DOI] [PubMed] [Google Scholar]
- 13. Hoffmann J. A., Kafatos F. C., Janeway C. A., Ezekowitz R. A. (1999) Phylogenetic perspectives in innate immunity. Science 284, 1313–1318 [DOI] [PubMed] [Google Scholar]
- 14. Tanaka H., Ishibashi J., Fujita K., Nakajima Y., Sagisaka A., Tomimoto K., Suzuki N., Yoshiyama M., Kaneko Y., Iwasaki T., Sunagawa T., Yamaji K., Asaoka A., Mita K., Yamakawa M. (2008) A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem. Mol. Biol. 38, 1087–1110 [DOI] [PubMed] [Google Scholar]
- 15. Ha Lee J., Hee Lee I., Noda H., Mita K., Taniai K. (2007) Verification of elicitor efficacy of lipopolysaccharides and peptidoglycans on antibacterial peptide gene expression in Bombyx mori. Insect Biochem. Mol. Biol. 37, 1338–1347 [DOI] [PubMed] [Google Scholar]
- 16. Taniai K., Wago H., Yamakawa M. (1997) In vitro phagocytosis of Escherichia coli and release of lipopolysaccharide by adhering hemocytes of the silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 231, 623–627 [DOI] [PubMed] [Google Scholar]
- 17. Ishii K., Hamamoto H., Kamimura M., Sekimizu K. (2008) Activation of the silkworm cytokine by bacterial and fungal cell wall components via a reactive oxygen species-triggered mechanism. J. Biol. Chem. 283, 2185–2191 [DOI] [PubMed] [Google Scholar]
- 18. Ishii K., Hamamoto H., Kamimura M., Nakamura Y., Noda H., Imamura K., Mita K., Sekimizu K. (2010) Insect cytokine paralytic peptide (PP) induces cellular and humoral immune responses in the silkworm Bombyx mori. J. Biol. Chem. 285, 28635–28642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakahara Y., Kanamori Y., Kiuchi M., Kamimura M. (2003) Effects of silkworm paralytic peptide on in vitro hematopoiesis and plasmatocyte spreading. Arch. Insect. Biochem. Physiol. 52, 163–174 [DOI] [PubMed] [Google Scholar]
- 20. Sekimizu K., Larranaga J., Hamamoto H., Sekine M., Furuchi T., Katane M., Homma H., Matsuki N. (2005) d-Glutamic acid-induced muscle contraction in the silkworm, Bombyx mori. J. Biochem. 137, 199–203 [DOI] [PubMed] [Google Scholar]
- 21. Watanabe T., Kimura K., Sumiya T., Nikaidou N., Suzuki K., Suzuki M., Taiyoji M., Ferrer S., Regue M. (1997) Genetic analysis of the chitinase system of Serratia marcescens 2170. J. Bacteriol. 179, 7111–7117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Lorenzo V., Herrero M., Jakubzik U., Timmis K. N. (1990) Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in Gram-negative eubacteria. J. Bacteriol. 172, 6568–6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Toratani T., Shoji T., Ikehara T., Suzuki K., Watanabe T. (2008) The importance of chitobiase and N-acetylglucosamine (GlcNAc) uptake in N,N′-diacetylchitobiose ((GlcNAc)2) utilization by Serratia marcescens 2,170. Microbiology 154, 1326–1332 [DOI] [PubMed] [Google Scholar]
- 24. Kurz C. L., Chauvet S., Andrès E., Aurouze M., Vallet I., Michel G. P., Uh M., Celli J., Filloux A., De Bentzmann S., Steinmetz I., Hoffmann J. A., Finlay B. B., Gorvel J. P., Ferrandon D., Ewbank J. J. (2003) Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 22, 1451–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishida S., Mizushima T., Miki T., Sekimizu K. (1997) Immotile phenotype of an Escherichia coli mutant lacking the histone-like protein HU. FEMS Microbiol. Lett. 150, 297–301 [DOI] [PubMed] [Google Scholar]
- 26. Nehme N. T., Liégeois S., Kele B., Giammarinaro P., Pradel E., Hoffmann J. A., Ewbank J. J., Ferrandon D. (2007) A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 3, e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamkanfi M., Declercq W., Kalai M., Saelens X., Vandenabeele P. (2002) Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 9, 358–361 [DOI] [PubMed] [Google Scholar]
- 28. Kanuka H., Hisahara S., Sawamoto K., Shoji S., Okano H., Miura M. (1999) Proapoptotic activity of Caenorhabditis elegans CED-4 protein in Drosophila. Implicated mechanisms for caspase activation. Proc. Natl. Acad. Sci. U.S.A. 96, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Igaki T., Kanda H., Yamamoto-Goto Y., Kanuka H., Kuranaga E., Aigaki T., Miura M. (2002) Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J. 21, 3009–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moreno E., Yan M., Basler K. (2002) Evolution of TNF signaling mechanisms. JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr. Biol. 12, 1263–1268 [DOI] [PubMed] [Google Scholar]
- 31. Morgenstein R. M., Clemmer K. M., Rather P. N. (2010) Loss of the waaL O-antigen ligase prevents surface activation of the flagellar gene cascade in Proteus mirabilis. J. Bacteriol. 192, 3213–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Easom C. A., Clarke D. J. (2008) Motility is required for the competitive fitness of entomopathogenic Photorhabdus luminescens during insect infection. BMC Microbiol. 8, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tabei S. M., Hitchen P. G., Day-Williams M. J., Merino S., Vart R., Pang P. C., Horsburgh G. J., Viches S., Wilhelms M., Tomás J. M., Dell A., Shaw J. G. (2009) An Aeromonas caviae genomic island is required for both O-antigen lipopolysaccharide biosynthesis and flagellin glycosylation. J. Bacteriol. 191, 2851–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abeyrathne P. D., Daniels C., Poon K. K., Matewish M. J., Lam J. S. (2005) Functional characterization of WaaL, a ligase associated with linking O-antigen polysaccharide to the core of Pseudomonas aeruginosa lipopolysaccharide. J. Bacteriol. 187, 3002–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Labbé K., Saleh M. (2008) Cell death in the host response to infection. Cell Death Differ. 15, 1339–1349 [DOI] [PubMed] [Google Scholar]
- 36. Majumdar T., Chattopadhyay P., Saha D. R., Sau S., Mazumder S. (2009) Virulence plasmid of Aeromonas hydrophila induces macrophage apoptosis and helps in developing systemic infection in mice. Microb. Pathog. 46, 98–107 [DOI] [PubMed] [Google Scholar]
- 37. Timmer A. M., Timmer J. C., Pence M. A., Hsu L. C., Ghochani M., Frey T. G., Karin M., Salvesen G. S., Nizet V. (2009) Streptolysin O promotes group A Streptococcus immune evasion by accelerated macrophage apoptosis. J. Biol. Chem. 284, 862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carbonell G. V., Falcón R., Yamada A. T., da Fonseca B. A., Yano T. (2004) Morphological and intracellular alterations induced by Serratia marcescens cytotoxin. Res. Microbiol. 155, 25–30 [DOI] [PubMed] [Google Scholar]
- 39. Katsuma S., Mita K., Shimada T. (2007) ERK- and JNK-dependent signaling pathways contribute to Bombyx mori nucleopolyhedrovirus infection. J. Virol. 81, 13700–13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pei Z., Reske G., Huang Q., Hammock B. D., Qi Y., Chejanovsky N. (2002) Characterization of the apoptosis suppressor protein P49 from the Spodoptera littoralis nucleopolyhedrovirus. J. Biol. Chem. 277, 48677–48684 [DOI] [PubMed] [Google Scholar]
- 41. Shinzawa N., Nelson B., Aonuma H., Okado K., Fukumoto S., Miura M., Kanuka H. (2009) p38 MAPK-dependent phagocytic encapsulation confers infection tolerance in Drosophila. Cell Host Microbe 6, 244–252 [DOI] [PubMed] [Google Scholar]
- 42. Cho K. S., Lee J. H., Kim S., Kim D., Koh H., Lee J., Kim C., Kim J., Chung J. (2001) Drosophila phosphoinositide-dependent kinase-1 regulates apoptosis and growth via the phosphoinositide 3-kinase-dependent signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 98, 6144–6149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wickstrum J. R., Bokhari S. M., Fischer J. L., Pinson D. M., Yeh H. W., Horvat R. T., Parmely M. J. (2009) Francisella tularensis induces extensive caspase-3 activation and apoptotic cell death in the tissues of infected mice. Infect. Immun. 77, 4827–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miao E. A., Ernst R. K., Dors M., Mao D. P., Aderem A. (2008) Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl. Acad. Sci. U.S.A. 105, 2562–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jia J., Alaoui-El-Azher M., Chow M., Chambers T. C., Baker H., Jin S. (2003) c-Jun NH2-terminal kinase-mediated signaling is essential for Pseudomonas aeruginosa ExoS-induced apoptosis. Infect. Immun. 71, 3361–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ishii K., Hamamoto H., Imamura K., Adachi T., Shoji M., Nakayama K., Sekimizu K. (2010) Porphyromonas gingivalis peptidoglycans induce excessive activation of the innate immune system in silkworm larvae. J. Biol. Chem. 285, 33338–33347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu G. Y., Doran K. S., Lawrence T., Turkson N., Puliti M., Tissi L., Nizet V. (2004) Sword and shield. Linked group B streptococcal β-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc. Natl. Acad. Sci. U.S.A. 101, 14491–14496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dao D. N., Kremer L., Guérardel Y., Molano A., Jacobs W. R., Jr., Porcelli S. A., Briken V. (2004) Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect. Immun. 72, 2067–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kostakioti M., Newman C. L., Thanassi D. G., Stathopoulos C. (2005) Mechanisms of protein export across the bacterial outer membrane. J. Bacteriol. 187, 4306–4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Labbate M., Queck S. Y., Koh K. S., Rice S. A., Givskov M., Kjelleberg S. (2004) Quorum sensing-controlled biofilm development in Serratia liquefaciens MG1. J. Bacteriol. 186, 692–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Labbate M., Zhu H., Thung L., Bandara R., Larsen M. R., Willcox M. D., Givskov M., Rice S. A., Kjelleberg S. (2007) Quorum-sensing regulation of adhesion in Serratia marcescens MG1 is surface dependent. J. Bacteriol. 189, 2702–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bott M., Dimroth P. (1994) Klebsiella pneumoniae genes for citrate lyase and citrate lyase ligase. Localization, sequencing, and expression. Mol. Microbiol. 14, 347–356 [DOI] [PubMed] [Google Scholar]
- 53. Mita K., Kasahara M., Sasaki S., Nagayasu Y., Yamada T., Kanamori H., Namiki N., Kitagawa M., Yamashita H., Yasukochi Y., Kadono-Okuda K., Yamamoto K., Ajimura M., Ravikumar G., Shimomura M., Nagamura Y., Shin-I T., Abe H., Shimada T., Morishita S., Sasaki T. (2004) The genome sequence of silkworm, Bombyx mori. DNA Res. 11, 27–35 [DOI] [PubMed] [Google Scholar]
- 54. Forbes S. J., Eschmann M., Mantis N. J. (2008) Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect. Immun. 76, 4137–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. DeFlaun M. F., Oppenheimer S. R., Streger S., Condee C. W., Fletcher M. (1999) Alterations in adhesion, transport, and membrane characteristics in an adhesion-deficient pseudomonad. Appl. Environ. Microbiol. 65, 759–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Watnick P. I., Lauriano C. M., Klose K. E., Croal L., Kolter R. (2001) The absence of a flagellum leads to altered colony morphology, biofilm development, and virulence in Vibrio cholerae O139. Mol. Microbiol. 39, 223–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seimon T. A., Obstfeld A., Moore K. J., Golenbock D. T., Tabas I. (2006) Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc. Natl. Acad. Sci. U.S.A. 103, 19794–19799 [DOI] [PMC free article] [PubMed] [Google Scholar]