Background: Cytoglobin plays cytoprotective roles under hypoxic/ischemic conditions, but the mechanisms remain unclear.
Results: Cytoglobin functions as a nitrite reductase leading to NO generation and soluble guanylyl cyclase activation under anaerobic or severely hypoxic conditions and this is increased by acidosis as occurs in ischemia.
Conclusion: Cytoglobin-mediated nitrite reduction generates NO that activates soluble guanylyl cyclase under hypoxic/ischemic conditions.
Significance: Cytoglobin serves a protective function by reducing nitrite to NO under ischemic conditions.
Keywords: Heme, Hypoxia, Ischemia, Nitric Oxide, Vascular Biology, Cytoglobin Function, Ischemic Protection, Nitrite Activation, Soluble Guanylyl Cyclase, Vasodilation
Abstract
Cytoglobin (Cygb) is a recently discovered cytoplasmic heme-binding globin. Although multiple hemeproteins have been reported to function as nitrite reductases in mammalian cells, it is unknown whether Cygb can also reduce nitrite to nitric oxide (NO). The mechanism, magnitude, and quantitative importance of Cygb-mediated nitrite reduction in tissues have not been reported. To investigate this pathway and its quantitative importance, EPR spectroscopy, spectrophotometric measurements, and chemiluminescence NO analyzer studies were performed. Under anaerobic conditions, mixing nitrite with ferrous-Cygb triggered NO formation that was trapped and detected using EPR spin trapping. Spectrophotometric studies revealed that nitrite binding to ferrous-Cygb is followed by formation of ferric-Cygb and NO. The kinetics and magnitude of Cygb-mediated NO formation were characterized. It was observed that Cygb-mediated NO generation increased linearly with the increase of nitrite concentration under anaerobic conditions. This Cygb-mediated NO production greatly increased with acidosis and near-anoxia as occur in ischemic conditions. With the addition of nitrite, soluble guanylyl cyclase activation was significantly higher in normal smooth muscle cells compared with Cygb knocked down cells with Cygb accounting for ∼40% of the activation in control cells and ∼60% in cells subjected to hypoxia for 48 h. Overall, these studies show that Cygb-mediated nitrite reduction can play an important role in NO generation and soluble guanylyl cyclase activation under hypoxic conditions, with this process regulated by pH, oxygen tension, nitrite concentration, and the redox state of the cells.
Introduction
Cytoglobin (Cygb)2 is a hexacoordinate hemeprotein in the globin family that is widely expressed in almost all tissues (1). Cygb is mainly located in the cytoplasm of cells (2). One possible function of Cygb is similar to myoglobin (Mb) transferring oxygen from arterial blood to the tissues. Recent studies suggest that Cygb is involved in nitric oxide (NO) metabolism and may play a cytoprotective role under ischemic/hypoxic conditions, but the underlying mechanisms remain unclear (3–5).
NO is a paramagnetic molecule that exerts a large number of important regulatory biological functions and also plays an important role in the pathogenesis of cellular injury (6–8). In addition to NO generated from NO synthases, under ischemic conditions where marked intracellular acidosis occurs, nitrite can be a major source of NO generation when NO synthase is impaired (9–27).
Multiple hemeproteins, including myoglobin (Mb), hemoglobin (Hb), cytochrome c (Cyt c), and neuroglobin (Ngb), have been reported to function as nitrite reductases in mammalian tissues and blood under anaerobic conditions (16–24, 28). However, there has been a lack of prior investigation regarding whether Cygb can also reduce nitrite to NO and the reaction mechanism and relative importance of this process.
Cygb is a hexacoordinate hemeprotein with both proximal and distal histidine residues occupying the fifth and sixth coordination sites of the heme iron (29). Thus, Cygb must be in a transient five-coordinate form to provide a binding site for nitrite. In Cygb, exogenous ligands have to displace the distal histidine to form a new complex. Therefore, alterations in the pH or redox state that affect the dissociation of the distal histidine can greatly affect the rate of nitrite binding to Cygb and the further Cygb oxidation by nitrite.
In cells, Cygb concentrations are reported to be in the micromolar range, and tissue hypoxia can further increase Cygb expression levels by up to 10-fold (2). It has been further reported that tissue globins contribute to the remarkable tolerance of mammals toward environmental hypoxia (4).
The principal receptor for NO, soluble guanylyl cyclase (sGC), which catalyzes conversion of GTP to the second messenger molecule cyclic GMP (cGMP), is localized in the cytoplasm, which is also the cellular site of Cygb. Therefore, NO generated by Cygb could readily bind to and activate sGC in cells. In vascular smooth muscle, this activation leads to muscle relaxation with vasodilation. Thus, Cygb-mediated NO formation under hypoxic conditions could serve as an important mechanism of hypoxic vasodilation.
Therefore, we performed studies to characterize the mechanism and magnitude of NO generation from Cygb-mediated nitrite reduction. Electron paramagnetic resonance (EPR) spin trapping and chemiluminescence NO analyzer were utilized to measure the magnitude and rate of NO generation, and immunoassays were used to measure cGMP formation. NO formation is shown to occur due to Cygb-mediated nitrite reduction. The kinetics of Cygb-mediated nitrite reduction and NO generation are characterized. It is determined that physiological levels of Cygb and nitrite can generate sufficient NO to play a critical role in sGC activation and vasodilatation under ischemic conditions.
EXPERIMENTAL PROCEDURES
Materials
Sodium nitrite, MES, and sodium dithionite were obtained from Sigma. sGC was obtained from Alexis Biochemical Corp. (San Diego). Direct cGMP assay kit was obtained from ENZO Life Science Int. (Farmingdale, NY), and cGMP production was quantified by immunoassay according to the protocol provided by the company. N-Methyl-d-glucamine dithiocarbamate (MGD) was synthesized using carbon disulfide and N-methyl-d-glucamine (30, 31). Ferrous ammonium sulfate was purchased from Aldrich (99.997%).
Expression and Purification of Recombinant Cytoglobin
The expression plasmid for Cygb (human Cygb cDNA in pET3a) was obtained from Dr. Thorsten Burmester (Mainz, Germany). Escherichia coli strain BL21(DE3)PLysS was transformed by the plasmid for Cygb expression. Cells were grown in Terrific Broth (47.6 g/liter) supplemented with glycerol (8 ml/liter), ampicillin (0.2 g/liter), and chloramphenicol (0.05 g/liter) in a total volume of 1 liter in a 37 °C shaker. The cells were induced with isopropyl 1-thio-β-d-galactopyranoside when their A600 was between 0.6 and 0.8. After induction, cells were grown overnight in a shaker at 25 °C. Cells were harvested by centrifugation and suspended in ∼100 ml of 50 mm Tris-HCl (pH 7.5), 0.5 m NaCl, 1 mm EDTA, 5 mm dithiothreitol, and complete protease inhibitor tablets (Roche Applied Science). After cell suspension, ∼0.1 volume of 10% (v/v) Triton X-100, 10% (v/v) deoxycholic acid, 500 mm Tris-HCl (pH 7.5), and 20 mm EDTA was added, and the cells were lysed by passage through a high pressure homogenizer (EmulsiFlex-C3 by Avestin, Inc.). The inclusion bodies were pelleted by centrifugation at 3300 × g for 15 min, and the pellet was resuspended and washed three times in 1% (v/v) Triton X-100, 50 mm Tris-HCl (pH 7.5), and 1 mm EDTA. Solubilization of the inclusion bodies was done in 6 m guanidinium hydrochloride, 100 mm NaCl, 0.1 mm EDTA, 50 mm Tris-HCl (pH 7.5), and 1% (v/v) 2-mercaptoethanol for at least 1 h in a cold room at 4 °C. After solubilization, a 1.4 m excess of hemin was added with gentle stirring for an hour prior to placing the solution in dialysis tubing. The human Cygb was dialyzed against 50 mm Tris-HCl (pH 7.5), 100 mm NaCl, and 0.1 mm EDTA at 4 °C. After dialysis, insoluble material was removed by centrifugation (45,000 × g for 1.5 h), and the human Cygb was concentrated using Amicon Ultra-15 centrifugal filters (Millipore Corp.) with a 10,000 molecular weight cutoff. Final purification was done on an AKTA purifier system (GE Healthcare) using a HiPrep 26/60 Sephacryl S-300 high resolution size-exclusion column eluted with 50 mm Tris-HCl (pH 7.5), 100 mm NaCl, and 0.1 mm EDTA. The protein was concentrated and stored in 50-μl aliquots in a −80 °C freezer. All assays of Cygb described below were performed in phosphate buffer (100 mm) unless noted otherwise.
EPR Spectroscopy
EPR measurements were performed using a Bruker EMX spectrometer with an ER 4119HS resonator operating at X-band. Measurements were performed at ambient temperature with a modulation frequency of 100 kHz, modulation amplitude of 2.5 G, microwave power of 20 milliwatts, and scan time of 80 s with 60 averages. NO generated from the reaction solution was purged out using argon to a vessel that contained 1 ml of 2 mm Fe2+-MGD spin-trap solution, as described previously (14). This setup was designed to isolate the reaction solution from the spin trap and thus avoid any possible perturbation caused by Fe2+-MGD complexes on the reaction system.
Spectrophotometric Studies
Cygb was reduced with excess dithionite and passed through a Sephadex G-25 column under anaerobic conditions in a glove box filled with argon. Different concentrations of nitrite were mixed with Cygb, and reaction kinetics of Cygb with nitrite was monitored by absorption spectroscopy in an air-tight cuvette. Optical spectra were measured using a Varian Cary 300 UV-visible spectrophotometer at 25 °C.
Chemiluminescence Measurements
The rate of NO production was measured using a Sievers 280B nitric oxide analyzer interfaced through a DT2821 A-to-D converter to a personal computer. In the analyzer, NO was reacted with ozone forming excited-state NO2, which emits light. Mixing of reagents and separation of NO from the reaction mixture were done at controlled temperature (37 °C) in a glass-purging vessel equipped with a heating jacket. The release of NO was quantified by analysis of the digitally recorded signal from the photomultiplier tube using specially designed data acquisition and analysis software developed in our laboratory. After an initial 30 s of equilibration of the flow from the purging vessel to the detector, the signal provides the rate of NO formation over time (32). Calibration of the magnitude of NO production was determined from the integral of the signal over time compared with that from nitrite concentration standards (13, 33).
Oxygen Control and Measurement
Oxygen-dependent NO generation from Cygb was investigated by purging with an argon/oxygen gas mixture as described in our previous investigations (14, 34). Using the highly sensitive technique of EPR oximetry with the glucose char probe, pO2 values can be measured down to millitorr values (35, 36). Using this EPR oximetry technique, we observed that our reaction vessel purged with argon was markedly hypoxic with a pO2 measured by EPR oximetry as 0.03 torr, which we consider as near-anoxic or for simplicity as anaerobic.
Human Aortic Smooth Muscle Cell Studies
Human aortic smooth muscle cells (SMCs) were obtained from Lonza Walkersville, Inc. (Walkersville, MD). Cells were cultured in high glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum and 50 IU/ml penicillin/streptomycin (50 IU/ml/50 mg/ml) and incubated at 37 °C in a humidified CO2 atmosphere. Experiments were performed using 70–80% confluent cells at passages 4–6.
Gene Knockdown Experiments
Cygb shRNA (short hairpin RNA; for stable transfection) plasmid, Mb or Cyt c siRNA (small interfering RNA; for transient transfection) (Santa Cruz Biotechnology), was used to knockdown corresponding genes in SMCs according to the manufacturer's recommendations. Briefly, for transfection of Cygb shRNA plasmid, a 6-μl aliquot of Lipofectamine RNAiMAX (Invitrogen) was added to 100 μl of antibiotic-free Opti-MEM medium, mixed gently by pipetting up and down, and incubated at room temperature for 5 min. An aliquot of Cygb shRNA plasmid (final concentration of 100 nm) was mixed with the Lipofectamine RNAiMAX/Opti-MEM mixture, incubated at room temperature for 30 min, and added to a 9.5-cm culture well containing 900 μl of antibiotic-free Opti-MEM medium and exponentially growing SMCs. For transfection of Mb or Cyt c siRNA, Lipofectamine RNAiMAX/Opti-MEM mixture volume (6 μl: 100 μl ratio) and siRNA (final concentration of 100 nm) were adjusted as cells were grown in 150-mm culture plates containing 10 ml of medium. Control SMCs were transfected with scrambled shRNA or siRNA. Transfected cells were incubated at 37 °C in a 5% CO2-humidified incubator for 7 h, and Opti-MEM medium was changed to growth medium containing 20% (v/v) serum. 48 h post-transfection, Mb and Cyt c siRNA or scrambled siRNA-transfected cells were collected for further studies. For Cygb shRNA or scrambled shRNA transfection, growth medium containing 3 μg/ml puromycin was added to SMCs at 48 h post-transfection, and cells were allowed to grow and expand. Cygb, Mb, and Cyt c protein expressions were evaluated in transfected SMCs by Western blotting.
Generation of Hypoxia in SMCs
Exponentially growing cells were placed in a modular incubator chamber (Billups-Rothenberg, Inc.). The chamber was sealed, flushed for ∼45 min with 5% CO2/balance N2 gas mixture until an O2 tension of 0.6% was achieved, and placed in a 37 °C incubator for 48 h. Cells were collected for Western blotting and nitrite-dependent sGC activation studies.
Western Blotting
Whole-cell lysate or pure proteins, in RIPA buffer, are quantitated using Bio-Rad DC protein assay kit (Bio-Rad). Proteins were separated in reducing graded (4–20%) polyacrylamide gel (Invitrogen) and electroblotted on PVDF membranes (Bio-Rad). The following antibodies (Santa Cruz Biotechnology) were used: rabbit polyclonal anti-Cygb and anti-Mb; mouse monoclonal anti-Cyt c and β-actin. Anti-mouse and anti-rabbit linked to HRP were used as secondary antibodies. Protein band intensities were quantified by a high resolution Pharos FX Plus Molecular Imager (Bio-Rad). The protein concentration was obtained by the quantization of the band intensities of Cygb and Mb and comparing them to band intensities of pure protein standards run in parallel.
Immunoassay of Guanylyl Cyclase Activity
Activation of sGC was investigated using an enzyme-linked immunoassay. In isolated enzyme studies, ferrous Cygb (10 μm) was incubated with 10 μm nitrite in 1 ml of reaction buffer (10 ng of sGC, 5 mm EDTA, 2 mm MgCl2, and 1 mm GTP in 100 mm phosphate buffer (pH 7.4 or pH 6.0)) at room temperature under anaerobic conditions. After a 30-min incubation, the proteins in the samples were removed using Millipore filters (centrifuged 2000 × g for 10 min at 4 °C). The measurements of cGMP in the solution were performed using a direct cGMP assay kit by immunoassay according to the manufacturer's protocol. The standard curve was obtained with known amounts of cGMP.
In cellular investigations, cells were trypsinized and counted, and 106/ml normal or Cygb, Mb, or Cyt c knockdown SMCs were incubated anaerobically with or without addition of 10 μm nitrite in phosphate-buffered saline (PBS) for 10 min at 37 °C. Cells were washed twice with PBS and collected by centrifuging at 1000 × g for 10 min at 4 °C. 106 cells were treated with 0.1 ml of 0.1 m HCl for ∼15 min at room temperature. Centrifugation at 1000 × g was performed to pellet the cellular debris. The cGMP in the supernatant was assayed immediately using a direct cGMP assay kit by immunoassay according to the manufacturer's protocol.
Statistical Analysis
Values are expressed as the mean of at least three repeated measurements and presented as ± S.D., unless noted otherwise. Statistical significance of difference was evaluated by the Student's t test. A p value of 0.05 or less was considered to indicate statistical significance.
RESULTS
NO Generation from Nitrite and Cygb
To investigate the mechanism and magnitude of NO generation from Cygb-mediated nitrite reduction, EPR spectroscopy was applied to measure NO generation under near-anoxic conditions. Ferrous-Cygb in phosphate buffer was prepared in an anaerobic glove box filled with argon as described under “Experimental Procedures.” The NO production from nitrite by ferrous-Cygb (50 μm) was measured by EPR spectroscopy. The NO generated was purged from the reaction vessel using argon to a secondary vessel containing the spin-trap Fe2+-MGD. NO is paramagnetic and binds with high affinity to the water-soluble spin trap Fe2+-MGD forming the mononitrosyl iron complex that exhibits a characteristic triplet NO-Fe2+-MGD spectrum, enabling detection of nitrite-derived NO formation (10, 37, 38).
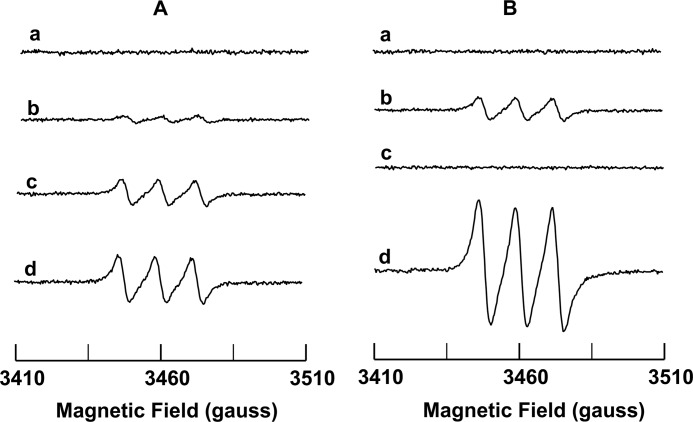
After 120 min of anaerobic incubation at pH 7.0, no significant NO generation was detected from nitrite (100 μm) alone (Fig. 1A, a). However, with the addition of 50 μm Cygb to 10, 50, or 100 μm nitrite, a clear NO triplet signal was seen that increased with the concentration of nitrite (Fig. 1A, b–d). This Cygb and nitrite-dependent NO generation was decreased to half as the pH value increased to 8.0, but it increased more than two times as the pH decreased to 6.0 (Fig. 1B, b and d). In matched experiments, no significant NO generation was detected in the absence of Cygb at pH 8 or 6 (Fig. 1B, a and c). Thus, Cygb can reduce nitrite with NO generation, and this process is facilitated by acidic conditions.
FIGURE 1.
EPR measurement of NO generated from nitrite under anaerobic conditions. A, anaerobic conditions in phosphate buffer (pH 7.0) with nitrite (100 μm) and without Cygb (a), nitrite (10 μm) with Cygb (50 μm) (b), nitrite (50 μm) and Cygb (50 μm) (c), and nitrite (100 μm) with Cygb (50 μm) (d). B, nitrite (100 μm) in phosphate buffer (pH 8.0) (a), nitrite (100 μm) with Cygb (50 μm) in phosphate buffer (pH 8.0) (b), nitrite (100 μm) in phosphate buffer (pH 6.0) (c), and nitrite (100 μm) with Cygb (50 μm) in phosphate buffer (pH 6.0) (d). Reactions were performed at 37 °C in the reaction vessel with NO continuously purged using argon from the reaction vessel into a trap vessel containing 1 ml of 2 mm (MGD)2-Fe2+. Samples were taken from the trap vessel after 120 min, and spectra of the (MGD)2-Fe2+-NO adducts formed are shown.
Spectrophotometric Measurements of Cygb Oxidation by Nitrite
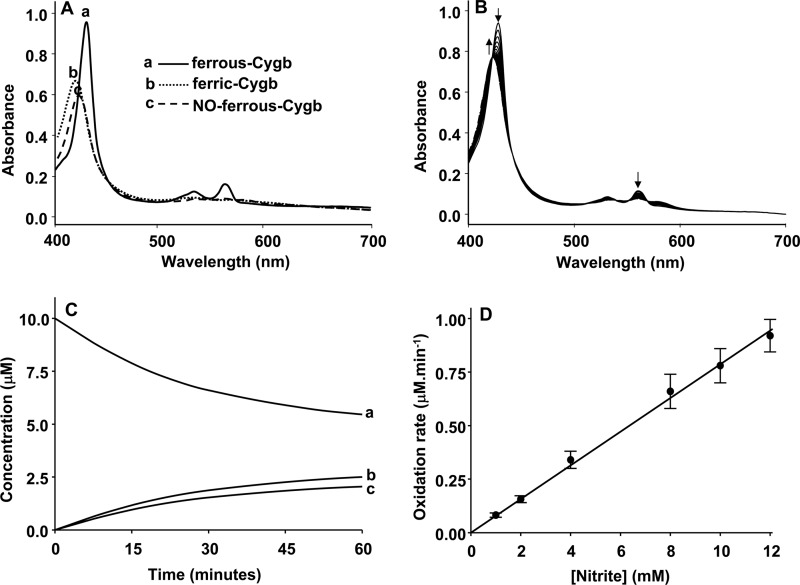
To investigate the mechanism of Cygb-mediated nitrite reduction and subsequent NO release, spectrophotometric studies were performed. Ferrous-Cygb was prepared in an anaerobic glove box as described above. Nitrosyl-Cygb was prepared by mixing Cygb with an excess of NO under anaerobic conditions.
The absorption spectra of 10 μm deoxy-Fe2+-Cygb (Fig. 2A, a), Fe3+-Cygb (Fig. 2A, b), and NO-Fe2+-Cygb (Fig. 2A, c) are shown in Fig. 2. The kinetics of oxidation of Cygb (10 μm) by 2 mm nitrite in phosphate buffer at pH 7.0 was investigated by monitoring the time-dependent change in the visible spectrum of Cygb (Fig. 2B). Spectra were deconvoluted by least squares analysis of multicomponent spectra (39, 40) using Sigma Plot. The time-dependent changes of deoxy-Fe2+-Cygb, Fe3+-Cygb, and NO-Fe2+-Cygb species were calculated by deconvolution of the reaction spectra (Fig. 2C). At pH 7.0, the initial Cygb oxidation rate increased linearly with the increase in nitrite concentration (Fig. 2D).
FIGURE 2.
Kinetics of cytoglobin oxidation by nitrite at pH 7.0 under anaerobic conditions. A, standard visible absorption spectra of deoxycytoglobin of ferrous-Cygb (10 μm) (a), ferric-Cygb (10 μm) (b), and NO-ferrous-Cygb (10 μm) in phosphate buffer (pH 7.0) (c). Measurements were made at room temperature under anaerobic conditions as described under “Experimental Procedures.” B, reaction of Cygb (10 μm) with nitrite (2 mm) in phosphate buffer (pH 7.0) under anaerobic conditions, and the visible absorbance spectra of the reaction mixture was measured every 4 min. C, time-dependent concentration change of ferrous-Cygb (a), ferric-Cygb (b), and NO-ferrous-Cygb (c) spectra in the reaction mixture of B. D, nitrite (1–12 mm) concentration-dependent Cygb (10 μm) oxidation rates in phosphate buffer (pH 7.0) under anaerobic conditions. The points show the measured experimental values from the mean of three repeated measurements ± S.D., and lines show the linear regression fit of the data points with correlation coefficient γ2 >0.98.
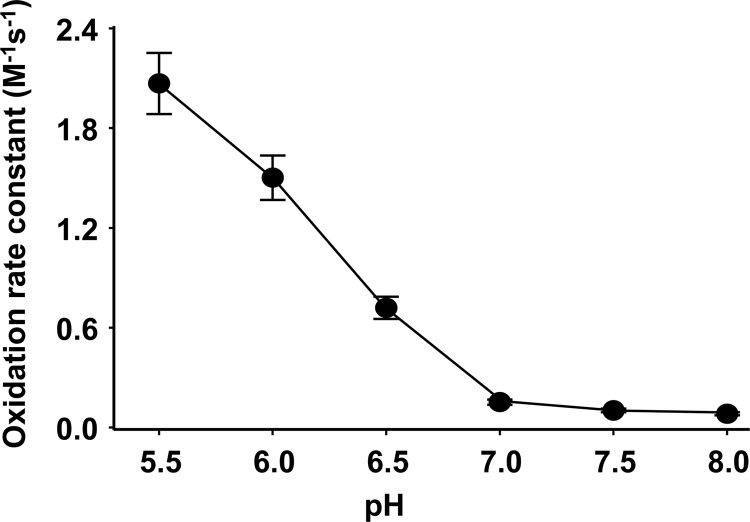
The effect of pH on the kinetics of Cygb oxidation was also investigated. 10 μm Cygb was incubated under anaerobic conditions with 1 mm nitrite at pH 8.0, 7.5, 7.0, 6.5, and 6.0 in phosphate buffer or at pH 5.5 in MES (50 mm). The oxidation rate of Cygb increased nearly 2-fold as pH decreased from 8.0 to 7.0, and it further increased ∼15 times from pH 7.0 to pH 5.5 (Fig. 3A.). From the oxidation rates and the initial concentrations of Cygb and nitrite, the apparent pH-dependent second-order rate constants of the reaction of Cygb and nitrite were calculated (Fig. 3B). At pH 7.0, the rate constant was ∼ 0.14 m−1 s−1, ∼20% higher than the reported reaction rate constant of six-coordinate Ngb oxidation by nitrite of 0.12 m−1 s−1 (40). Under acidic conditions, the apparent rate constant of Cygb oxidation by nitrite increased to 0.7 at pH 6.5, 1.5 at pH 6.0, and 2.1 m−1 s−1 at pH 5.5.
FIGURE 3.
Effect of pH on cytoglobin oxidation. Rate constants of pH-dependent Cygb (10 μm) oxidation by nitrite (1 mm) at pH 5.5 in MES (50 mm) or at pH 6.0, 6.5, 7.0, 7.5, 8.0 in phosphate buffer under anaerobic conditions. Values shown are the mean of three repeated measurements ± S.D.
Kinetics of Nitrite-dependent NO Generation
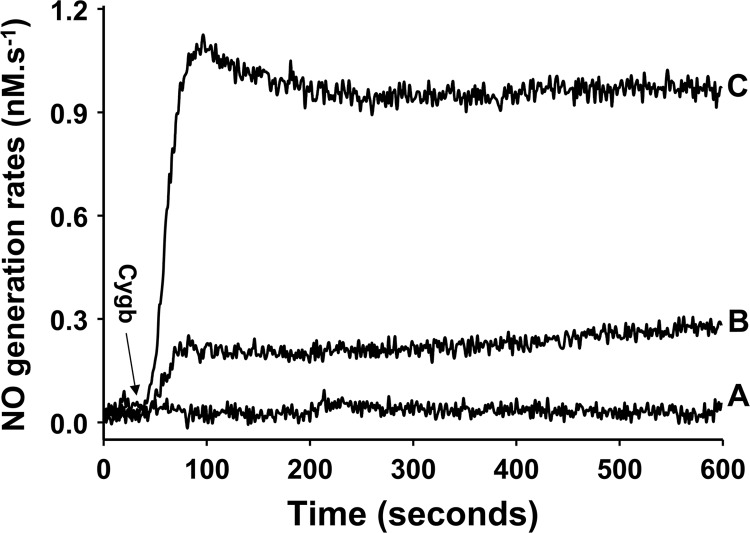
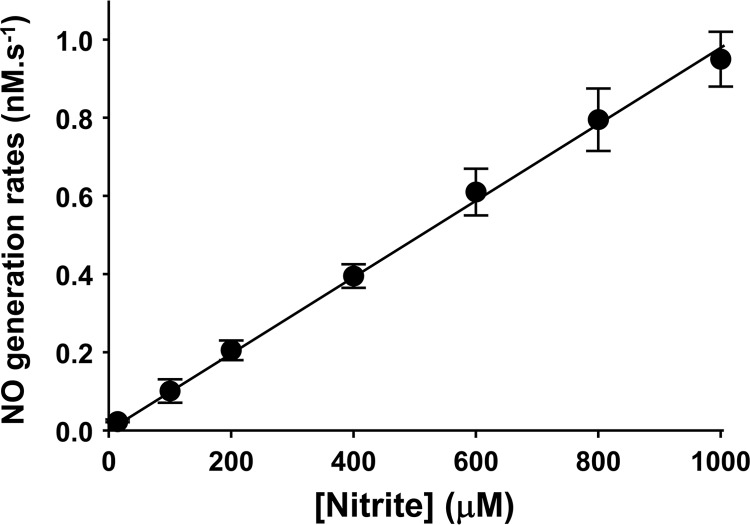
To further quantitate the rate of NO generation from Cygb-mediated nitrite reduction, studies were performed using a chemiluminescence NO analyzer. NO was purged from the solution by argon and then reacted with ozone in the analyzer to form excited-state NO2, which emits light. For the purging vessel and flows used, it took about 30 s for NO to reach the detector. Only after this 30-s delay, the signal recorded by the detector was proportional to the rate of NO generation in the reaction chamber. This method provided direct measurement of the rate of NO generation as a function of time. With nitrite (10 μm) alone in phosphate buffer (pH 7.0), no measurable rate of NO formation was observed (Fig. 4A). With the addition of Cygb (10 μm), prominent NO formation was seen from 200 μm (Fig. 4B) or 1 mm (Fig. 4C) nitrite at pH 7.0. To further characterize the mechanism and magnitude of nitrite-dependent NO formation, kinetic studies of nitrite concentration-dependent NO generation were performed. The rate of NO formation derived from nitrite reduction was measured under anaerobic conditions using the NO analyzer. Following addition of nitrite (0.1-1 mm), prominent generation of NO was detected from 10 μm Cygb at pH 7.0 (Fig. 5). It was determined that the rates of NO release increased linearly with increasing nitrite concentrations (0.1–1 mm).
FIGURE 4.
Measurement of the rate of NO generation from cytoglobin-mediated nitrite reduction. This was performed using the chemiluminescence NO analyzer under anaerobic conditions in phosphate buffer (pH 7.0) with 10 μm nitrite (A), 200 μm nitrite (B), and 1 mm nitrite (C) with 10 μm Cygb in 5 ml of phosphate buffer at 37 °C.
FIGURE 5.
Kinetics of nitrite-dependent NO generation from cytoglobin. Nitrite (0.1–1 mm) concentration-dependent NO generation from Cygb (10 μm) was measured by the chemiluminescence NO analyzer at 37 °C under anaerobic conditions. Linear dependence of NO generation rate versus nitrite concentration was observed, with linear regression γ2 >0.99.
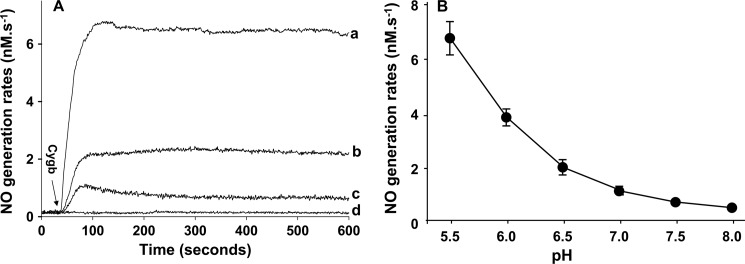
Effect of pH on Cygb-mediated NO Generation
Under ischemic conditions, marked intracellular acidosis occurs, and pH values in tissues, such as the heart, can fall to levels of 5.5 or below (9). To assess the NO formation under different physiological or pathological conditions and to further characterize the mechanism of Cygb-mediated NO generation from nitrite, experiments were performed to measure the effect of different pH values on the magnitude of Cygb-mediated NO generation under anaerobic conditions. It was observed that the rate of NO generation shows a marked increase with decreasing pH values (Fig. 6A). In a series of experiments, the NO generation rate was found to double as the pH value decreased from 8.0 to 7.0, and it increased another two times as the pH decreased from 7.0 to 6.0 (Fig. 6B). Thus, under acidic conditions, increased NO generation occurs. In contrast, under alkaline conditions, prominent inhibition was seen.
FIGURE 6.
Effect of pH on NO generation from cytoglobin-mediated nitrite reduction under near-anaerobic conditions. Measurements were performed with 10 μm Cygb in the presence of 1 mm nitrite at pH 5.5 in 5 ml of MES (50 mm) or at pH 6.0, 6.5, 7.0, 7.5, or 8.0 in 5 ml of phosphate buffer at 37 °C under anaerobic conditions. The maximum rates of NO production were measured by the chemiluminescence NO analyzer under anaerobic conditions. A, chemiluminescence measurements of NO generation from 1 mm nitrite with 10 μm Cygb at pH 5.5 (a), pH 6.5 (b), and pH 7.0 (c) or without addition of Cygb at pH 5.5 (d). B shows the graph of pH-dependent NO generation rates from pH 5.5 to 8.0.
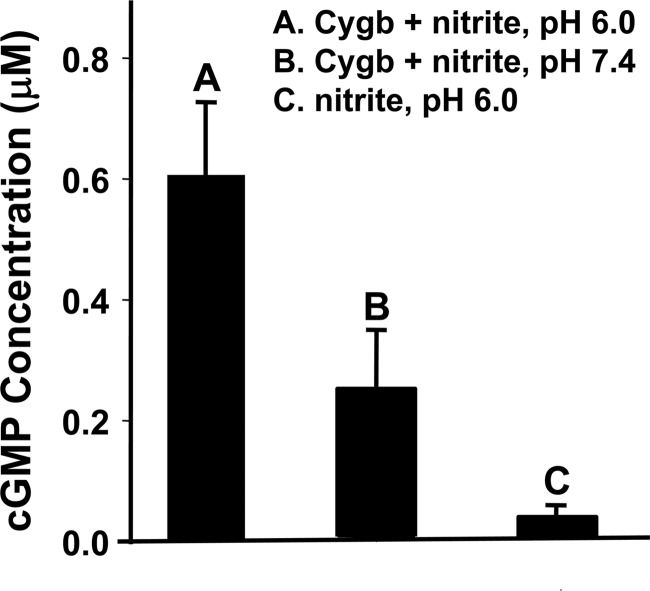
Effect of Nitrite Reduction on sGC Activity
To determine the effect of Cygb-mediated nitrite reduction on sGC activation, enzyme-linked immunoassays were performed to measure cGMP formation. After incubation of 10 μm nitrite with 10 μm Cygb in the reaction buffer under anaerobic conditions for 30 min, measurements of the formation of the sGC product cGMP were performed. Increased cGMP was detected, suggesting that sGC was activated by pathophysiological levels of nitrite in the presence of Cygb at pH 7.4 (Fig. 7A) or pH 6.0 (Fig. 7B). With 100 μm nitrite in the reaction buffer in the absence of Cygb, no significant cGMP formation was detected at pH 7.4 and only a trace amount of cGMP at pH 6.0. These results reveal that physiological levels of Cygb and nitrite can generate NO that is sufficient to activate sGC in tissues, and this activation is greatly facilitated under anaerobic and acidic conditions.
FIGURE 7.
Measurement of sGC activation by cytoglobin-mediated nitrite reduction. sGC activation was determined from measurements of cGMP formation. Reactions were performed in 1 ml of phosphate buffer with EDTA (5 mm), MgCl2 (2 mm), sGC (10 ng), and GTP (1 mm), purged with argon, and incubated with the following: nitrite (10 μm) and Cygb (10 μm) at pH 6.0 (A), pH 7.4 (B), or nitrite (10 μm) without Cygb at pH 6.0 (C). All were incubated for 30 min.
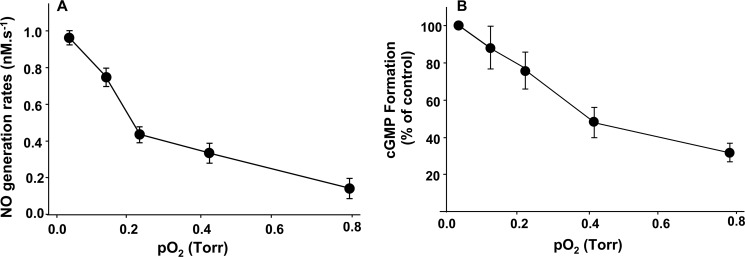
Effect of O2 Tension on Cygb-mediated NO Generation
In biological tissues subjected to ischemia, low O2 tensions occur with a fall to values of 0.4 and 0.1 torr reported in the heart, respectively, after 10 or 30 min of global ischemia (35). To quantitatively determine the effect of low pathophysiological levels of O2 on the process of Cygb-mediated nitrite reduction, chemiluminescence measurements of the rate of NO production were performed in the presence of 10 μm nitrite. Under hypoxic conditions with pO2 values of 0.03 to 0.79 torr, NO formation from Cygb-mediated nitrite reduction was measured. It was observed that the rate of Cygb-mediated NO generation from nitrite was only decreased by ∼25% in the presence of 0.13 torr O2 from that under near-anoxic conditions of 0.03 torr O2. This Cygb-dependent NO generation further decreased with further increase in O2 level (Fig. 8A).
FIGURE 8.
Effect of oxygen on NO generation and sGC activation. Oxygen tension was controlled by purging with argon/oxygen gas mixtures. A, Cygb (10 μm) was incubated with 1 mm nitrite at pH 7.0 in 5 ml of phosphate buffer at 37 °C with pO2 values from 0.03 to 0.79 torr. The rates of NO generation were measured by the chemiluminescence NO analyzer. B, sGC activation was determined from measurements of cGMP formation. Reactions were performed in 1 ml of phosphate buffer with EDTA (5 mm), MgCl2 (2 mm), sGC (10 ng), and GTP (1 mm), incubated with nitrite (10 μm) and Cygb (10 μm) at pH 7.4, and purged with given argon/oxygen gas mixtures with a 30-min incubation time.
Under a similar range of hypoxia with pO2 values of 0.03–0.79 torr, sGC activation by Cygb-mediated nitrite reduction was observed. After incubation of 10 μm nitrite with 10 μm Cygb in the reaction buffer (10 ng/ml sGC, 5 mm EDTA, 2 mm MgCl2, and 1 mm GTP in phosphate buffer (pH 7.4)) under different O2 tensions, measurements of the formation of the sGC product cGMP were performed. With an O2 tension of 0.13 torr, the nitrite-stimulated cGMP production remained ∼90% of that under near-anoxic conditions, but it dropped ∼50% at 0.43 torr pO2 (Fig. 8B). Thus, with pO2 values that occur in ischemic tissues, Cygb-mediated nitrite reduction occurs with NO generation sufficient to activate sGC.
Effect of Nitrite Reduction on sGC Activity in Human SMCs
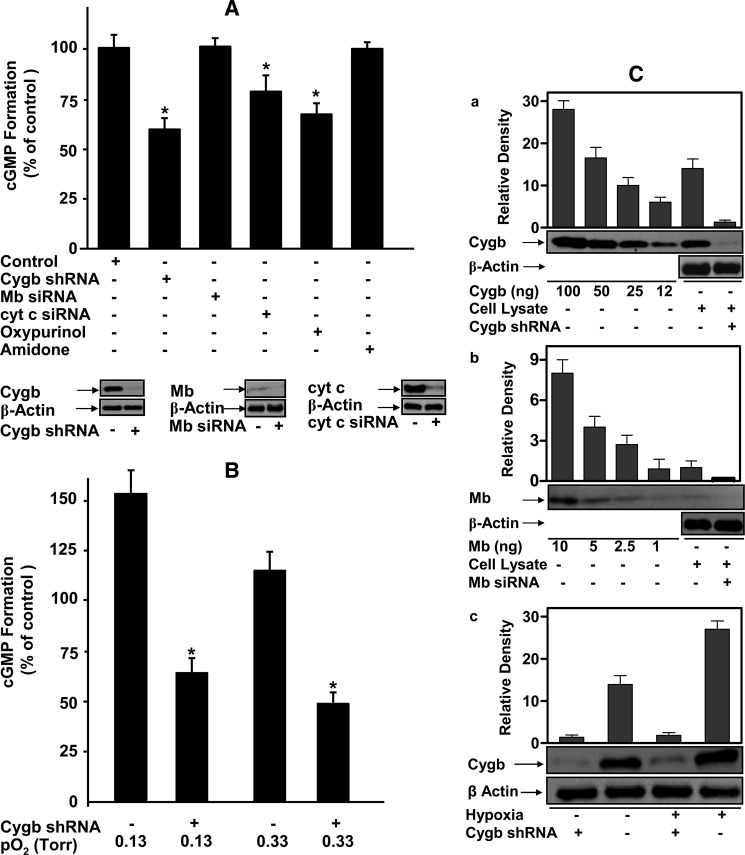
To further investigate the physiological role of Cygb-mediated nitrite reduction in sGC activation, studies were performed using human SMCs. Normal SMCs or Cygb-knockdown SMCs were incubated with or without addition of 10 μm nitrite in PBS for 10 min at 37 °C under marked hypoxic conditions by gently purging the cells with argon. In this SMC experiment with argon purging for 10 min, the pO2 was measured to be 0.13 torr by EPR oximetry. This pO2 was similar to that reported in the ischemic heart after 30 min (35). Without addition of nitrite, only trace cGMP levels of ∼2–3 nm were detected in the supernatant of cell lysates. With the addition of nitrite (10 μm), cGMP increased over 10-fold in normal control SMCs, although clearly less increase was seen in the Cygb-knockdown cells. A significantly higher concentration of cGMP was detected in the control SMCs compared with the Cygb-knockdown cells, p < 0.02, with an ∼40% decrease accompanying the 90% knockdown in Cygb, as visualized on immunoblotting (Fig. 9A).
FIGURE 9.
sGC activation by cytoglobin-mediated nitrite reduction in human smooth muscle cells. 1 × 106 control human smooth muscle cells or cells with different gene knockdown were cultured either under normoxic or hypoxic conditions. Gene knockdown was performed using either shRNA or siRNA as described under “Experimental Procedures.” A, cells were incubated with 10 μm nitrite in 1 ml of PBS in the presence or absence of oxypurinol (0.2 mm) or amidone (20 μm) for 10 min at 37 °C under near-anaerobic conditions by gently purging with argon. * indicates significant difference compared with corresponding control cells (p < 0.05). B, cells were cultured under hypoxic conditions (0.6% O2, pO2 = 4.6 torr) for 48 h, harvested, and then incubated with 10 μm nitrite in 1 ml of PBS for 10 min at 37 °C under severe hypoxia with pO2 0.13 or 0.33 torr. * indicates significant difference of Cygb knockdown cell compared with corresponding cells without treatment of shRNA (p < 0.01). A and B, nitrite dependent-sGC activation was determined from the increased cGMP formation compared with cells incubated under similar conditions without the addition of nitrite. C shows expression level of Cygb (a) or Mb (b) in control or with gene knockdown of normoxic cultured cells; different concentrations of pure Cygb or Mb were run in parallel as standards. c shows expression level of Cygb in normoxic or hypoxic cultured cells with or without Cygb shRNA. Band intensities were quantified by a high resolution Pharos FX-plus Molecular Imager.
Under hypoxia, Cygb expression can be greatly up-regulated (2, 4). After preincubation of SMCs for 48 h under hypoxic conditions, pO2 4.6 torr, Western blotting results showed that the level of Cygb in SMCs doubled (Fig. 9C). Concomitant with the increased levels of Cygb, it was observed that Cygb-mediated nitrite reduction was increased with this contributing over 60% of the total nitrite-dependent sGC activation as shown by the cGMP production from these hypoxia-pretreated cells compared with that in matched cells with Cygb knockdown (Fig. 9B). With similar hypoxia-pretreated cells incubated with nitrite in the presence of higher pO2 levels of 0.33 torr, nitrite-dependent cGMP production remained ∼80% that of similar cells at 0.13 torr (Fig. 9B). Thus, Cygb levels are increased by prior periods of hypoxia. In addition, Cygb-mediated nitrite reduction and sGC activation are seen to occur under the hypoxic conditions present in ischemic tissues (35).
Myoglobin is plentiful in cardiac and skeletal muscle, but its presence in the vessel wall remains controversial. From prior studies, myoglobin is reported to be present at only very low levels in vascular smooth muscle (41). Our quantitative Western blotting results indicate that there is ∼1 ng of Mb, versus ∼45 ng of Cygb in 106 SMCs. Given the single cell volume reported for smooth muscle cells of ∼400 μm3 (42), the concentration of Mb can be estimated to be ∼0.14 μm, which is ∼40 times lower than the level of Cygb (∼5.3 μm) in normal SMCs and ∼80 times lower than the level of Cygb in hypoxia-cultured SMCs (Fig. 9C). Nitrite-dependent sGC activation studies indicate that there was no significant difference in cGMP formation in Mb knockdown SMCs compared with control SMCs (Fig. 9A). Although aldehyde oxidase inhibitor amidone (20 μm) showed no inhibition of nitrite-dependent sGC activation, the xanthine oxidase inhibitor oxypurinol (0.2 mm) conferred about 33% inhibition of sGC activation by nitrite. Accompanying knockdown of Cyt c, a 24% decrease of sGC activation was seen (Fig. 9A). These results indicate that Cygb plays an important role in nitrite-mediated sGC activation in smooth muscle under low oxygen conditions, and this process would be expected to further increase with cellular acidosis.
DISCUSSION
Cygb is a recently identified six-coordinate globin widely expressed in mammalian tissues. To characterize the mechanism and magnitude of Cygb-mediated nitrite reduction, and to further characterize the pathological role of this NO generation pathway under ischemic conditions, we performed a series of studies using EPR spectroscopy, chemiluminescence NO analyzer, and immunoassay to measure the magnitude of NO generation and the activation of sGC. It was observed that addition of nitrite (0.1–12 mm) triggered NO production from ferrous-Cygb under anaerobic conditions (Figs. 1 and 4–6).
Spectrophotometric studies reveal that ferrous-Cygb was oxidized in the presence of nitrite with the formation of ferric-Cygb and nitrosyl-ferrous-Cygb (Fig. 2). This Cygb-mediated nitrite reduction under anaerobic conditions can be described as shown in Reactions 1–3,
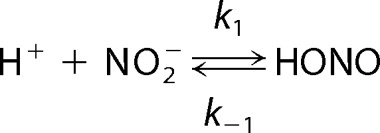 |
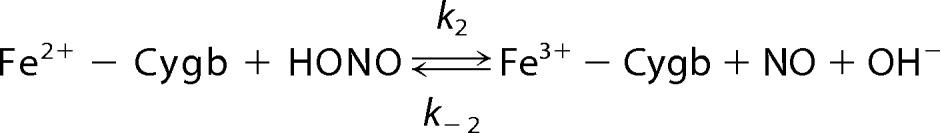 |
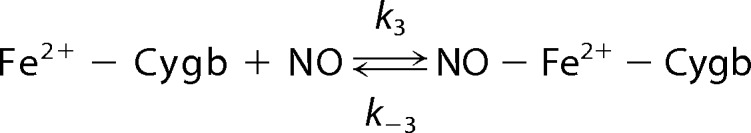 |
Cygb is a hexacoordinate hemeprotein with proximal and distal histidine residues occupying the fifth and sixth coordination sites of the heme iron (29). Thus, six-coordinate Cygb has to be in a transient five-coordinate state for nitrite to bind. The redox state and pH in the cells that affect the coordination of the heme can have a great effect on the rate of Cygb reaction with nitrite. During ischemic conditions with acidosis, protonation of nitrite (Reaction 1) and transformation of six-coordinated Cygb to five-coordinated Cygb can greatly facilitate Cygb-mediated nitrite reduction. From the initial oxidation rates and the concentrations of Cygb and nitrite in the mixture, the apparent pH-dependent second-order rate constants of the reaction of Cygb and nitrite were calculated. We observed that the oxidation rates of Cygb increased nearly two times as the pH decreased from 8.0 to 7.0, and it increased a further ∼15 times from pH 7.0 to pH 5.5 (Fig. 3).
Multiple hemeproteins have been reported to function as nitrite reductases in mammalian tissues and blood (16–24, 28). Several members of the globin family, including Hb, Mb, and Ngb, have shown the ability to reduce nitrite to NO, eliciting vasodilatation under ischemic conditions (16, 21, 28, 39, 40, 43). Compared with the reaction rate of these hemeproteins with nitrite, Cygb had a rate constant similar to that of six-coordinate Ngb, two times faster than six-coordinate mitochondrial Cyt c, but much lower than five-coordinate hemeproteins Hb and Mb (Table 1).
TABLE 1.
Reaction rate constants for nitrite reduction by deoxy-hemeproteins
| Hemeprotein | Nitrite reduction rate constant |
|---|---|
| m−1 s−1 | |
| Cytoglobin (human) | 0.14, 25 °C, pH 7.0 |
| Hemoglobin (human adult) | 0.12 at T state, 6 at R state, 25 °C, pH 7.4 (21) |
| Myoglobin (horse heart) | 6, 25 °C, pH 7.4 (21, 39) |
| Neuroglobin (human) | 0.12, 25 °C, pH 7.4 (40) |
| Cytochrome c (horse heart) | 0.07, 25 °C, pH 7.4a |
a H. Li, C. Hemann, T. M. Abdelghany, M. A. El-Mahdy, and J. L. Zweir, unpublished data.
In addition to the critical function of nitrite reduction, five-coordinate Hb and Mb are extremely effective NO scavengers (44–47). Using isolated Hb, our previous studies revealed the formation of nitrosyl-hemoglobin from the reaction of deoxyhemoglobin with nitrite, but only with nitrite levels well above the Hb concentration could NO release be detected (22). Conversely, six-coordinate Cygb has to be in a transient five-coordinate state to free a binding site of the heme to trap NO as shown in Reaction 4.
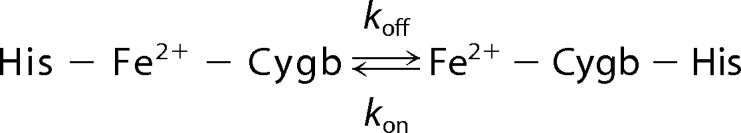 |
The observed kon/koff is 165 at 37 °C (pH 7.0) (48), and thus ∼99.4% of Cygb is present as a six-coordinate that cannot react and scavenge NO. Compared with Mb and Hb, Cygb is a much weaker NO scavenger with only 0.6% of Cygb present as a five-coordinate to react with and trap NO as shown in Reaction 5,
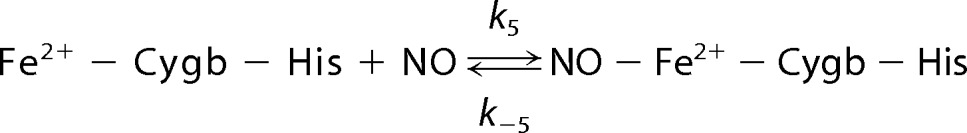 |
We observed a significant amount of NO release from the reaction mixture upon purging with argon (Figs. 4–6). This Cygb-dependent NO generation increases linearly with the increase of nitrite concentration. Of note, as pH decreased from 8.0 to 5.5, Cygb oxidation rates increased about 30 times, but detected NO release rates only increased ∼12 times. This difference may be due to the increased trapping of NO caused by the increase of five-coordinate Cygb at the lower pH.
The effect of pO2 on Cygb-mediated NO generation and sGC activation was investigated (Fig. 8). Our previous studies have shown that after 10 min of ischemia, the pO2 in the myocardium of the isolated rat heart falls to ∼0.2 torr and continues to fall over 20 min to a value of ∼0.12 torr (35). Decreased NO generation and sGC activation were observed with increased pO2 levels. At a pO2 value of 0.13 torr, the rate of Cygb-mediated NO generation from nitrite remained about 75% of that under near-anoxic conditions (Fig. 8A), and cGMP production remained ∼90% (Fig. 8B). Thus, under severe hypoxic conditions as occur in ischemic tissues, Cygb-mediated nitrite reduction can be an important source of NO that induces sGC activation that can in turn lead to compensatory vasodilation. However, as pO2 levels further increase toward normoxic levels, this NO generation pathway would be greatly inhibited as O2 binding to the heme of Cygb would lead to nitrite oxidation and prevent nitrite binding and reduction.
Cygb concentrations in cells, such as smooth muscle, have been reported to be in the micromolar range (∼1–10 μm); however, there are further reports that Cygb can be up-regulated up to an order of magnitude by tissue hypoxia (2, 4). In control smooth muscle cells, we estimated a cellular Cygb concentration of ∼5 μm. From the reported Cygb concentrations and kinetic data, as well as the tissue levels of nitrite, Cygb-mediated NO generation can be estimated. Tissue nitrite levels of ∼10 μm have been reported in heart (9, 10). Based on the data from Fig. 6, Cygb-mediated NO production from 10 μm nitrite with 5 μm Cygb would be ∼7 pm/s in smooth muscle cells at normal pH. The acidic and markedly hypoxic conditions in ischemia would further enhance Cygb-mediated nitrite reduction with Cygb-mediated NO generation from 10 μm nitrite reaching levels of 35 pm/s at pH 5.5. With a 10-fold increase in Cygb levels as has been reported in chronic hypoxia (2, 4), one would estimate NO production rates as high as 350 pm/s. Of note, under hypoxic conditions NO decay would be slow so that NO levels would accumulate over time. Because the Cygb is present in the cytosol in close proximity to sGC, these levels of NO, although low, would be sufficient to activate sGC. Measurements in smooth muscle cells confirm this as knockdown of Cygb clearly decreased nitrite-mediated cGMP formation (Fig. 9A).
To assess the importance of Cygb-mediated nitrite reduction in the process of NO formation and sGC activation, measurement of sGC activation was performed from physiological levels of nitrite and Cygb, both 10 μm as considered above. The addition of nitrite markedly increased cGMP production (Fig. 8), and this Cygb-dependent cGMP production increased significantly with decreasing pH. NO release was observed from Cygb-mediated nitrite reduction (Figs. 1 and 4–6) and escapes trapping by Cygb. sGC is present in the cytoplasm at the same cellular compartment where Cygb is localized. NO generation from Cygb can diffuse only a limited distance to reach and activate sGC without being trapped by scavengers in cells, blood, or tissues as shown in Reaction 6,
Cygb-dependent sGC activation was seen to occur with physiological intracellular levels of nitrite (Fig. 9). Human aortic smooth muscle cell studies demonstrated that sGC activation was much higher in Cygb-containing cells compared with Cygb-knockdown cells accounting for just over 40% of the total in control normoxic cells (Fig. 9). Furthermore, in cells subjected to 48 h of hypoxia, a 2-fold cytoglobin increase occurred with Cygb accounting for over 60% of the total sGC activation.
Five-coordinate hemeproteins Hb and Mb can reduce nitrite with a higher rate than Cygb (Table 1), but Mb and Hb are strong NO scavengers, trapping NO at a rate close to the diffusion limit (44–47). This NO scavenging function can overwhelm the nitrite reductase function. Only a trace amount of Mb (∼0.14 μm) was detected in SMCs, which is about 40 times lower than the Cygb concentration in SMCs (Fig. 9). There was no significant difference for nitrite-dependent sGC activation in normal control cells compared with that in Mb-knockdown cells (Fig. 9). This result indicates that Mb-mediated nitrite reduction does not play a significant role in sGC activation under controlled experimental conditions.
Six-coordinate Cyt c has been reported to function as a nitrite reductase (24, 49). Most of Cyt c is normally intramitochondrial, and the amount in the cytosol that can react with nitrite and activate sGC is unknown. In this study, an ∼24% decrease of sGC activation was seen accompanying knockdown of Cyt c (Fig. 9A).
It has been previously demonstrated that the molybdenum oxidoreductases xanthine oxidase and aldehyde oxidase can function as potent nitrite reductases in tissues (11, 12, 14, 34). In smooth muscle cells, we observed that xanthine oxidase inhibition blocked ∼33% of sGC activation by nitrite, although aldehyde oxidase inhibition had no significant effect.
Although Cygb was found to be the largest contributor to the process of nitrite-mediated sGC activation, Cyt c and xanthine oxidase also significantly contributed to this process. With hypoxia, the induction of higher Cygb expression levels increased the contribution of Cygb to >60%.
In conclusion, our studies demonstrate that Cygb can reduce nitrite leading to NO formation under anaerobic conditions. This process of NO generation from nitrite reduction in tissues would be regulated by pH, nitrite concentration, and the redox state of the cells. Nitrite-derived NO production from Cygb could serve as an alternative source of NO to activate sGC and induce vasodilatation under ischemic conditions that are accompanied by severe hypoxia and acidosis.
Acknowledgment
We thank Ahmed Esmat for help in performing the Western blotting experiments.
This work was supported by National Institutes of Health Grants HL63744, HL65608, and HL38324 (to J. L. Z.).
- Cygb
- cytoglobin
- Ngb
- neuroglobin
- Mb
- myoglobin
- Cyt c
- cytochrome c
- NO
- nitric oxide
- sGC
- soluble guanylyl cyclase
- MGD
- N-methyl-d-glucamine dithiocarbamate
- SMC
- smooth muscle cell.
REFERENCES
- 1. Burmester T., Ebner B., Weich B., Hankeln T. (2002) Cytoglobin. A novel globin type ubiquitously expressed in vertebrate tissues. Mol. Biol. Evol. 19, 416–421 [DOI] [PubMed] [Google Scholar]
- 2. Schmidt M., Gerlach F., Avivi A., Laufs T., Wystub S., Simpson J. C., Nevo E., Saaler-Reinhardt S., Reuss S., Hankeln T., Burmester T. (2004) Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia. J. Biol. Chem. 279, 8063–8069 [DOI] [PubMed] [Google Scholar]
- 3. Burmester T., Gerlach F., Hankeln T. (2007) Regulation and role of neuroglobin and cytoglobin under hypoxia. Adv. Exp. Med. Biol. 618, 169–180 [DOI] [PubMed] [Google Scholar]
- 4. Avivi A., Gerlach F., Joel A., Reuss S., Burmester T., Nevo E., Hankeln T. (2010) Neuroglobin, cytoglobin, and myoglobin contribute to hypoxia adaptation of the subterranean mole rat Spalax. Proc. Natl. Acad. Sci. U.S.A. 107, 21570–21575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Trent J. T., 3rd, Hargrove M. S. (2002) A ubiquitously expressed human hexacoordinate hemoglobin. J. Biol. Chem. 277, 19538–19545 [DOI] [PubMed] [Google Scholar]
- 6. Ignarro L. J., Byrns R. E., Buga G. M., Wood K. S. (1987) Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacological and chemical properties identical to those of nitric oxide radical. Circ. Res. 61, 866–879 [DOI] [PubMed] [Google Scholar]
- 7. Moncada S., Higgs E. A. (1991) Endogenous nitric oxide. Physiology, pathology, and clinical relevance. Eur. J. Clin. Invest. 21, 361–374 [DOI] [PubMed] [Google Scholar]
- 8. Palmer R. M., Ferrige A. G., Moncada S. (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524–526 [DOI] [PubMed] [Google Scholar]
- 9. Zweier J. L., Samouilov A., Kuppusamy P. (1999) Nonenzymatic nitric oxide synthesis in biological systems. Biochim. Biophys. Acta 1411, 250–262 [DOI] [PubMed] [Google Scholar]
- 10. Zweier J. L., Wang P., Samouilov A., Kuppusamy P. (1995) Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1, 804–809 [DOI] [PubMed] [Google Scholar]
- 11. Millar T. M., Stevens C. R., Benjamin N., Eisenthal R., Harrison R., Blake D. R. (1998) Xanthine oxidoreductase catalyzes the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 427, 225–228 [DOI] [PubMed] [Google Scholar]
- 12. Li H., Samouilov A., Liu X., Zweier J. L. (2001) Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J. Biol. Chem. 276, 24482–24489 [DOI] [PubMed] [Google Scholar]
- 13. Li H., Samouilov A., Liu X., Zweier J. L. (2003) Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrate reduction. Evaluation of its role in nitrite and nitric oxide generation in anoxic tissues. Biochemistry 42, 1150–1159 [DOI] [PubMed] [Google Scholar]
- 14. Li H., Samouilov A., Liu X., Zweier J. L. (2004) Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation. J. Biol. Chem. 279, 16939–16946 [DOI] [PubMed] [Google Scholar]
- 15. Li Y., Zhang D., Jin W., Shao C., Yan P., Xu C., Sheng H., Liu Y., Yu J., Xie Y., Zhao Y., Lu D., Nebert D. W., Harrison D. C., Huang W., Jin L. (2006) Mitochondrial aldehyde dehydrogenase-2 (ALDH2) E504K polymorphism contributes to the variation in efficacy of sublingual nitroglycerin. J. Clin. Invest. 116, 506–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cosby K., Partovi K. S., Crawford J. H., Patel R. P., Reiter C. D., Martyr S., Yang B. K., Waclawiw M. A., Zalos G., Xu X., Huang K. T., Shields H., Kim-Shapiro D. B., Schechter A. N., Cannon R. O., 3rd, Gladwin M. T. (2003) Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 9, 1498–1505 [DOI] [PubMed] [Google Scholar]
- 17. Gladwin M. T. (2005) Hemoglobin as a nitrite reductase regulating red cell-dependent hypoxic vasodilation. Am. J. Respir. Cell Mol. Biol. 32, 363–366 [DOI] [PubMed] [Google Scholar]
- 18. Huang K. T., Keszler A., Patel N., Patel R. P., Gladwin M. T., Kim-Shapiro D. B., Hogg N. (2005) The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J. Biol. Chem. 280, 31126–31131 [DOI] [PubMed] [Google Scholar]
- 19. Crawford J. H., Isbell T. S., Huang Z., Shiva S., Chacko B. K., Schechter A. N., Darley-Usmar V. M., Kerby J. D., Lang J. D., Jr., Kraus D., Ho C., Gladwin M. T., Patel R. P. (2006) Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107, 566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gladwin M. T., Schechter A. N., Kim-Shapiro D. B., Patel R. P., Hogg N., Shiva S., Cannon R. O., 3rd, Kelm M., Wink D. A., Espey M. G., Oldfield E. H., Pluta R. M., Freeman B. A., Lancaster J. R., Jr., Feelisch M., Lundberg J. O. (2005) The emerging biology of the nitrite anion. Nat. Chem. Biol. 1, 308–314 [DOI] [PubMed] [Google Scholar]
- 21. Huang Z., Shiva S., Kim-Shapiro D. B., Patel R. P., Ringwood L. A., Irby C. E., Huang K. T., Ho C., Hogg N., Schechter A. N., Gladwin M. T. (2005) Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Invest. 115, 2099–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H., Cui H., Kundu T. K., Alzawahra W., Zweier J. L. (2008) Nitric oxide production from nitrite occurs primarily in tissues not in the blood. Critical role of xanthine oxidase and aldehyde oxidase. J. Biol. Chem. 283, 17855–17863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li H., Liu X., Cui H., Chen Y. R., Cardounel A. J., Zweier J. L. (2006) Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J. Biol. Chem. 281, 12546–12554 [DOI] [PubMed] [Google Scholar]
- 24. Chen Y. R., Chen C. L., Liu X., Li H., Zweier J. L., Mason R. P. (2004) Involvement of protein radical, protein aggregation, and effects on NO metabolism in the hypochlorite-mediated oxidation of mitochondrial cytochrome c. Free Radic. Biol. Med. 37, 1591–1603 [DOI] [PubMed] [Google Scholar]
- 25. Godber B. L., Doel J. J., Sapkota G. P., Blake D. R., Stevens C. R., Eisenthal R., Harrison R. (2000) Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 275, 7757–7763 [DOI] [PubMed] [Google Scholar]
- 26. Sergeev N. S., Ananiadi L. I., L'vov N. P., Kretovich W. L. (1985) The nitrate reductase activity of milk xanthine oxidase. J. Appl. Biochem. 7, 86–92 [PubMed] [Google Scholar]
- 27. Zhang Z., Naughton D., Winyard P. G., Benjamin N., Blake D. R., Symons M. C. (1998) Generation of nitric oxide by a nitrite reductase activity of xanthine oxidase. A potential pathway for nitric oxide formation in the absence of nitric oxide synthase activity. Biochem. Biophys. Res. Commun. 249, 767–772 [DOI] [PubMed] [Google Scholar]
- 28. Petersen M. G., Dewilde S., Fago A. (2008) Reactions of ferrous neuroglobin and cytoglobin with nitrite under anaerobic conditions. J. Inorg. Biochem. 102, 1777–1782 [DOI] [PubMed] [Google Scholar]
- 29. de Sanctis D., Dewilde S., Pesce A., Moens L., Ascenzi P., Hankeln T., Burmester T., Bolognesi M. (2004) Crystal structure of cytoglobin. The fourth globin type discovered in man displays heme hexa-coordination. J. Mol. Biol. 336, 917–927 [DOI] [PubMed] [Google Scholar]
- 30. Shinobu L. A., Jones S. G., Jones M. M. (1984) Sodium N-methyl-d-glucamine dithiocarbamate and cadmium intoxication. Acta Pharmacol. Toxicol. 54, 189–194 [DOI] [PubMed] [Google Scholar]
- 31. Zweier J. L., Wang P., Kuppusamy P. (1995) Direct measurement of nitric oxide generation in the ischemic heart using electron paramagnetic resonance spectroscopy. J. Biol. Chem. 270, 304–307 [DOI] [PubMed] [Google Scholar]
- 32. Samouilov A., Zweier J. L. (1998) Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal. Biochem. 258, 322–330 [DOI] [PubMed] [Google Scholar]
- 33. Samouilov A., Kuppusamy P., Zweier J. L. (1998) Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch. Biochem. Biophys. 357, 1–7 [DOI] [PubMed] [Google Scholar]
- 34. Li H., Kundu T. K., Zweier J. L. (2009) Characterization of the magnitude and mechanism of aldehyde oxidase-mediated nitric oxide production from nitrite. J. Biol. Chem. 284, 33850–33858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zweier J. L., Chzhan M., Ewert U., Schneider G., Kuppusamy P. (1994) Development of a highly sensitive probe for measuring oxygen in biological tissues. J. Magn. Reson. 105, 52–57 [DOI] [PubMed] [Google Scholar]
- 36. Kuppusamy P., Chzhan M., Samouilov A., Wang P., Zweier J. L. (1995) Mapping the spin-density and line shape distribution of free radicals using 4D spectral-spatial EPR imaging. J. Magn. Reson. 107, 116–125 [DOI] [PubMed] [Google Scholar]
- 37. Xia Y., Zweier J. L. (1995) Substrate control of free radical generation from xanthine oxidase in the postischemic heart. J. Biol. Chem. 270, 18797–18803 [DOI] [PubMed] [Google Scholar]
- 38. Lancaster J. R., Jr., Langrehr J. M., Bergonia H. A., Murase N., Simmons R. L., Hoffman R. A. (1992) EPR detection of heme and non-heme iron-containing protein nitrosylation by nitric oxide during rejection of rat heart allograft. J. Biol. Chem. 267, 10994–10998 [PubMed] [Google Scholar]
- 39. Shiva S., Huang Z., Grubina R., Sun J., Ringwood L. A., MacArthur P. H., Xu X., Murphy E., Darley-Usmar V. M., Gladwin M. T. (2007) Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 100, 654–661 [DOI] [PubMed] [Google Scholar]
- 40. Tiso M., Tejero J., Basu S., Azarov I., Wang X., Simplaceanu V., Frizzell S., Jayaraman T., Geary L., Shapiro C., Ho C., Shiva S., Kim-Shapiro D. B., Gladwin M. T. (2011) Human neuroglobin functions as a redox-regulated nitrite reductase. J. Biol. Chem. 286, 18277–18289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ormerod J. O., Ashrafian H., Maher A. R., Arif S., Steeples V., Born G. V., Egginton S., Feelisch M., Watkins H., Frenneaux M. P. (2011) The role of vascular myoglobin in nitrite-mediated blood vessel relaxation. Cardiovasc. Res. 89, 560–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tornig J., Gross M. L., Simonaviciene A., Mall G., Ritz E., Amann K. (1999) Hypertrophy of intramyocardial arteriolar smooth muscle cells in experimental renal failure. J. Am. Soc. Nephrol. 10, 77–83 [DOI] [PubMed] [Google Scholar]
- 43. Dezfulian C., Raat N., Shiva S., Gladwin M. T. (2007) Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc. Res. 75, 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma V. S., Traylor T. G., Gardiner R., Mizukami H. (1987) Reaction of nitric oxide with hemeproteins and model compounds of hemoglobin. Biochemistry 26, 3837–3843 [DOI] [PubMed] [Google Scholar]
- 45. Joshi M. S., Ferguson T. B., Jr., Han T. H., Hyduke D. R., Liao J. C., Rassaf T., Bryan N., Feelisch M., Lancaster J. R., Jr. (2002) Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc. Natl. Acad. Sci. U.S.A. 99, 10341–10346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu X., Yan Q., Baskerville K. L., Zweier J. L. (2007) Estimation of nitric oxide concentration in blood for different rates of generation. Evidence that intravascular nitric oxide levels are too low to exert physiological effects. J. Biol. Chem. 282, 8831–8836 [DOI] [PubMed] [Google Scholar]
- 47. Cooper C. E. (1999) Nitric oxide and iron proteins. Biochim. Biophys. Acta 1411, 290–309 [DOI] [PubMed] [Google Scholar]
- 48. Liu X., Follmer D., Zweier J. R., Huang X., Hemann C., Liu K., Druhan L. J., Zweier J. L. (2012) Characterization of the function of cytoglobin as an oxygen-dependent regulator of nitric oxide concentration. Biochemistry 51, 5072–5082 [DOI] [PubMed] [Google Scholar]
- 49. Basu S., Azarova N. A., Font M. D., King S. B., Hogg N., Gladwin M. T., Shiva S., Kim-Shapiro D. B. (2008) Nitrite reductase activity of cytochrome c. J. Biol. Chem. 283, 32590–32597 [DOI] [PMC free article] [PubMed] [Google Scholar]