Background: The NLRP3 inflammasome is a critical mediator of inflammation in response to pathogens, tissue damage, and metabolic diseases.
Results: Non-transcriptional priming and activation signals stimulate NLRP3 deubiquitination.
Conclusion: The NLRP3 inflammasome is non-transcriptionally primed and activated by a deubiquitination mechanism.
Significance: Understanding the mechanism of activation of the NLRP3 inflammasome is crucial for the development of therapeutics to alleviate NLRP3-dependent inflammatory diseases.
Keywords: Caspase, Inflammation, Innate Immunity, Toll-like Receptor (TLR), Ubiquitination, NLRP3, Caspase-1, Deubiquitination, Inflammasome
Abstract
The NLRP3 inflammasome is a key component of the innate immune response to pathogenic infection and tissue damage. It is also involved in the pathogenesis of a number of human diseases, including gouty arthritis, silicosis, atherosclerosis, and type 2 diabetes. The assembly of the NLRP3 inflammasome requires a priming signal derived from pattern recognition or cytokine receptors, followed by a second signal derived from extracellular ATP, pore-forming toxins, or crystalline materials. How these two signals activate the NLRP3 inflammasome is not yet clear. Here, we show that in mouse macrophages, signaling by the pattern recognition receptor TLR4 through MyD88 can rapidly and non-transcriptionally prime NLRP3 by stimulating its deubiquitination. This process is dependent on mitochondrial reactive oxygen species production and can be inhibited by antioxidants. We further show that signaling by ATP can also induce deubiquitination of NLRP3 by a mechanism that is not sensitive to antioxidants. Pharmacological inhibition of NLRP3 deubiquitination completely blocked NLRP3 activation in both mouse and human cells, indicating that deubiquitination of NLRP3 is required for its activation. Our findings suggest that NLRP3 is activated by a two-step deubiquitination mechanism initiated by Toll-like receptor signaling and mitochondrial reactive oxygen species and further potentiated by ATP, which could explain how NLRP3 is activated by diverse danger signals.
Introduction
Inflammasomes are key components of the innate immune response to tissue damage or infection by microbial or viral pathogens (1). These large multiprotein complexes form rapidly in response to danger and pathogen-associated signals, serving as scaffolds to promote maturation of caspase-1, a cysteine protease that processes inactive pro-IL-1β and pro-IL-18 to their active proinflammatory cytokines, IL-1β and IL-18, respectively. The most widely studied is the NLRP3 (cryopyrin, NALP3) inflammasome. NLRP3 is critical for innate immunity against pathogens (1), but inappropriate activation of NLRP3 has been implicated in the pathogenesis of a number of human diseases, including gouty arthritis, silicosis, and neurodegeneration, and in many metabolic disorders, such as atherosclerosis, type 2 diabetes, and obesity (2, 3). In addition, abnormal activation of the NLRP3 inflammasome due to mutations in NLRP3 is responsible for several autoinflammatory diseases or periodic fever syndromes (4).
NLRP3 is activated by diverse signals, including infection with intracellular microbial and viral pathogens; Toll-like receptor (TLR)2 agonists plus potassium-depleting agents, such as ATP, nigericin, and maitotoxin; and crystalline materials, such as monosodium urate, silica, and cholesterol crystals (5). How these diverse stimuli activate the NLRP3 inflammasome is not yet clear. Studies in mouse bone marrow-derived macrophages showed that activation of the NLRP3 inflammasome requires two signals, a priming signal (or signal 1) from a pattern recognition or cytokine receptor, followed by a second signal (or signal 2) from the purinergic P2X7 receptor or pore-forming toxins (6). Neither of the two signals alone can significantly activate the NLRP3 inflammasome. Although it is widely believed that signal 1 is important for transcriptional up-regulation of NLRP3, we show here that signal 1 can non-transcriptionally prime NLRP3 in mouse macrophages by stimulating its deubiquitination. We further show that signal 1 is regulated by mitochondrial reactive oxygen species (mtROS) and that signal 2 can also stimulate NLRP3 deubiquitination by a mtROS-independent mechanism.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Antibodies against human caspase-1 p30, mouse NLRP3, and mouse caspase-1 p20 were described previously (6–8). HRP-conjugated anti-ubiquitin antibody (P4D1) was from Santa Cruz Biotechnology. The following reagents were from the indicated sources: anti-FLAG® M2-agarose affinity gel, nigericin, ATP, pyridaben, N-ethylmaleimide, and N-acetylcysteine (NAC) from Sigma; rotenone from Tocris; Mito-TEMPO from Enzo Life Sciences; PR-619 from LifeSensors; WP1130 from BioVision; and ultrapure LPS from InvivoGen.
Cells, Generation of Stable Cell Lines, and Drug Treatment
Primary bone marrow macrophages were isolated from mouse femurs and grown in Dulbecco's modified Eagle's medium (Invitrogen) as described previously (9). The immortalized WT, Nlrp3 knock-out (Nlrp3-KO) and Myd88/Trif double knock-out (Myd88/Trif-dKO) macrophages were described previously (6, 9, 10). The stable NLRP3-reconstituted N1-8, N1-9, and NG5 cell lines, which express a C-terminally FLAG-tagged mouse NLRP3 protein, and Myd88/Trif-dKO+Myd88 cells were generated by retroviral transduction as described previously (8, 9). The 293T-caspase-1-ASC cell line, which stably expresses human caspase-1 and ASC, and the 293T-caspase-1-ASC-NLRP3 cell line, which stably expresses human caspase-1, ASC, and FLAG-tagged human NLRP3, were described previously (10). Cells were treated with the following drugs as indicated in each experiment: ultrapure LPS (500 ng/ml), ATP (5 mm), cycloheximide (5 μm), rotenone (20 μm), pyridaben (10 μm), PR-619 (15 μm), WP1130 (10 μm), NAC (25 μm), Mito-TEMPO (100 μm), and nigericin (10 μm). In all experiments using cycloheximide, NAC, Mito-TEMPO, PR-619, or WP1130, cells were pretreated with these drugs for 10 min before stimulation with LPS, ATP, or LPS plus ATP.
Immunoblot Analysis of Active Caspase-1
Cell culture supernatants from treated macrophages were precipitated and analyzed by immunoblotting as described (10).
Assay of NLRP3 Ubiquitination
NLRP3 ubiquitination was assayed by immunoprecipitation of NLRP3 from cells using anti-FLAG M2-agarose affinity gel, followed by immunoblotting with HRP-conjugated anti-ubiquitin antibody. Briefly, cells (7 × 106) were lysed in 0.6 ml of denaturation buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 1% SDS, and 10 mm N-ethylmaleimide) and then boiled for 10 min, followed by dilution with 10 volumes of binding buffer (50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 0.5% Nonidet P-40, and 10 mm N-ethylmaleimide). The diluted lysates were immunoprecipitated with anti-FLAG M2-agarose beads at 4 °C for 4 h, and the beads were then washed three times with binding buffer, followed by elution with SDS sample buffer and fractionation on a 4–15% gradient Mini-PROTEAN® TGXTM SDS-polyacrylamide gel (BIO-RAD). The gel was immunoblotted using standard immunoblotting techniques.
RESULTS
Rapid Non-transcriptional Priming of the NLRP3 Inflammasome
We previously reported that cell priming with multiple TLR ligands for 2 or more hours induces up-regulation of NLRP3 expression through activation of NF-κB, which we identified to be an important determinant for NLRP3 activation (6). Although up-regulation of NLRP3 expression by TLR signaling can act as a priming signal, subsequent time course analysis of the effect of LPS, which stimulates TLR4, on activation of the NLRP3 inflammasome by ATP showed that a lengthy incubation period with LPS is not necessary for priming. In both primary bone marrow-derived macrophages and immortalized wild-type macrophages, only 10 min of incubation with LPS was sufficient to prime the NLRP3 inflammasome, which activated caspase-1 when ATP was added to the cells (Fig. 1, A and B). As up-regulation of NLRP3 expression by LPS did not occur until after 2 or more hours of stimulation (Fig. 1A), these results suggest that the effect of LPS on the NLRP3 inflammasome is not dependent on NLRP3 up-regulation. Supporting this, simultaneous stimulation of macrophages with LPS plus ATP or treatment with ATP first followed by LPS activated the NLRP3 inflammasome (Fig. 1B). Furthermore, inhibition of new protein synthesis by the protein synthesis inhibitor cycloheximide failed to prevent priming of the NLRP3 inflammasome by LPS (Fig. 1C). The apparent inconsistency between our results and previously published results that showed inhibition of NLRP3 inflammasome activity by cycloheximide (6, 11, 12) can be explained by differences in the length of treatment with cycloheximide. In the previous studies, cells were treated with cycloheximide and LPS for >3 h before ATP stimulation compared with 20 min in our studies. Prolonged treatment with cycloheximide leads to down-regulation of NLRP3 expression and cell death, which might be responsible for inhibition of caspase-1 activation (supplemental Fig. 1). Altogether, these results indicate that LPS primes the NLRP3 inflammasome by a non-transcriptional mechanism.
FIGURE 1.
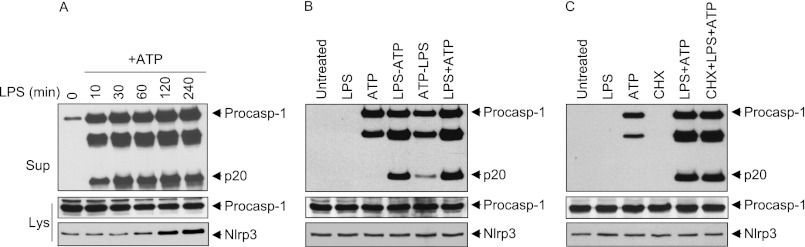
LPS-induced priming of the NLRP3 inflammasome does not require new protein synthesis. A and C, immunoblots of caspase-1 in the culture supernatants (Sup; upper panels) or cell lysates (Lys; middle panels) of WT primary bone marrow-derived macrophages stimulated with LPS for the indicated times, followed by ATP (45 min) (A), or immortalized WT macrophages stimulated with LPS for 10 min in the presence or absence of cycloheximide (CHX), followed by ATP (45 min) (C). B, immunoblot of caspase-1 in the culture supernatants (upper panel) or cell lysates (middle panel) of WT macrophages unstimulated (Untreated; first lane) or stimulated with LPS (45 min; second lane), ATP (45 min; third lane), LPS for 10 min followed by ATP for 35 min (fourth lane), ATP for 10 min followed by LPS for 35 min (fifth lane), or simultaneously with LPS and ATP for 45 min (sixth lane). The lower panels in A–C show immunoblots of NLRP3 in the cell lysates of the same macrophages. Procasp-1, procaspase-1.
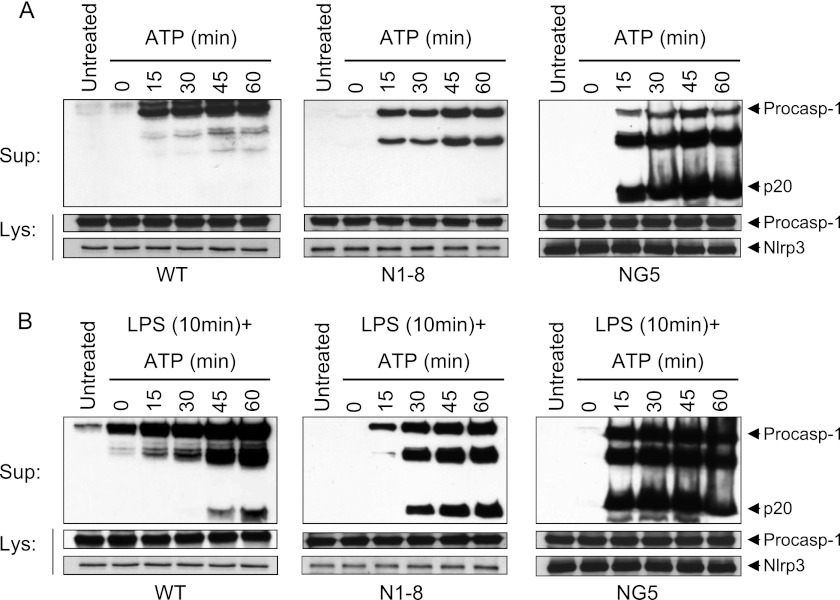
To provide further evidence for the non-transcriptional priming mechanism, we stably reconstituted immortalized Nlrp3-KO macrophages with NLRP3 and isolated macrophage clones that express different levels of NLRP3 (supplemental Fig. 2A). The N1-8 macrophages express NLRP3 at a level comparable with that present in uninduced WT macrophages (basal level), whereas NG5 macrophages express NLRP3 at a level comparable with that present in 6-h LPS-induced WT macrophages (fully induced level). Consistent with our previous work (6, 9), the NG5 macrophages, which express induced levels of NLRP3, activated caspase-1 with ATP treatment alone without prior priming with LPS (Fig. 2A, right panel). In contrast, the N1-8 macrophages, which express basal levels of NLRP3, and WT macrophages did not activate NLRP3 with ATP treatment alone as evidenced by the absence of caspase-1 processing to the p20 form (Fig. 2A, middle and left panels, respectively). However, when these macrophages were primed for 10 min with LPS, they activated caspase-1 after ATP was added (Fig. 2B, middle and left panels, respectively), but with slower kinetics and intensity compared with NG5 macrophages (Fig. 2B, right panel, and supplemental Fig. 2B), indicating that increased NLRP3 expression accelerates the kinetics and level of caspase-1 activation. As the level of NLRP3 in the N1-8 macrophages is not regulated transcriptionally by LPS-TLR4 signaling, these results indicate that LPS can prime the NLRP3 inflammasome by a transcription-independent mechanism at a low expression level of NLRP3. Consistent with this, another cell line, N1-9, which expresses intermediate levels of NLRP3 (between N1-8 and NG5 macrophages), activated caspase-1 with ATP treatment alone without prior priming with LPS, but also with slower kinetics compared with NG5 macrophages (Fig. 2A, right panel, and supplemental Fig. 2C). Prior priming with LPS for 10 min accelerated the kinetics of caspase-1 activation in N1-9 macrophages (supplemental Fig. 2D) to a level similar to that in NG5 macrophages (Fig. 2B, right panel).
FIGURE 2.
Time course analysis of NLRP3 activation in WT and stable NLRP3-reconstituted Nlrp3-KO macrophages. A and B, immunoblots of caspase-1 in the culture supernatants (Sup; upper panels) of WT, N1-8, and NG5 macrophages stimulated with ATP alone for the indicated times (A) or stimulated with LPS for 10 min followed by ATP for the indicated times (B). The middle and lower panels show immunoblots of caspase-1 and NLRP3 in the cell lysates (Lys) of the same macrophages. Procasp-1, procaspase-1.
mtROS Are Required for Non-transcriptional Priming of NLRP3
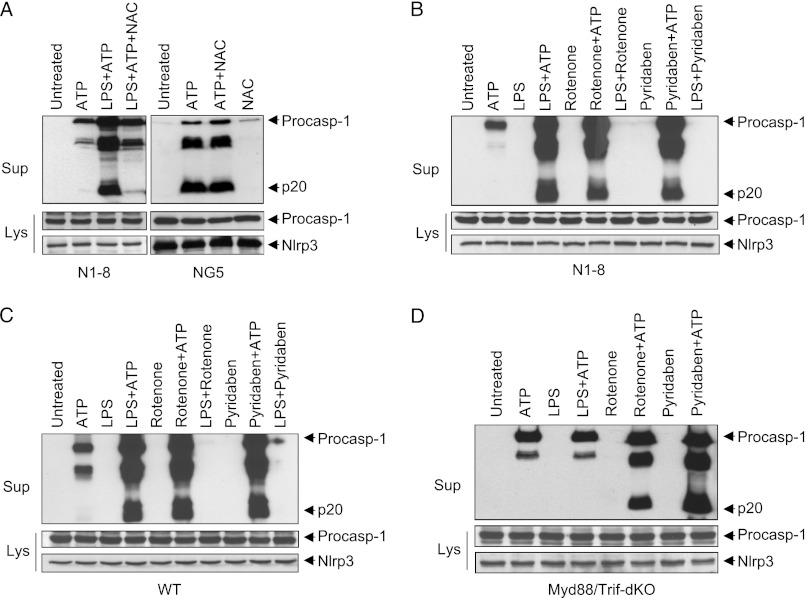
mtROS have been implicated in regulation of the NLRP3 inflammasome (13, 14), but the mechanism is still unclear. One study proposed that mtROS regulate priming, but not activation, of NLRP3 by regulating its transcriptional induction (12). However, further investigation revealed that scavenging of ROS by the antioxidant NAC inhibited inflammasome activation in N1-8 macrophages, in which NLRP3 priming does not depend on transcriptional induction of NLRP3, in response to LPS plus ATP (Fig. 3A, left panel). Consistent with the previous study (12), NAC had no effect on NLRP3 activation in NG5 cells, which express up-regulated levels of NLRP3, in response to ATP (Fig. 3A, right panel). Similar results were obtained with the mitochondrial targeted antioxidant Mito-TEMPO (supplemental Fig. 3, A and B).
FIGURE 3.
Intact TLR4 signaling and mtROS are required for non-transcriptional priming of NLRP3. A, immunoblots of caspase-1 in the culture supernatants (Sup) of N1-8 (left panels) and NG5 (right panels) macrophages stimulated with LPS (10 min) plus ATP (45 min) or with ATP alone (45 min) in the presence or absence of NAC as indicated. B–D, immunoblots of caspase-1 in the culture supernatants of N1-8 (B), WT (C), and Myd88/Trif-dKO (D) macrophages stimulated with LPS, rotenone, or pyridaben for 10 min followed by ATP as indicated. The middle and lower panels in A–D show immunoblots of caspase-1 and NLRP3 in the cell lysates (Lys) of the same macrophages. Procasp-1, procaspase-1.
The above results suggest that LPS triggers NLRP3 priming through a mechanism that depends on mtROS. To investigate whether this mechanism requires intact signaling downstream of TLR4, we stimulated WT, N1-8, and Myd88/Trif-dKO macrophages with LPS for 10 min, followed by ATP. This treatment resulted in activation of NLRP3 in the N1-8 and WT macrophages (Fig. 3, B and C), but not in the Myd88/Trif-dKO macrophages (Fig. 3D). Reconstitution of the Myd88/Trif-dKO macrophages with FLAG-tagged MyD88 allowed activation of the NLRP3 inflammasome by LPS plus ATP (supplemental Fig. 4), indicating that MyD88 is important and sufficient for non-transcriptional priming of NLRP3. Next, we investigated whether TLR4 signaling is required for induction of mtROS by treating these macrophages with the mitochondrial complex I inhibitors rotenone and pyridaben, which directly stimulate mtROS production. Both rotenone and pyridaben were able to prime the NLRP3 inflammasome in all of these macrophages (Fig. 3, B–D). The effect of rotenone and pyridaben on NLRP3 was inhibited by NAC and Mito-TEMPO (supplemental Fig. 3, A, C, and D). Taken together, these results indicate that LPS-induced priming of the NLRP3 inflammasome is dependent on intact TLR4 signaling and mtROS.
Signals 1 and 2 Induce NLRP3 Deubiquitination
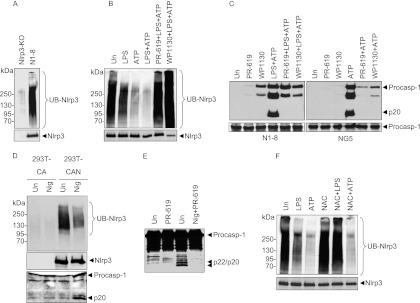
Post-translational modification by ubiquitination can regulate the activity of many cellular proteins (15). Immunoprecipitation of NLRP3 from N1-8 macrophages followed by immunoblotting with anti-ubiquitin antibody revealed that NLRP3 is ubiquitinated (Fig. 4A). To investigate whether TLR4 signaling might prime the NLRP3 inflammasome by regulating its ubiquitination, we analyzed the ubiquitination status of NLRP3 in N1-8 macrophages after treatment with LPS, ATP, or LPS plus ATP. Treatment with LPS or ATP alone induced notable deubiquitination of NLRP3, which was further enhanced by co-treatment with both stimuli (Fig. 4B, first through fourth lanes). Treatment with the general deubiquitinating (DUB) enzyme inhibitors PR-619 and WP1130 inhibited the LPS/ATP-induced deubiquitination of NLRP3 and further increased NLRP3 ubiquitination (Fig. 4B, fifth and sixth lanes). These inhibitors were also able to block NLRP3 activation in N1-8 and NG5 cells in response to LPS plus ATP or ATP treatment alone (Fig. 4C), respectively, indicating that deubiquitination of NLRP3 by the priming and activation signals is critical for its activation. In contrast, they were not able to inhibit activation of the NLRC4 inflammasome in Salmonella-infected cells (supplemental Fig. 5A), although they reduced the amount of caspase-1 activation perhaps because Salmonella can activate both NLRC4 and NLRP3 inflammasomes (16). These drugs were also unable to inhibit activation of caspase-1 in vitro by the ASC pyroptosome (supplemental Fig. 5B) (7), ruling out the possibility that these drugs inhibit the autocatalytic activity of caspase-1 or its recruitment to ASC. To provide additional support for the role of deubiquitination in NLRP3 activation, we analyzed the ubiquitination status of human NLRP3 in the 293T-caspase-1-ASC cell line stably reconstituted with FLAG-tagged NLRP3 (293T-caspase-1-ASC-NLRP3 cells) (10). These cells are responsive to the NLRP3-activating toxin nigericin (supplemental Fig. 5C). Immunoprecipitation of proteins from the 293T-caspase-1-ASC-NLRP3 cells or the parental 293T-caspase-1-ASC cells followed by immunoblotting with anti-ubiquitin antibody showed ubiquitinated NLRP3 in the 293T-caspase-1-ASC-NLRP3 cells, but not in the 293T-caspase-1-ASC cells (Fig. 4D). Treatment with nigericin induced notable deubiquitination of NLRP3 (Fig. 4D, third and fourth lanes), which was associated with caspase-1 activation (Fig. 4D, lower panel). Nigericin-induced caspase-1 activation in these cells was also blocked by PR-619 (Fig. 4E). Taken together, these results indicate that NLRP3 activation in mouse and human cells is regulated by deubiquitination.
FIGURE 4.
Deubiquitination of NLRP3 is required for its priming and activation. A, B, and F, anti-ubiquitin (UB) antibody (upper panels) and anti-NLRP3 antibody (lower panels) immunoblots of anti-FLAG immunoprecipitates from Nlrp3-KO and N1-8 macrophages (A, first and second lanes, respectively) and N1-8 macrophages stimulated with LPS, ATP, or LPS plus ATP in the presence or absence of the deubiquitination inhibitors PR-619 and WP1130 (B) and the antioxidant NAC (F) as indicated. C, immunoblots of caspase-1 in the culture supernatants of N1-8 and NG5 macrophages stimulated with LPS plus ATP (N1-8) or ATP (NG5) in the presence or absence of PR-619 or WP1130 as indicated (upper panels). The lower panels show immunoblots of caspase-1 in the cell lysates of the same macrophages. D, anti-ubiquitin antibody (upper panel) and anti-NLRP3 antibody (middle panel) immunoblots of anti-FLAG immunoprecipitates from 293T-caspase-1-ASC (293T-CA) and 293T-caspase-1-ASC-NLRP3 (293T-CAN) cells stimulated with nigericin (Nig) for 60 min or left untreated (Un). The lower panel shows a caspase-1 immunoblot in the culture supernatants of the same cells. E, immunoblot of caspase-1 in cell lysates of 293T-caspase-1-ASC-NLRP3 cells treated with nigericin in the presence or absence of PR-619 as indicated. Procasp-1, procaspase-1.
As mtROS are important for the priming of NLRP3 (Fig. 3), we next examined whether scavenging of mtROS by NAC or Mito-TEMPO would inhibit LPS-induced deubiquitination of NLRP3. Treatment with NAC or Mito-TEMPO inhibited deubiquitination of NLRP3 in response to LPS stimulation, but not to ATP stimulation (Fig. 4D and supplemental Fig. 6). These results are consistent with the observations that NAC or Mito-TEMPO can inhibit inflammasome activation in N1-8 cells, but not in NG5 cells (Fig. 3 and supplemental Fig. 3). The results also suggest that LPS activates an antioxidant-sensitive DUB enzyme, whereas ATP activates an antioxidant-insensitive DUB enzyme.
DISCUSSION
In this work, we identified a new regulatory mechanism that controls NLRP3 inflammasome activation. We have shown that TLR4 signaling through MyD88 can rapidly prime the NLRP3 inflammasome at basal NLRP3 expression levels through a non-transcriptional mechanism. This early priming mechanism is likely involved in the secretion of constitutively expressed cytokines, such as IL-18, and other inflammatory mediators (e.g. HMGB1). This mechanism appears to require mtROS production, as scavenging of mtROS with antioxidants blocks NLRP3 activation, whereas stimulation of mtROS production with complex I inhibitors promotes NLRP3 activation. However, because ROS scavengers and inducers have potentially off-target effects, more evidence is required to support a link between ROS and NLRP3.
We have also shown that both signal 1 (priming) and signal 2 stimulate NLRP3 deubiquitination. Pharmacological inhibition of NLRP3 deubiquitination completely blocked NLRP3 activation in both mouse and human cells, indicating that deubiquitination of NLRP3 is required for its activation. At high NLRP3 expression levels, prior priming with TLR4 agonist is not required, and treatment with ATP alone can activate NLRP3. A possible explanation for this is that at high expression levels, NLRP3 is partially ubiquitinated, and ATP-induced deubiquitination is sufficient to activate it. However, at basal expression levels, NLRP3 might be highly ubiquitinated at different domains by different polyubiquitin chains (e.g. Lys-48 and Lys-63). TLR4 signaling might activate a DUB enzyme that targets a specific polyubiquitin chain and/or a specific domain in NLRP3, whereas ATP signaling might activate a second DUB enzyme that deubiquitinates a different domain. Therefore, treatment with LPS or ATP alone will not normally activate NLRP3, but treatment with both activates it. Consistent with this hypothesis, we found that LPS-induced NLRP3 deubiquitination is inhibited by mtROS scavengers (NAC and Mito-TEMPO), whereas ATP-induced NLRP3 deubiquitination is not inhibited by these agents. These results suggest that the DUB enzyme that is potentially involved in LPS-induced priming of NLRP3 is different from that involved in ATP-induced activation of NLRP3. These DUB enzymes might target different types of ubiquitin chains in NLRP3 and/or different domains of NLRP3. Further characterization of the NLRP3 deubiquitination mechanism and the identification of the NLRP3-deubiquitinating enzymes should enhance our understanding of the mechanism of NLRP3 activation and facilitate the development of novel therapeutics that target the NLRP3-deubiquitinating enzymes to alleviate NLRP3-dependent inflammatory diseases.
This work was supported, in whole or in part, by National Institutes of Health Grants AG14357 and AR055398 (to E. S. A.).

This article contains supplemental Figs. 1–6 and additional references.
- TLR
- Toll-like receptor
- mtROS
- mitochondrial reactive oxygen species
- NAC
- N-acetylcysteine
- KO
- knock-out
- dKO
- double KO
- DUB
- deubiquitinating.
REFERENCES
- 1. Franchi L., Muñoz-Planillo R., Núñez G. (2012) Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13, 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wen H., Ting J. P., O'Neill L. A. (2012) A role for the NLRP3 inflammasome in metabolic diseases–did Warburg miss inflammation? Nat. Immunol. 13, 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strowig T., Henao-Mejia J., Elinav E., Flavell R. (2012) Inflammasomes in health and disease. Nature 481, 278–286 [DOI] [PubMed] [Google Scholar]
- 4. Aksentijevich I., Putnam C. D., Remmers E. F., Mueller J. L., Le J., Kolodner R. D., Moak Z., Chuang M., Austin F., Goldbach-Mansky R., Hoffman H. M., Kastner D. L. (2007) The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 56, 1273–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tschopp J., Schroder K. (2010) NLRP3 inflammasome activation: the convergence of multiple signaling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 [DOI] [PubMed] [Google Scholar]
- 6. Bauernfeind F. G., Horvath G., Stutz A., Alnemri E. S., MacDonald K., Speert D., Fernandes-Alnemri T., Wu J., Monks B. G., Fitzgerald K. A., Hornung V., Latz E. (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandes-Alnemri T., Wu J., Yu J. W., Datta P., Miller B., Jankowski W., Rosenberg S., Zhang J., Alnemri E. S. (2007) The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yu J. W., Fernandes-Alnemri T., Datta P., Wu J., Juliana C., Solorzano L., McCormick M., Zhang Z., Alnemri E. S. (2007) Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol. Cell 28, 214–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juliana C., Fernandes-Alnemri T., Wu J., Datta P., Solorzano L., Yu J. W., Meng R., Quong A. A., Latz E., Scott C. P., Alnemri E. S. (2010) Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 285, 9792–9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandes-Alnemri T., Yu J. W., Datta P., Wu J., Alnemri E. S. (2009) AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franchi L., Eigenbrod T., Núñez G. (2009) Cutting edge: TNF-α mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J. Immunol. 183, 792–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bauernfeind F., Bartok E., Rieger A., Franchi L., Núñez G., Hornung V. (2011) Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 187, 613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakahira K., Haspel J. A., Rathinam V. A., Lee S. J., Dolinay T., Lam H. C., Englert J. A., Rabinovitch M., Cernadas M., Kim H. P., Fitzgerald K. A., Ryter S. W., Choi A. M. (2011) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 [DOI] [PubMed] [Google Scholar]
- 15. Pickart C. M. (2004) Back to the future with ubiquitin. Cell 116, 181–190 [DOI] [PubMed] [Google Scholar]
- 16. Broz P., Newton K., Lamkanfi M., Mariathasan S., Dixit V. M., Monack D. M. (2010) Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]