Abstract
Background
Our objective was to determine the quality of literature in costing of the economic burden of patient safety.
Methods
We selected 15 types of patient safety targets for our systematic review. We searched the literature published between 2000 and 2010 using the following terms: “costs and cost analysis,” “cost-effectiveness,” “cost,” and “financial management, hospital.” We appraised the methodologic quality of potentially relevant studies using standard economic methods. We recorded results in the original currency, adjusted for inflation, and then converted to 2010 US dollars for comparative purposes (2010 US$1.00 = 2010 €0.76). The quality of each costing study per patient safety target was also evaluated.
Results
We screened 1948 abstracts, and identified 158 potentially eligible studies, of which only 61 (39%) reported any costing methodology. In these 61 studies, we found wide estimates of the attributable costs of patient safety events ranging from $2830 to $10,074. In general hospital populations, the cost per case of hospital-acquired infection ranged from $2132 to $15,018. Nosocomial bloodstream infection was associated with costs ranging from $2604 to $22,414.
Conclusion
There are wide variations in the estimates of economic burden due to differences in study methods and methodologic quality. Greater attention to methodologic standards for economic evaluations in patient safety is needed.
Keywords: patient safety, burden of illness, review, quality
Introduction
Patient safety has received considerable public, professional, political, and scientific attention over the past decade. Although the human burden associated with adverse events is well established, the economic cost of patient safety has received less attention. Despite the substantial effort that has been expended to develop and implement safety improvements, there is uncertainty about both the economic impact of unsafe care and the improvement strategies that offer the best value. Significant resources have been expended across the world to reduce patient safety events through interventions, without clear improvements.1,2
A fuller understanding of the economic burden of patient safety may inform health policy, health services research priorities, safety improvement priorities, and patient safety priorities. High quality data on economic burden of a condition are an essential component of comparative economic analyses such as cost-effectiveness analyses.
The objective of an economic burden study is to describe the economic impact of a patient safety target. These types of studies generally examine the overall cost of the condition to an environment (eg, acute care setting, society).
Economic burden studies should be based on rigorous analytical methods, be impartial and credible in the use of data, and be transparent for and accessible by the reader.3 Economic burden studies are conducted using recognized frameworks which can be modified for specific target conditions. 4,5 Drummond and Jefferson and Drummond et al constructed a checklist of economic parameters used worldwide. 6,7 Economic burden studies should clearly outline the resource studies, the method for attributing costs to these resources, the method for measuring the resources used, the time frame for measuring the resources, and the economic perspective (hospital, third-party payer, or society) from which the resources were measured.
Our objective was to determine the quality of literature in costing of the economic burden of patient safety targets in the acute care environment.
Methods
We developed a list of patient safety targets based on prior systematic reviews,8 and existing national and international safety initiatives.9,10 Patient safety targets were based on three characteristics: (1) a clinical outcome (eg, hospital-acquired methicillin-resistant Staphylococcus aureus [MRSA] infection) or a surrogate with an established link to a clinical outcome (eg, MRSA colonization); (2) high specificity as a measure of patient safety, as opposed to being a naturally occurring condition; and (3) a sufficiently long history of measuring this outcome in the literature, such that some studies on the economic burden could be expected.
Patient safety targets included: adverse events, adverse drug events, ventilator-associated pneumonia, nosocomial urinary tract infection, antibiotic-resistant organism colonization, antibiotic-resistant organism infection, catheter-associated bloodstream infection, nosocomial Clostridium difficile-associated disease, surgical site infection, nosocomial pressure ulcers, wrong site surgery, retained surgical foreign bodies, contrast-induced nephropathy, nosocomial venous thromboembolism, and nosocomial fall-related injuries. We also included six improvement strategies (hand hygiene, rapid response teams, bundles, check-lists, automatic stop orders and bar coding) to ensure that we obtained all relevant economic literature that may not be captured through searches based solely on patient safety targets.
We sought burden-of-illness or cost-of-illness studies. A search was performed using the MEDLINE database for articles published between 2000 and 2010 using the following search terms for costs: “costs and cost analysis” (Medical Subject Headings [MeSH]), “cost-effectiveness” (text word), “cost” (text word), and “financial management, hospital” (MeSH). We also searched the Agency for Healthcare Research and Quality Patient Safety Network (see http://psnet.ahrq.gov) using the term “cost.”
Reviews, editorials, and articles with no costing information in the abstract were excluded. One member of the study team (MK) excluded reviews, editorials, and articles with no costing information in the abstract. Two independent members of the study team (MK and EE) reviewed the remaining abstracts and obtained the full publication of any abstract considered potentially relevant by either member. Full publications of any abstracts considered potentially relevant were retrieved. Two investigators (EE and NM) independently evaluated each publication, using adapted relevant methodologic features (n = 21) as described by Drummond and Jefferson.6 Each feature was arbitrarily scored one point, for a maximum score of 21. Features from the original list were excluded if they were not applicable to economic burden studies. If the two reviewer scores were within five points of one another, the higher score was taken. Otherwise, reviewers met to discuss and resolve discrepancies.
We report all cost data in 2010 United States dollars for comparative purposes between patient safety targets. The original year and currency is stated in the summary data. Each cost was first converted to US dollars of the same year as indicated in the publication using the Bank of Canada currency converter.11 Then, each converted cost was inflated to 2010 US dollars using the United States Department of Labor Bureau of Labor Statistics inflation calculator.12
Results
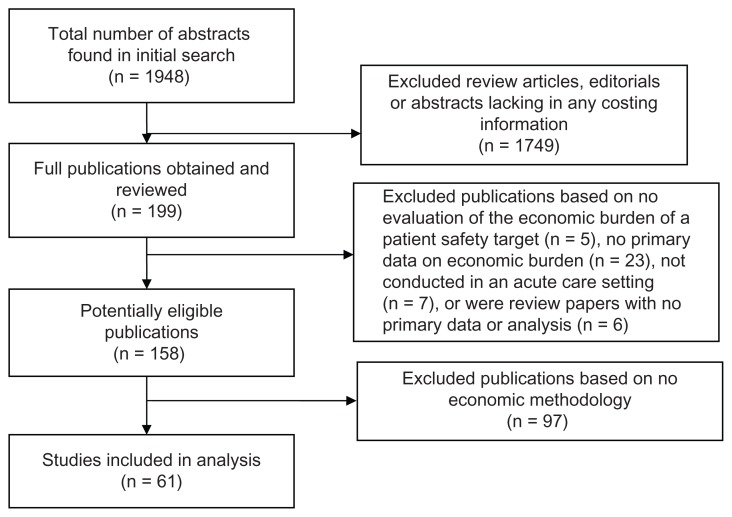
Our initial search yielded 1948 citations. We screened out 1749 abstracts that were review articles, editorials, or lacking in any costing information or results. We obtained and reviewed the remaining 199 publications. We excluded 41 publications for the following reasons: no evaluation of the economic burden of a patient safety target (n = 5), no primary data on economic burden (n = 23), not conducted in an acute care setting (n = 7) or were review papers with no primary data or analysis (n = 6). This left 158 potentially eligible publications. Ninety-seven (61%) of the 158 potentially eligible publications described no economic methodology despite reporting an estimate of economic burden and were excluded from further review (Figure 1).
Figure 1.
Results of screening and exclusion process.
Methodologic quality
For the remaining 61 studies, the median methodologic feature score was 15/21 (mean 14.6 ± 21, range 9–20). All studies had essential methodologic features such as a statement of the research question, a statement of the economic importance of the research question, and a justification of the economic viewpoint. Fewer than 50% of these 61 studies reported productivity changes, discussed the relevance of productivity changes, provided details of inflation adjustments or currency conversions, or described any sensitivity analyses. Studies used different methods for identifying attributable cost, including propensity scores, case–controls, and regression analysis (Tables 1 and 2).
Table 1.
Summary of studies of economic burden of patient safety targets in acute care (detailed summary of each study is in Table 3)
| Number of studies | Mean methodologic feature score [median, range] | Study design | |
|---|---|---|---|
| Adverse events and adverse drug events | 8 | 13 [13, 12–16] | Retrospective cohort study (n = 5), prospective cohorts with nested cases and controls (n = 2), case series (n = 1) |
| Nosocomial infections (not otherwise specified) | 10 | 14 [15, 12–16] | Prospective study (n = 1), retrospective cohort (n = 5), retrospective case control study (n = 3), decision model (n = 1) |
| Surgical site infections | 8 | 14 [14.5, 11–17] | Prospective study (n = 1), retrospective cohort (n = 3), retrospective case control study (n = 2), nested case control (n = 2) |
| Nosocomial bloodstream infections | 10 | 14 [15, 9–18] | Prospective study (n = 1), retrospective cohort (n = 3), retrospective case control study (n = 5), case series (n = 1) |
| Nosocomial sepsis | 2 | 17 [17.5, 15–20] | Prospective cohort (n = 1), retrospective cohort (n = 1) |
| Nosocomial rotavirus infections | 3 | 14 [14, 13–15] | Prospective cohort (n = 1), prospective case series (n = 1), nested case control (n = 1) |
| Nosocomial urinary tract infection | 4 | 13 [15, 9–15] | Prospective cohort (n = 1), retrospective cohort (n = 2), retrospective case control (n = 1) |
| Nosocomial pneumonia | 4 | 14 [13–15] | Prospective cohort (n = 2), prospective/ retrospective case control (n = 1), retrospective case control (n = 1) |
| Nosocomial respiratory tract infection | 3 | 15 [15, 15–15] | Retrospective cohort (n = 1), retrospective case control (n = 1), one case control (n = 1) |
| Miscellaneous nosocomial infections | 12 | 15 [14.5, 12–20] | Prospective nested case control (n = 1), case control (n = 1), retrospective case series (n = 4), retrospective case control (n = 2), retrospective cohort (n = 3), retrospective nested case control (n = 1) |
| Nosocomial venous thromboembolism | 2 | 17 [17, 16–18] | Decision analysis (n = 1), retrospective observational cohort study (n = 1) |
| Nosocomial falls | 3 | 15 [15, 14–16] | Prospective cohort (n = 1), case series (n = 2) |
| Total | 68a |
Note:
Sixty-one studies in total, of which three reported outcomes for more than one type of infection, therefore the total listed in Table 2 is 68.
Table 2.
Methodologic characteristics of studies of economic burden of patient safety events in acute care (n = 61)
| Methodologic feature | Studies with this feature present % (n) | Example of information used in the analysis |
|---|---|---|
| Study design | ||
| The research question is stated | 100% (61) | “The aim of this study was to evaluate the epidemiology, additional length of stay, incremental costs and outcomes due to hospitalacquired infections, and to estimate the potential impact of infection control on community hospitals and medical centers.”26 |
| The economic importance of the research question is stated | 100% (61) | “More recently, an argument has been made to focus on direct costs (primarily consumables), because they are most subject to savings by implementation of effective infection control interventions.”37 |
| The viewpoint(s) of the analysis are clearly stated and justified | 100% (61) | “The main focus of costs calculated in this study was the health care sector.”13 |
| The form of economic evaluation used is stated | 67% (41) | “Economic burden of surgical site infections at a European university hospital.”32 |
| The choice of form of economic evaluation is justified in relation to the questions addressed | 67% (41) | “To quantify the economic and medical burden of SSIs in a European university hospital, we conducted a matched casecontrol study nested in a larger prospective observational study.”32 |
| Data collection | ||
| The primary outcome measure(s) for the economic evaluation are clearly stated | 74% (45) | “Data on the predicted number of cases of hospital-acquired infection were combined with data on the estimated prolongation of stay due to hospital-acquired infection. This produced an estimate of the number of bed days attributable to hospital-acquired infection. Valuations of the opportunity cost of the resources used to supply a bed day were applied to derive a monetary estimate of the opportunity cost of hospital-acquired infection.”30 |
| Productivity changes (if included) are reported separately | 11% (7) | “Lost productivity costs due to hospital staff members on sick leave totaled €9,264.”58 |
| The relevance of productivity changes to the study question is discussed | 15% (9) | “Additional expenses were €18,375 for increased nursing care (extra staffing of temporary isolation ward).”58 |
| Quantities of resources are reported separately from their unit costs | 72% (44) | “All patients staying more than 24 hours in a 19-bed MICU at Barnes-Jewish Hospital from Jul 1, 1997 to Dec 31, 1999 were eligible. All health care workers and visitors were required to wear gowns and gloves on entry into the rooms of patients colonized or infected with VRE from Jul 1, 1997 to Jun 30, 1998 and from Jul 1, 1999 to Dec 31, 1999. All patients were actively screened[…]. A matched cohort study design was used to determine the attributable cost of VRE. Patients without VRE from the same [medical intensive care unit] population were matched to patients with VRE by diagnosis-related group (DRG) code, APACHE score, and age.VRE-attributable length of MICU stay (d): 4.0, 18.9, 35.3 VRE-attributable LOS (d): 8.3, 38.2, 18.9.”67 |
| Methods of the estimation of quantities and unit costs are described | 88% (54) | “Overall costs for the vancomycin-resistant Enteroccoci surveillance and infection control program were estimated using the hospital’s step-down cost allocation system, which recorded line-item cost data per resource consumed and total cost per hospital admission. MICU costs were estimated from these data by dividing the patient’s total hospitalization cost by total days of hospitalization and then multiplying the quotient by the patient’s total MICU-days. This data system also provided hospital reimbursement data, type of insurance, case-mix index, and DRG. Variable cost
|
| Currency and price data are recorded | 80% (49) | “Costs are reported in 2001 Euros (1€ = US $0.95).”46 |
| Details of currency of price adjustments for inflation or currency conversion are given | 39% (24) | “All costs were adjusted to 1999 dollars using the consumer price index for health care.”44 |
| Analysis and interpretation of results | ||
| Time horizon of costs and benefits is stated | 87% (53) | “In the hospital X, a total of 90 persons with symptoms and signs consistent with norovirus gastroenteritis with clinical onsets in the time period from Dec 1, 2006 to Feb 13, 2007 were reported.”58 |
| Details of statistical tests and confidence intervals are given for stochastic data | 95% (58) | “Using logistic regression, preoperative antibiotic therapy (cefazolin/metronidazole vs cefotetan), patient demographics, surgical procedure, obesity, and modified SECNIC score were examined as predictors of LOS ≥ 1 week and cost ≥$15,000.”34 |
| The approach to sensitivity analysis is given | 30% (18) | “Models 2 and 3 incorporated additional cost predictors sequentially: suspected and confirmed HAI and ICU treatment.”23 |
| The choice variables for sensitivity analysis is justified | 30% (18) | “Several parameters were changed to determine the impact of our four main assumptions on the net benefits of gowns.”67 |
| The ranges over which the variables are varied are stated | 31% (19) | “The variation between 60 to 140 patient contacts yielded net benefits of $388,664 and $450,017, respectively. The variable of 1 to 4 cultures per patients resulted in net benefits of $418,188 and $421,464, respectively. The variation in costs of labor and materials results in net benefits of $406,488 and $435,426, respectively.”67 |
| Major outcomes are presented in a disaggregated as well as aggregated forma | 54% (33) | “Cost data were available for all of the 164 patients admitted after Jul 1, 1999.”45 |
| The answer to the study question is given | 98% (60) | “In conclusion, in the presence of prompt catheter removal and initiation of antimicrobial therapy, no significant attributable mortality could be documented in critically ill patients. However, increases in the durations of ICU and hospital stay contribute to an important economic burden. These significant increases in cost underscore the need to vigorous application of evidence-based cost-effective preventive measures.”38 |
| Conclusions follow from the data reported | 98% (60) | “Prevention of MRSA infection is essential if we are to minimize its major impact on individual patients and if we are to get the most effective use of health care resources.”64 |
| Conclusions are accompanied by the appropriate caveats | 93% (57) | “It should be understood that the cost of an infection, if avoided, will not be realized as a cash saving. Many of the costs/benefits are fixed and it is principally the variable costs/benefits (for example drugs and other consumables), which represent a small proportion of the total costs, that would show as cash savings, and as such an expenditure that could be avoided.”27 |
Notes:
When the major outcomes are presented in aggregated form the overall cost is stated, for example, surgical site infections cost $10,000. If the major outcomes are presented in disaggregated form the different components that make up the overall cost is stated. For example, surgical site infections cost $10,000, of that professional costs were $3000, medication costs were $2000 and hospitalization costs were $5000. (Numbers used are for illustration only).
Abbreviations: LOS, length of stay; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus.
There was heterogeneity in study populations, resources incorporated, methods and results, so economic burden summary for all patient safety targets could not be calculated. We summarize the methodologic feature scores and range of results for each type of adverse event in Table 1. The maximum score was 21. We provide detailed summaries of each study organized by patient safety target (Table 3).
Table 3.
Detailed summary of systematic review
| Study, methodologic feature score | Design | Method for estimating attributable cost | Resources measured | Source of resource cost (currency, year) | Sample population (time horizon) | Case definition | Incidence | Attributable LOS | Estimated attributable cost |
|---|---|---|---|---|---|---|---|---|---|
| General studies of adverse events and adverse drug events | |||||||||
| Hoonhout13 Methodologic feature score = 16 | Retrospective cohort | Multivariate multilevel analysis | Direct medical costs, based on additional LOS and additional medical procedures | Dutch guideline prices of 2003, corrected for 2004 (€, 2004) | 7926 patients of which 451 patients with AEs in 21 Dutch hospitals (Aug 2005–Oct 2006) | Any AE: an unintended injury resulting in temporary/ permanent disability, death, extra LOS, caused by health care | 5.7% | University hospitals: 10.1 additional days General: 8.9 additional days |
Attributable costs of all AEs: mean €4446 per AE Excess costs of preventable AEs: mean €3634 per AE |
| Ehsani14 Methodologic feature score = 13 | Retrospective cohort | Simple linear regression modeling | Total cost of per-patient care from database (not further described) | Patient-level costing dataset of the Victorian Department of Human Services (AU$, year unclear) | Of 979,834 admissions, 67,609 had an AE, 45 hospitals in Victoria, Australia (Jun 2003– Jul 2004) | Any AE, identified via diagnosis codes | 6.9% had at least 1 AE | 10 additional days | AU$11,846 per AE |
| New15 Methodologic feature score = 12 | Retrospective cohort | Ordinary least squares regression analysis | LOS, surgical and medical procedures, laboratory tests | Hospital accounting database (AU$, 2004) | Of 1605 SCI patients, 610 with one complication, in 45 campuses of 26 AU health services (Jun 2003–Jun 2004) | At least one AE or HAC in a patient with SCI | 38% of multiday SCI episodes had at least 1 incident complication | 32 additional days | Additional costs, any complication: AU$7359 UTI: AU$23,705 Procedural complications: AU$21,821 Anemia: AU$18,047 Pressure ulcer: AU$17,882 |
| Pappas16 Methodologic feature score = 12 | Retrospective cohort | Regression analysis | Nursing staff hours per patient day, clinical outcomes, patient-level data | Cost accounting system/ l Eclipsys TSI (US$, year unstated) | Of 3200 inpatients. Medical patients: 688 Surgical patients: 461; 2 hospitals from hospital databases (24 month) |
Nosocomial AEs including medication error, fall, UTI, pneumonia, and pressure ulcer | Medical patients: 21.5% surgical patients: 14.4% | Not available | Medical patients: $1029 per AE Surgical patients: $903 per AE |
| Morris17 Methodologic feature score = 12 | Retrospective cohort | Unclear | Hospital charges, costs, legal fees and indemnity payments, legal write-offs | Unclear (assumed US$, year unstated) | 130 cases out of 32,100 patients over age 13 (Jan 1, 1995–Dec 6, 1999) | Surgical AEs, not further specified | 0.4% | Not available | Total legal payment for the study group (126) was $8.2 million |
| Aoki18 Methodologic feature score = 15 | Case series | Multivariate logistic analysis | Legal compensation in medical disputes | Medical dispute records (US$, 2007; converted from JP yen) | 155 resolved medical dispute cases in Japan (1989–1998) | Any medical dispute case resolved during the study period | Not available | Not available | Legal compensation for an AE claim was mean $38,937, median $7417 |
| Kaushal19 Methodologic feature score = 14 | Prospective with nested case control | Matched case-control, linear regression model | Charges, actual variable costs, actual fixed costs, actual direct variable costs and actual direct fixed costs | Hospital TSI database (US$, 2003) | 108 cases matched with 375 controls in 1 hospital MICU and cardiac care unit (Jul 2002–Jun 2003) | Any AE, detected via observation, reports, and guided implicit chart abstraction | Not available | MICU, AEs: 0.77 additional days Cardiac care unit AEs: 1.08 additional days |
$3961 in the MICU, $3857 in the cardiac care unit |
| Senst20 Methodologic feature score = 13 | Prospective with nested case control | Case control, multiple linear regression model | Charges converted to costs | Prospectively recorded charges (US$, year unclear) | Of 3187 admissions 134 had an ADE, in 1 US health care network including 4 hospitals and 26 clinics (53-day study period, 1998) | ADE: an injury caused by the use, disuse or misuse of a drug via error or despite proper usage | 4.2% | 1.2 additional days | $2162 per ADE |
| Nosocomial infections (not otherwise specified)a | |||||||||
| Chen21 Methodologic feature score = 15 | Retrospective analytic cohort | Stratified analysis and regression model | LOS, physician services, medical and surgical procedures, lab and radiology, unit costs | Hospital database (US$, 2001) | 778 patients admitted to 3 ICUs in 1 hospital between Oct 2001 and Jun 2002 | Any nosocomial infection (such as BSI, UTI, SSI) confirmed by culture, symptoms and an attending physician | 10.2% had at least one nosocomial infection | 18.2 additional days | $3306 additional costs per nosocomial infection |
| Chen22 Methodologic feature score = 15 | Retrospective analysis of a prospectively assembled cohort | Generalized linear modeling | Medical and surgical procedures, medications, lab investigation, ICU bed-days | Hospital database, microcosting (US$, 2007, converted from Taiwanese dollars) | 401 NIs in 320 of 2757 patients, in 4 ICUs in one hospital in Taiwan (2003–2004) |
BSI, UTI, SSI, respiratory tract infection “and others” diagnosed ≥ 48 hours after admission to ICU | 14.5 NI episodes per 100 admissions | Not available | $10,015 attributable cost per case |
| Roberts23 Methodologic feature score = 16 | Retrospective cohort | Ordinary least-squares regression and economic models | Location of care (ICU, ward, etc), lab and radiography tests, procedures, consultations and medication | Data abstracted from medical records, microcosting (US$, 1998) | 25 patients with HAI in one urban teaching hospital (Jan–Dec 1998) | Any HAIs, according to the CDC’s NNIS | 15.2% | 10.7 additional days | Incremental costs attributable to suspected HAI: $6767 confirmed HAI: $15,275 |
| Kilgore24 Methodologic feature score = 13 | Retrospective cohort | Multivariable regression models and restricted models | Total, variable costs of inpatient care, LOS | Cardinal Health-MedMined database (US$, 2007) | Of 1,355,647 admissions, 58,293 had an NIM. Over 69 months from 55 hospital databases (Mar 2001–Jan 2006) | Any nosocomial infection, identified via NIM | Overall NIM rate was 4.3% | 5.4 additional days | NIMs are associated with attributable costs of US$12,197 |
| Esatoglu25 Methodologic feature score = 12 | Retrospective case control | Matched 1:1 by age, sex, clinic, primary diagnosis of the infected patients | LOS, medical goods/materials, drugs, tests, beds, treatments and other costs. | Unspecified, presumably hospital accounting database (US$, 2001) | 57 patients with HAI matched 1:1, in one hospital in Ankara, Turkey (Sep–Dec 2001) | Any HAI, not further described | Not available | Mean 23 additional days | HAI mean additional cost: US$2026.70 |
| Sheng26 Methodologic feature score = 12 | Retrospective case control | Matched 1:1 by age ± 2, sex, underlying illness, operation(s), admission date 28 days, ward, diagnosis and severity | Costs of stay, medication, lab procedures, materials and services, nursing care | Hospital finance departments (US$, 2002) | 273 adult case-control pairs, from 2 community hospitals and 1 tertiary medical center (Oct–Dec 2002) | Patients aged ≥ 16 years with onset of any infection ≥ 48 hours after admission or within 1 week of discharge | Not available | 19.67 additional days | US$5189 in mean additional costs |
| Plowman27 Methodologic feature score = 16 | Prospective cohort | Linear regression model | Resources, LOS, care and treatment, paid staff time, nursing costs, unit costs for lab, radiology and other diagnostic procedures | Costs estimated for specialty via interviewing health care professionals, hospital database (GBP, year unclear) | 4000 adults in one general hospital in London, England, of which 312 had an HAI (Apr 1994– May 1995) | Any HAI | Incidence of HAIs: 7.8% | 14.1 additional days | Mean additional costs due to HAI at any site: £3154 (model estimate £2917) |
| Lee28 Methodologic feature score = 13 | Retrospective cohort | Linear regression models | Third part payer’s overall hospital costs, increased LOS (postsurgical), antibiotic costs | Quality Indicator/Improvement Project database (US$, 2007, converted from JP yen) | 1058 gastrectomy patients from 10 JP hospitals, of which 215 had any HAI (Apr 2004–Jan 2007) | Diagnosed with any HAI | HAI incidence 20.3% | 10.6 days attributable | Attributable HAI costs: US$2767 (range $1035–$6513) |
| Mahieu29 Methodologic feature score = 15 | Retrospective cohort with nested case control | Matched by gestational age and early post-natal co-morbidity factors | Charges and LOS | Charges from hospital discharge abstracts and patient files (€, 1995) | Of 515 neonates in one Belgian NICU, 69 had one or more HAI (Oct 1993–Dec 1995) | Infections ≥ 48 hours after admission to NICU and treated with IV antibiotics for 5+ days were considered nosocomial | 13% incidence of one or more HAI | Mean 24 additional days | Mean extra charge with HAI was €11,750 |
| Graves30 Methodologic feature score = 15 | Decision model | Monte Carlo simulation | Estimated literature cost per bed-day, literature estimates of increased LOS, medical and surgical services | Database and literature values for NZ hospitals (US$, year unstated) | Any/all recorded admissions, NZ hospitals (1998–1999) | HAI reported in database | No overall incidence reported | Not collected in study | Not reported per case. Estimated national costs of HAI over fiscal year in NZ, medical patients: NZ$4,569,826. Surgical: NZ$3,900,922 |
| Surgical site infections | |||||||||
| Chen22 Methodologic feature score = 15 | Retrospective analysis of a prospectively assembled cohort | Generalized linear modeling | Medical and surgical procedures, medications, lab investigation, ICU bed-days | Hospital database, microcosting (US$, 2007, converted from Taiwanese dollars) | 401 NIs in 320 of 2,757 patients, in 4 ICUs in one hospital in Taiwan (2003–2004) |
BSI, UTI, SSI, respiratory tract infection “and others” diagnosed ≥ 48 hours after admission to ICU | 14.5 NI episodes per 100 admissions | Not available | US$10,015 attributable cost per case |
| Defez31 Methodologic feature score = 15 | Retrospective case control | Matched 1:1 by age, sex, ward, LOS before infection, DRG, and McCabe index | Lab tests, radiology, surgery, antimicrobial agents, rate per day of hospital bed | Reimbursement from La Nomeclature Générale des Actes Professionnels and hospital pharmacy accounting database (€, 2004) | 1703 infected patients from previous study; 30 randomly chosen for each infection site, total 150. One French hospital. (2001–2003) | Patients with single-site nosocomial infection | Not available | Not available | Attributable cost (mean €) by site of infection, UTI: €574 Surgical site: €1814 Respiratory tract: €2421 Bloodstream: €953 Other: €1259 |
| Weber32 Methodologic feature score = 17 | Prospective with nested case control | Matched 1:1 by age ± 5 years, procedure code, and NNIS risk index | LOS, ICU LOS, patient charges, antibiotic costs | Microcosting from hospital accounting database (Swiss franc, assumed 2001) | 6283 surgical procedures in one Swiss hospital, 187 with SSI (2000–2001) | All surgical site infections at one Swiss hospital | 3.0% | 16.8 additional days | Mean additional hospital cost was 19,638 Swiss francs |
| Whitehouse33 Methodologic feature score = 13 | Prospective case control | Matched 1:1 by type of operative procedure, NNIS risk index, age ± 5, surgery within the same year, surgeon | Total direct costs from database, representing sum of costs required to provide health care services | Hospital accounting database, microcosting (US$, 1997) | 59 cases, each matched with 1 control, in one US hospital (1997–1998) | Orthopedic SSI: superficial incisional, deep incisional, or organ/space | Literature rates of SSI following orthopedic surgery: 0.7% (low-risk, hip replacement) to 7.9% (high-risk, spinal fusion) | Median 1 extra day during initial hospital stay, median of 14 extra days during follow-up period | Median direct cost was US$24,344 for a case, compared with US$6636 for uninfected patients |
| Mahmoud34 Methodologic feature score = 12 | Retrospective analytic cohort | Logistic regression | Medical and surgical procedures, hotel costs, nursing, pharmacy, ICU, supplies, lab procedures | Large US hospital database: Premier Perspective database (US$, 2005/6) | 25,825 patients undergoing colorectal procedures, of which 956 have SSI, in US database of 196 hospitals (Jan 2005–Jun 2006) | Incisional SSIs, superficial or deep as defined by the US CDC | SSI incidence: 3.7% | LOS with postoperative complications is 3–11 days longer than without | Mean total direct costs incurred by treating SSI: US$13,746 |
| Penel35 Methodologic feature score = 15 | Prospective cohort with a post hoc analysis | Unclear | LOS. Estimation of per diem cost, including rooming, lab, medications and procedure costs | Macro costing: LOS multiplied by estimation of per diem cost (€, 2005) | 261 head/neck cancer surgery patients in one hospital SSI: 94 PP: 34 SSI and PP: 13 (Jan 1997–Dec 1999) |
Based on the CDC 1992; SSI, PP | SSI: 36% PP: 13% SSI and PP: 5% |
SSI: 16 days in additional mean LOS PP: 17 days SSI and PP: 31 days |
SSI: €16,000 increase in mean direct medical costs PP: €17,000 Both SSI and PP: €35,000 |
| Jenney36 Methodologic feature score = 11 | Retrospective cohort with nested case control | Matched 1:1 by sex, age ± 5, NNIS risk index scores | LOS, antibiotic costs, salaries, utilities and overhead costs | Hospital finance department (AU$, 1999) | 1377 CABG procedures, of which 956 had a SSI; 125 cases in an AU hospital (1996–1998) | SSI after CABG, defined according to the CDC | SSI incidence: 9.1% | 1.36 mean additional days | Mean excess cost: AU$12,419/case |
| Olsen37 Methodologic feature score = 12 | Retrospective cohort | Generalized least squares and propensity score matched-pairs | Department actual cost components multiplied by patient charge codes (pharmacy, room and board, procedures) | Barnes-Jewish Hospital cost accounting database (US$, 2008) | 1616 women who under-went low transverse cesarean delivery at one tertiary care hospital, SSI: 81 EM: 123 (Jul 1999–Jun 2001) |
Patients diagnosed with SSI and/or EM after surgery | Incidence of SSI: 5.0% EM: 7.6% | Not available | SSI: attributable cost US$3529 by generalized least squares, US$2852 by propensity method. EM: US$3956 by generalized least squares, US$3842 by propensity method |
| Nosocomial bloodstream infections | |||||||||
| Chen22 Methodologic feature score = 15 | Retrospective analysis of a prospectively assembled cohort | Generalized linear modeling | Medical and surgical procedures, medications, lab investigation, ICU bed-days | Hospital database, microcosting (US$, 2007, converted from Taiwanese dollars) | 401 NIs in 320 of 2757 patients, in 4 ICUs in one hospital in Taiwan (2003–2004) | BSI, UTI, SSI, respiratory tract infection “and others” diagnosed ≥ 48 hours after admission to ICU | 14.5 NI episodes per 100 admissions | Not available | US$10,015 attributable cost per case |
| Defez31 Methodologic feature score = 15 | Retrospective case control | Matched 1:1 by age, sex, ward, LOS before infection, DRG, and McCabe index | Lab tests, radiology, surgery, antimicrobial agents, rate per day of hospital bed | Reimbursement from La Nomeclature Générale des Actes Professionnels and hospital pharmacy accounting database (€, 2004) | 1703 infected patients from previous study; 30 randomly chosen for each infection site, total 150. One French hospital. (2001–2003) |
Patients with single-site nosocomial infection | Not available | Not available | Attributable cost (mean €) by site of infection, UTI: €574 Surgical site: €1814 Respiratory tract: €2421 Bloodstream: €953 Other: €1259 |
| Blot38 Methodologic feature score = 18 | Retrospective case control | Linear regression analysis, and matched 1:1 or 1:2 by APACHE II score, principal diagnosis, ICU LOS | Duration of mechanical ventilation, LOS, hospital costs | Patient hospital invoices (€, 2002) | 36,836 patients (192 cases) were admitted to one general ICU in Belgium. (1992–2002) | Catheter-related bloodstream infection: positive culture results and clinical signs of sepsis | 5.2 cases BSI per 1000 admissions, or 1 case per 1000 catheter-days | 10 days attributable | Attributable costs €13,585 |
| Orsi39 Methodologic feature score = 15 | Retrospective case control | Matched 1:2 by pre-infection LOS, primary diagnosis, ward, CVC, age ± 5, sex | Single-day hospital cost, increased LOS | Data from clinical and micro-biological records collected by infection control team (€, year unclear) | 105 included cases, each matched with 2 controls at one teaching hospital in Rome, Italy (Jan 1994–Jun 1995) | Bloodstream infection: isolated pathogen(s) in the blood, plus one or more related symptom, ≥48 hours after admission | Diagnosed in 2% of screened patients | Attributable LOS 19.1–19.8 days (mean), 13–15 days (median) | Attributable €15,413 expenditure per case |
| Pirson40 Methodologic feature score = 9 | Retrospective case control | Matched (ratio unstated) by APR-DRG | Administrative, general services costs, medical charges, LOS, drugs | Hospital cost centers, medical records data and invoicing data (€, 2001) | 46 cases of HAB in one Belgian hospital (2001) | An infection of bacteremia developed ≥ 48 hours after admission | 0.56% incidence | 21.1 additional days | Average attributable costs: €12,853 |
| Pirson41 Methodologic feature score = 12 | Retrospective case control | Matched 1:1 by APR-DRG and severity of illness | Salaries, hotel costs, drugs, ICU, medical and surgical procedures, laboratory, diagnostics | Université Libre de Bruxelles costing database (€, 2003) | 326 cases in 2003 and 277 cases in 2004; 3 Belgian hospitals (2003 and 2004) | Cases were defined as bacteremia that developed ≥ 48 hours after admission | Incidence of HAB: 1.4% and 1.2% in 2003 and 2004 | Attributable LOS: 6.1 days (ICU); 30 days (non-ICU) | Mean additional cost of HAB was €16,709 |
| Kilgore42 Methodologic feature score = 12 | Retrospective cohort | Regression analysis | “Fixed and variable costs of care” | Hospital accounting database (US$, 2006) | 1,355,647 admissions during 69 months from 55 hospital databases (Mar 2001– Jan 2006) | Nosocomial BSIs, nonduplicate isolate collected ≥ 3 days after admission | Nosocomial BSIs identified in 0.93% of admissions | Not available | Incremental costs: US$19,427 |
| Elward43 Methodologic feature score = 15 | Prospective cohort | Multiple linear regression analysis | Direct medical costs of pediatric ICU and hospital-stay | Hospital accounting database (US$, 1999/2000) | 911 admissions, including 56 case patients under age 18 in one US PICU (Sep 1, 1999–May 31, 2000) | Bloodstream infections in PICU patients, recognized pathogen isolated from blood > 48 hours postadmission | Rate of BSI: 13.8 per 1000 central venous catheter days | Not available | Attributable PICU direct costs: US$39,219 |
| Payne44 Methodologic feature score = 15 | Retrospective cohort | Multiple regression | Charges converted to costs | Hospital charges (converted to costs), Centers for Medicare and Medicaid Services (US$, 1999) | 2809 VLBW infants in 17 NICUs, 553 with nosocomial BSI (1998–1999) | BSI after 3rd postnatal day, with symptoms of infection and 5+ days antibiotic treatment after diagnosis | Nosocomial BSI: 19.7% | The mean additional LOS of VLBW infants with BSI was 32.5 days | The mean attributable cost was US$54,539 |
| Chu45 Methodologic feature score = 16 | Prospective case series | Not stated | All infection-related diagnostic tests and surgical procedures, inpatient and outpatient costs | Hospital accounting system (US$, 2002) | 298 patients with a prosthetic implant and S. aureus bacteremia (nosocomial acquired) (Sep 1994–Sep 2002) | Positive blood culture for S. aureus bacteremia, ≥72 hours postadmission, in a patient with ≥1 prosthetic implant | Not available | Mean 33 additional days | Attributable cost per case: US$67,439 |
| Nosocomial sepsis | |||||||||
| Adrie46 Methodologic feature score = 20 | Retrospective analytic cohort of prospective database | Model, multiple linear regression | Direct ICU and medical costs, unit costs of ICU resources, overheads and other fixed costs | Prospective database, microcosting (€, 2001. 1€ = 1 US$) | Of 1698 patients hospitalized for more than 48 hours in 6 ICUs, 340 had sepsis. (Apr 1997–Dec 2000) | Severe sepsis: infection, ≥2 criteria for systemic inflammatory response syndrome and ≥1 criterion for organ dysfunction | 20.0% | Not available | US$27,510 |
| Brun-Buisson47 Methodologic feature score = 15 | Prospective cohort with retrospective measurement of costs | Costing model (Chaix et al, 1999) | All resources used and direct costs (of fluids, drugs, blood products and procedures) | Hospital accounting database and previously built costing model created in this ICU (€, 2001) | 424 patients in one Paris, France ICU (1997–1998) | Patients with sepsis, clinically or microbiologically documented, ≥48 hours after admission | ICU-acquired sepsis: 23% | 19 additional days compared to patients with no sepsis | Nosocomial cases incurred average total costs were €39,908 higher than patients with no sepsis |
| Nosocomial rotavirus infection | |||||||||
| Festini48 Methodologic feature score = 14 | Prospective cohort | Unclear | LOS, estimated cost of hospital day based on DRG, lost productivity of patients’ parents | Hospital accounting databases, wage data provided by Italian Central Bank (€, year unclear) | 608 children under 30 months of age in four Italian hospitals (2006–2008) | Hospital-acquired, positive rapid rotavirus testing | Incidence of nRVI was 5.3% | 1.7 attributable days | National burden of nosocomial rotavirus in Italy, based on attributable LOS, is estimated at €8,019,155 |
| Fruhwirth49 Methodologic feature score = 13 | Prospective case series | Unclear | Direct medical costs, direct nonmedical eg, food, indirect costs eg, productivity loss | Hospital database, microcosting (€, 1997/1998) | 33 cases of nosocomial rotavirus infection in children < 48 months, in Austria (Dec 1997– May 1998) | Rotavirus-positive diarrhea, nosocomial if onset was >48 hours after admission | Risk for contracting nRVI was 2.59 per 1000 hospital days during peak rotovirus season (Dec–May), <48 months of age | Not available | Case cost average €2442 |
| Piednoir50 Methodologic feature score = 15 | Prospective cohort with nested case control | Matched 1:1 by primary diagnoses, date of admission ± 7 days, age ± 3 months, sex, pre-infection LOS | All expenses sustained by the hospital: medical, preventative, staff costs and fixed costs | Medical records and hospital accounting database (€, 2001/2002) | 23 cases matched 1:1, in one French pediatric hospital (Dec 1, 2001– Mar 31, 2002) | Rotavirus-positive stool via qualitative enzyme-linked immunosorbent assay (ELISA) ≥48 hours postadmission | Attack rate: 6.6% Incidence: 15.8 per 1000 hospital days | 4.9 additional days | Mean attributable cost due to nosocomial rotavirus infection: €1930 |
| Nosocomial urinary tract infections | |||||||||
| Chen22 Methodologic feature score = 15 |
Retrospective analysis of a prospectively assembled cohort | Generalized linear modeling | Medical and surgical procedures, medications, lab investigation, ICU bed-days | Hospital database, microcosting (US$, 2007, converted from Taiwanese dollars) | 401 NIs in 320 of 2,757 patients, in 4 ICUs in one hospital in Taiwan (2003–2004) | BSI, UTI, SSI, respiratory tract infection “and others” diagnosed ≥ 48 hours after admission to ICU | 14.5 NI episodes per 100 admissions | Not available | US$10,015 attributable cost per case |
| Defez31 Methodologic feature score = 15 | Retrospective case control | Matched 1:1 by age, sex, ward, LOS before infection, DRG, and McCabe index | Lab tests, radiology, surgery, antimicrobial agents, rate per day of hospital bed | Reimbursement from La Nomeclature Générale des Actes Professionnels and hospital pharmacy accounting database (€, 2004) | 1703 infected patients from previous study; 30 randomly chosen for each infection site, total 150. One French hospital. (2001–2003) |
Patients with single-site nosocomial infection | Not available | Not available | Attributable cost (mean €) by site of infection UTI: €574 Surgical site: €1814 Respiratory tract: €2421 Bloodstream: €953 Other: €1259 |
| Tambyah51 Methodologic feature score = 15 | Prospective cohort data analyzed retrospectively | Patient records reviewed by investigators | Laboratory costs, LOS, medications | Hospital charges were converted to costs via cost-to-charge ratio (US$, 1998) | 1497 catheterized patients in one US university hospital, of which 223 had UTI (1997–1998) | Nosocomial UTI, defined as new bacteriuria or funguria exceeding 103 CsFU/mL | 14.9% of catheterized patients | Not available | Average attributable treatment cost: US$589 |
| Morse52 Methodologic feature score = 9 | Retrospective cohort | Unclear | Only “overall costs” of hospital stay after operation; not detailed further | Hospital case costing system (US$, year unstated) | 118 bowel surgery patients aged 65 to 79, and 33 aged > 80, with Medicare in one hospital; total of 64 patients experience a “never event” (Jan 2008–Mar 2009) | “Never events:” hospital-acquired complications that are not reimbursed by Medicare | 42.4% of study patients experienced a “never event” | Not available | Catheter-related UTI: US$14,300 extra costs; vascular catheter infection: US$16,400 extra costs |
| Nosocomial pneumonia | |||||||||
| Rosenthal53 Methodologic feature score = 13 | Prospective with nested case control | Matched 1:1 by ICU type, hospital and year of admittance, sex, age, and severity of illness score | Fixed cost per bed-day, defined daily antibiotic doses, LOS | Hospital finance department (Argentinian pesos [$], year unclear) | 307 case patients (pneumonia), 307 control patients in 3 hospitals over 5 years (1998–2002) | Nosocomial pneumonia according to definition from the CDC | 5.79% developed nosocomial pneumonia | Mean 8.95 additional days | Mean attributable cost for cases was ARS$2255 |
| Penel35 Methodologic feature score = 15 | Prospective cohort with a post hoc analysis | Unclear | LOS. Estimation of per diem cost, including rooming, lab, medications and procedure costs | Macro costing: LOS multiplied by estimation of per diem cost (€, 2005) | 261 head/neck cancer surgery patients in one hospital SSI: 94 PP: 34 SSI and PP: 13 (Jan 1997–Dec 1999) |
Based on the CDC 1992; SSI, PP | SSI: 36% PP: 13% SSI and PP: 5% |
SSI: 16 days in additional mean LOS PP: 17 days SSI and PP: 31 days |
SSI: €16,000 increase in mean direct medical costs PP: €17,000 Both SSI and PP: €35,000 |
| Dietrich54,b Methodologic feature score = 14 | Prospective case control | Matched 1:1 based on severity of disease, age, primary ward, status of ventilation, immunosuppression, sex, LOS | All resources consumed for diagnosis, treatment, nursing and hospital stay, including materials and personnel | Hospital accounting database (DM, 1998/1999) | 48 cases and 66 controls (resulting in 29 matched pairs) in one German teaching hospital, 5 ICUs (May 1998– Mar 1999) | Nosocomial pneumonia, diagnosed according to the criteria of the CDC | Not available | 5.00 additional ventilation days, 6.55 additional days in ICU and 7.40 additional days in hospital | Attributable cost per case: DM 14,606 from the hospital perspective |
| Dietrich54,b Methodologic feature score = 14 | Retrospective case control | Matched 1:1 based on severity of disease, age, primary ward, status of ventilation, immunosuppression, sex, LOS | All resources consumed for diagnosis, treatment, nursing and hospital stay, including materials and personnel | Hospital accounting database (DM, 1998/1999) | 37 matched pairs in one German teaching hospital, admitted to one of 2 neurosurgical wards (Feb 1997–Dec 1998) | Nosocomial pneumonia, diagnosed according to the criteria of the CDC | Not available | 5.00 additional ventilation days, 14.03 additional days in ICU and 10.14 additional days in hospital excess days | Attributable cost per case: DM 29,610 from hospital perspective |
| Brilli55 Methodologic feature score = 15 | Retrospective case control | Matched by primary and underlying diagnoses, ventilation days. When possible: surgical procedure, PRISM score, age, sex | Hotel costs, surgical, medical and laboratory procedures, supplies, blood products, radiology, other professional fees | Microcosting from hospital accounting database (US$, year unspecified) | 13 case patients matched to control patients 1:1 in one pediatric ICU (FY 2005–FY 2007) | Pediatric ICU patients with VAP | 7.8 cases per 1000 ventilator days in FY 2005 | 8.7 attributable days | Attributable VAP costs per patient: US$51,157. |
| Nosocomial respiratory tract infection | |||||||||
| Defez31 Methodologic feature score = 15 | Retrospective case control | Matched 1:1 by age, sex, ward, LOS before infection, DRG, and McCabe index | Lab tests, radiology, surgery, antimicrobial agents, rate per day of hospital bed | Reimbursement from La Nomeclature Générale des Actes Professionnels and hospital pharmacy accounting database (€, 2004) | 1703 infected patients from previous study; 30 randomly chosen for each infection site, total 150. One French hospital. (2001–2003) | Patients with single-site nosocomial infection | Not available | Not available | Attributable cost (mean €) by site of infection, UTI: €574 Surgical site: €1814 Respiratory tract: €2421 Bloodstream: €953 Other: €1259 |
| Chen22 Methodologic feature score = 15 | Retrospective analysis of a prospectively assembled cohort | Generalized linear modeling | Medical and surgical procedures, medications, lab investigation, ICU bed-days | Hospital database, microcosting (US$, 2007, converted from Taiwanese dollars) | 401 NIs in 320 of 2757 patients, in 4 ICUs in one hospital in Taiwan (2003–2004) | BSI, UTI, SSI, respiratory tract infection “and others” diagnosed ≥ 48 hours after admission to ICU | 14.5 NI episodes per 100 admissions | Not available | US$10,015 attributable cost per case |
| McCartney56 Methodologic feature score = 15 | Case control | Matched 1:1 by age, principal discharge diagnosis, same RSV season, number of secondary diagnoses | Direct medical costs | Hospital accounting database (US$, 1996) | All patients admitted to one Philadelphia pediatric hospital over 8 RSV seasons (1988–1996) | Nosocomial RSV infection | 88 nosocomial RSV cases out of 90,174 patients | Attributable LOS for nosocomial RSV was 7.8 days | Mean attributable cost to hospital per RSV NI was US$9419/case |
| Miscellaneous nosocomial infections | |||||||||
| Bou57 Methodologic feature score = 13 | Retrospective case series | Multiple linear regression analysis | ICU hospital costs only: treatments and diagnostic procedures | Hospital finance department, microcosting (€, year unspecified) | 67 ICU patients during a P. aeruginosa outbreak at one ICU in Spain (Jul– Sep 2003) | Any patient who developed the infection after ≥48 hours mechanical ventilation | Incidence of outbreak associated with pseudomonas infection; 17/67 | 38 additional days | €18,408 average extra ICU costs per case patient |
| Fretz58 Methodologic feature score = 16 | Retrospective case series | Unclear | Revenue loss, nursing, diagnostic procedures, pharmacy, costs of creating an isolation ward | Hospital department-specific costs (€, year unspecified) | 90 infected patients and staff of an Austrian hospital during a norovirus outbreak (Dec 2006– Feb 2007) | Positive stool specimen for norovirus ≥ 48 hours following admission | Not applicable | Not available | The total cost of the outbreak for the Department of Internal Medicine was €80,138 |
| Zingg59 Methodologic feature score = 17 | Retrospective case control | Matched 1:2 by age, sex, LOS, underlying disease category | Direct impact on hospital resources: loss of revenue, additional microbiological diagnosis | Hospital database, microcosting (US$, 2002) | 16 case patients and 32 control patients during a norovirus outbreak (2001 and 2002) | A person who developed acute diarrhea, nausea and vomiting during the norovirus outbreak | Attack rate 13.9% among patients and 29.5% among health care workers | Not available | US$2452 per case (derived from US$40,675 total direct outbreak costs ÷ 16 case patients) |
| Anil60 Methodologic feature score = 12 | Retrospective case control | Matched 1:1 based on birth weight ± 10%, sex, gestational age ± 2 weeks, ventilation, anti-microbial therapy, use of CVC/TPN | Charges per patient and actual financial burden of outbreak; not detailed further | Hospital discharge abstracts via the hospital’s central finance service (US$, assumed 2005) | 22 cases in one Turkish NICU, drug-resistant S. typhimurium outbreak (Mar 15–29, 2005) | Positive stool/rectal swab or fluid culture for S. typhimurium | Attack rate 30.5% | 9.8 additional days | US$1081.84 more charges per case compared to control |
| Baggett61 Methodologic feature score = 18 | Retrospective case series | Interviews with hospital staff and review of contact tracing logs | Direct costs: personnel time, laboratory and medication costs; Indirect: hospital staff furloughs | Hospital database, microcosting (US$, 2004) | Two hospitals experiencing a nosocomial pertussis outbreak (Jul 25– Sep 15, 2004) | A cough illness lasting ≥ 14 days with symptoms of whooping cough and/or isolation of B. pertussis or confirmed by PCR or culture | Incidence was 10/1475 persons exposed | Not available | Attributable cost per nosocomial case, Hospital A: US$43,893 Hospital B: US$30,282 |
| Spearing62 Methodologic feature score = 15 | Retrospective cohort | Unclear | Direct costs including medical costs, outbreak investigation, lost productivity costs, and misc | Medical records data and Medicare costs (AU$, 1996) | 52 cases in a 600-bed tertiary care complex during an outbreak of Salmonella (Dec 1996) | Not detailed; cases of Salmonella during the outbreak | Not available | Not available | AU$2308 (US$1827) per case (total outbreak cost AU$120,000 or US$95,000 ÷ 52 cases) |
| Wilson63 Methodologic feature score = 13 | Retrospective with nested case control | Matched 1:1 to controls with ≥20% total body surface burns | Hospital charges converted to costs | Hospital finance department; unclear costing methods (US$, 2001) | 34 burn patients (Jan–Dec 2004) | Hospital-acquired nosocomial MDRAB infection | 16% of 217 burn patients acquired MDRAB infection | 11 additional days | Mean additional cost: US$98,575 |
| Watters64 Methodologic feature score = 13 | Retrospective cohort | Unclear | Antibiotics, high dependency unit and intensive therapy unit facility use, prolonged LOS | Unspecified, presumably hospital accounting/finance database (GBP, year unstated) | 55 patients who had undergone head and neck surgery in one Irish hospital (over 1 year; year unspecified) | Positive MRSA screening in postoperative period after head and neck surgery | 25 patients (45%) became MRSA positive in the postoperative period | Difference in mean LOS: 45 days | Attributable cost: £6485 Mean extra antibiotic cost: £1700 |
| Mauldin65 Methodologic feature score = 17 | Retrospective case series | Segmented regression analysis for interrupted time series, univariate and multivariate | LOS, ICU LOS, drug costs, lab and medical procedures, adjusted hospital charges | Hospital database (US$, 2005) | 187 patients with MRSA, 19 patients with VRE infections in one US hospital (2000–2005) | Patients diagnosed with either VRE or MRSA | Not available | Not available | Total mean costs, MRSA patients: US$110,493 VRE patients: US$115,260 |
| Vonberg66 Methodologic feature score = 13 | Prospective with nested case control | Matched 1:3 by DRG in 2006, pre-infection LOS, Charlson comorbidity index | “General charge for each day of care,” and “some patient costs” (unclear) | Hospital finance department (€, year unstated) | 45 CDAD cases, 1:3 case: control in one German tertiary care hospital (Jan–Dec 2006) | Positive EIA or culture for CDAD, nosocomial if onset is ≥72 hours after admission | 10%–16% of patients are carriers of C. difficule, at risk for CDAD; incidence of CDAD not available | Median 7 additional days | Median incremental cost: €7147/CDAD case |
| Puzniak67 Methodologic feature score = 20 | Case control | Matched 1:1 by DRG, APACHE II score, age | Patient’s total hospitalization costs, microbiology costs, health care staff time, LOS, and MICU LOS | Hospital database, step-down cost allocation system (US$, year unstated) | Patients admitted ≥ 24 hours to a US MICU (Jul 1, 1997–Dec 31, 1999) | Positive screening for VRE | Not available | 4 attributable MICU days 8.3 attributable hospital days | MICU: US$7873 attributable Hospital: US$11,989 attributable |
| Fuller68 Methodologic feature score = 14 | Retrospective cohort | Linear regression model | Charges converted to costs | Health Services and Cost Review Commission, Maryland; Office of Statewide Planning and Development, California (US$, 2008) | Of 2,496,212 admissions about 139,788 had a complication 54,971 had multiple complications (Maryland: FY2008; California: FY2006) | Any negative event or outcome that results from the process of inpatient care | 4.0%–5.6% of patients had 1 hospital-acquired potentially preventable complication; Another 1.6%–2.2% had multiple complications | Not available | Maryland: US$626,416,710 (9.63% of total claims) associated with potentially preventable complications |
| Nosocomial venous thromboembolism | |||||||||
| Caprini69 Methodologic feature score = 18 | Decision analysis (Markov) | Univariate analysis | Patient care protocols, health care staff time, diagnostic tests, supplies, hospitalizations, procedures | Literature data (US$, year unstated) | Two hypothetical cohorts similar to all US patients undergoing total hip replacement surgery in the US (1995–1996) | DVT | Unclear | Unclear | Annual per-patient cost of DVT: US$3798 |
| MacDougall70 Methodologic feature score = 16 | Retrospective observational cohort study | Linear model with log-link function and gamma distribution | Treatment strategy, length of hospital stay, physician office, emergency room, outpatient claims, ancillary services, pharmacy utilization | Actual health care plan payments for services only | 16,063 DVT alone, 7889 PE alone, 3006 DVT and PE (Jan 1, 1997–Mar 31, 2004) | DVT and PE | Unclear | Mean LOS DVT = 10 days, PE = 9 days, DVT and PE = 10 days |
Annual direct medical costs of US$16,832 ($24,411 CAN) for DVT, US$18,221 ($26,426 CAN) for PE, US$24,874 ($36,074 CAN) for combined DVT and PE, and US$4726 ($6854 CAN) |
| Nosocomial related falls | |||||||||
| Nadkarni71 Methodologic feature score = 12 | Case series | Unclear | Operation procedures, nonoperative treatment, LOS | Southport and Ormskirk Hospital Risk Management Department; Hospital Finance Department (GBP, year unspecified) | 42 cases, of Southport and Ormskirk Hospital Risk Management Department incident forms (Jan 2000–Dec 2002) | Orthopedic injuries sustained by in-patients falling on the hospital wards | Not available | Mean 4.1, median 3 additional weeks | £1667 per case (total £70,000/42 cases) |
| Oliver72 Methodologic feature score = 13 | Case series | N/A | Legal payments | NHS Litigation Authority Database of clinical negligence claims (GBP, year unspecified) | 479 clinical negligence claims resulting from in-hospital falls in England (1995–2006) | Any closed clinical negligence claim resulting from in-hospital falls within the time period | Not applicable | Not applicable | 60.5% of claims resulted in payment of costs or damages, with mean payment £12,945/claim |
| Nurmi73 Methodologic feature score = 13 | Prospective cohort | Unclear | Emergency room visits, outpatient visits, LOS, radiology | Hospital accounting database (€, 1999) | 554 falls occurred among 218 patients treated in four institutions in Finland during study period (Feb 1, 1993–Jan 31, 1994) | Falls among ambulatory patients over 60 years within the study period | 1398 falls per 1000 person years. 30% of falls resulted in injury | Not available | Average cost per treating a fall: €944 |
Notes:
61 studies in total, of which three reported outcomes for more than one type of infection, therefore the total listed in Table 2 is 68;
Dietrich, 2002 is one paper detailing two different studies. The studies were separated in this table for clarity;
only gives incidence of PTS, PEs given post-surgical DVT.
Abbreviations: ADE, adverse drug event; AE, adverse event; APACHE, Acute Physiology and Chronic Health Evaluation; APR, all patient refined; ARS, Argentine peso; AU, Australian; BSI, blood stream infection; CABG, coronary artery bypass graft; CDAD, Clostridium difficile-associated disease; CDC, Centers for Disease Control and Prevention; CVC/TPN, central venous catheter/total parenteral nurtition; DM, Deutsche Mark; DRG, disease-related group; DVT, deep vein thrombosis; EM, endometriosis; FY, financial year; GBP, Great Britain Pound; HAB, hospital-acquired bacteremia; HAC, hospital-acquired complication; HAI, hospital-acquired infection; ICU, intensive care unit; JP, Japanese; LOS, length of stay; MICU, medical intensive care unit; MDRAB, multidrug-resistant Acinetobacter bowmanii; MRSA, methicillin-resistant Staphylococcus aureus; NHS, National Health Service; NI, nosocomial infection; NICU, neonatal ICU; NIM, Nosocomial Infection Marker; NNIS, National Nosocomial Infections Surveillance; nRVI, nosocomial rotovirus infection; NZ, New Zealand; PE, pulmonary embolism; PICU, perinatal ICU; PP, postoperative pneumonia; PTS, postthrombotic syndrome; RSV, respiratory syntactical virus; SCI, spinal cord injury; SSI, surgical site infection; SSTI, skin soft tissue infection; TSI, Transition Systems Incorporated; UTI, urinary tract infection; VAP, ventilator-associated pneumonia; VLBW, very low birth weight; VRE, vancomycin-resistant Enterococcus.
General studies of adverse events and adverse drug events
We identified eight studies of the economic burden of adverse events and adverse drug events published since 2000. Five of these studies used a retrospective cohort study design, and relied on regression analyses to determine the attributable costs. Of these, two articles broadly focused on any adverse event or hospital-acquired complication.13,14 An additional article evaluated the economic burden of a broad range of adverse events in patients with spinal cord injuries.15 One article included five specific adverse events: medication errors, patient falls, urinary tract infection, pneumonia, and pressure ulcers.16 Another article evaluated costs related only to surgical adverse events, but did not further define them.17 The three remaining studies related to adverse events were either case series18 or prospective cohorts with nested cases and controls.19,20 Two of these studies defined a case as any adverse events19 or a case leading to a medical dispute.18 One study specifically evaluated adverse drug events.20
Costs attributable to adverse events were $457119 and $10,07414 in two studies. In patients with spinal cord injury, the cost attributable to adverse events was $6258, but were significantly higher for specific complications; for example, procedural complications in these patients were associated with additional costs of $20,183.15 The cost attributable to adverse drug events was $2830.20 In another study, medication error in medical and surgical cases were associated with costs of $361 and $568, respectively.16 Attributable length of stay related to adverse events ranged from 0.77 days to 32 days.15,19,20 Three of the eight articles did not record length-of-stay data.16–18
Nosocomial infections (not otherwise specified)
We identified ten studies of the economic burden of general nosocomial infections not otherwise specified by type of infection. These included one prospective design, five retrospective cohort designs, three retrospective case control designs, and one decision model. Analytic methods included regression analysis, such as linear regression, multivariate regression, and ordinary least-squares regression analysis.
In general hospital populations, the cost per case of hospital-acquired infection ranged from $2132 to $15,018.21–27 Hospital-acquired infections cost $2910 in gastrectomy patients28 and $21,85629 in neonates. The estimated costs of hospital-acquired infections over one fiscal year in New Zealand in medical patients were US$5,626,640 and surgical patients were US$4,803,046.30
Surgical site infections
We found eight studies of the economic burden of surgical site infections. Study designs included prospective cohort (n = 1), retrospective cohorts (n = 3), retrospective case control (n = 2), and two nested case control designs. The average cost per case of surgical site infection in a general patient population was reported to be $1105,22 $2604,31 and $14,42232 in three studies. In orthopedic patients, the median attributable cost of surgical site infection was $24,058.33 Surgical site infection in patients after colorectal procedures was $14,868,34 head-and-neck cancer-related surgery was $22,234,35 coronary artery bypass graft procedures were $10,245,36 and low transverse cesarean delivery were $2888 to $357437 per case. The latter study found similar attributable costs for surgical site infections using two different statistical methods for attributing costs (attributable cost for surgical site infection was $3529 by regression and $2852 by propensity score).37
Nosocomial bloodstream infections
We found ten studies of the economic burden of nosocomial blood stream infections (one prospective, three retrospective cohorts, five retrospective case controls, and one case series).
In general European patient populations, nosocomial bloodstream infection was associated with costs ranging from $2604 to $22,414.22,31,38–41 One American study reported average incremental costs of $21,013.42 In a pediatric intensive care unit (ICU), nosocomial bloodstream infection was estimated to cost $49,663.43 Very-low-birth-weight infants with nosocomial bloodstream infection incurred average total costs $71,384 higher than those without the infection.44 S. aureus bacteremia in patients with prosthetic implants was associated with $81,743 in costs per nosocomial case in one prospective case series.45
Nosocomial sepsis
We found two studies of the economic burden of nosocomial sepsis. In one retrospective cohort study, nosocomial sepsis was associated with mean additional costs of $33,87246 per case. In one prospective cohort, ICU-acquired sepsis was associated with a mean increase of $44,178 in total costs per case.47
Nosocomial rotavirus infection
We reviewed three studies of nosocomial rotavirus infection. One prospective cohort study estimated the costs associated with nosocomial rotavirus infection in children under 30 months of age, but did not provide a per-case result; this study estimated that the national cost of all cases in 1 year in Italy is $11,952,319.48 Rotavirus in children under 48 months of age was associated with $3591 in costs per case in one prospective case series.49 One prospective study with a nested case control reported $2210 in mean excess costs per case.50
Nosocomial urinary tract infection
We found four studies of the economic burden of nosocomial urinary tract infections. These included one prospective, two retrospective cohorts, and one retrospective case control study. The average costs attributable to urinary tract infection ranged from $788 to $18,717.22,31,51,52
Nosocomial pneumonia
We found four studies of the economic burden of nosocomial pneumonia. Two studies were prospective cohort studies and found that nosocomial pneumonia was associated with average additional costs of $85653 and $23,624.35 One German article detailed both a prospective case control and a retrospective case control, reporting average excess costs of $10,387 and $21,057, respectively.54 In one study, the average cost attributable to ventilator-associated pneumonia in a pediatric ICU was $55,333.55
Nosocomial respiratory tract infection
We included three studies on the economic burden of nosocomial respiratory tract infections: one retrospective cohort, one retrospective case control, and one case control study.
Respiratory tract infections were associated with additional mean costs of $347631 and $450922 in two studies, respectively. In one additional study, a case was defined as an infection of nosocomial respiratory syncytial virus; this infection was associated with mean costs of $13,083 per case.56
Miscellaneous nosocomial infections
We included 12 studies that described the economic burden of miscellaneous nosocomial infections. During a Pseudomonas aeruginosa outbreak, it was retrospectively estimated that infected patients who had been on mechanical ventilation incurred excess costs of $26,522.57 Another retrospective case series investigated the economic impact of a norovirus outbreak that affected patients and staff, and did not provide a per-case cost estimate; dividing the total outbreak costs by the given number of case infections yielded a crude estimate of $1282 per case;58 another similar study yielded an estimate of $2972 per case of outbreak-related norovirus.59 In one drug-resistant Salmonella typhimurium outbreak in a Turkish neonatal ICU, cases incurred charges $1208 higher than controls.60 A pertussis outbreak incurred total hospital costs of $34,956 and $50,668 in two hospitals.61 The attributable costs during a Salmonella outbreak in one Australian tertiary care complex were reported in total costs rather than per case, and dividing by the number of cases yields an estimate of $2552 per case.62 One retrospective case control study defined a case as a multidrug-resistant infection of Acinetobacter baumannii in burn patients, and reported a mean additional cost per case of $121,371.63 In one Irish hospital, postoperative MRSA infection incurred additional costs of $13,651.64 Another retrospective case series reported the attributable cost of MRSA to be $123,367 and vancomycin-resistant Enterococcus (VRE) to be $128,690.65 One prospective study with a nested case control reported a median incremental cost of $9708 per case of Clostridium difficile-associated disease (CDAD).66 One case control study reported the attributable costs of VRE in the medical ICU and in hospital to be $9543 and $14,532, respectively.67 One retrospective cohort study examined the cost associated with potentially preventable complications and found it to be $634,432,559.68
Nosocomial venous thromboembolism (VTE)
We identified two burden studies published since 2000. One study focused on nosocomial deep vein thrombosis after hip replacement surgery.69 The cost of deep vein thrombosis was modeled in patients undergoing total hip replacement surgery, with Markov decision and univariate analyses. The article reported the annual per-patient cost of deep vein thrombosis to be $4676. Also provided are the discounted lifetime costs of $3779, as well as costs specific to deep vein thrombosis-related complications, namely post-thrombotic syndrome with ulcer ($4700) and pulmonary embolism ($8131). A retrospective US study of deep vein thrombosis (n = 15,679), pulmonary embolism (n = 7653) and post-thrombotic syndrome (n = 624) found annual attributable direct medical costs of $19,430 for deep vein thrombosis, $21,033 for pulmonary embolism, $28,713 for combined deep vein thrombosis, and pulmonary embolism, and $5455 for patient safety target. This study did not explicitly distinguish cases of nosocomial deep vein thrombosis, but 78% of the study cohort had abdominal or orthopedic surgery prior to the index venous thrombosis event.70
Nosocomial falls
We reviewed three burden-of-illness studies related to nosocomial falls. Two studies were case series.71,72 The third study had a prospective cohort design.73 One study73 identified cases only in patients aged over 60 years. One study focused on legal compensation rather than hospital-related costs,72 and neither of the other two articles71,73 clearly stated what methods were used for determining attributable costs. There was one additional case control study reported attributable length of stay, but not costs.74
Oliver found that 60.5% of legal claims related to in-hospital falls resulted in payment of costs or damages, with mean payment of $25,793.72 Nurmi provided the cost per treating an in-hospital fall, estimated at $1359.73 The third study did not describe costs per case or per fall, but did provide the total estimated attributable cost of all cases included in the study; dividing by the provided number of cases yields a crude estimate of $3230 per case.71
We did not find any eligible studies for the following target conditions: nosocomial pressure ulcers, wrong site surgery, retained surgical foreign bodies, contrast inducted nephropathy.
Discussion
We found that 61% of published studies on the economic burden of patient safety targets in acute care describe or report no clear costing methodology. Among the remaining 61 studies (39%), which did report costing methodology, there were wide variations in methodologic features and methods for attributing costs. These studies report wide estimates of the economic burden of patient safety in acute care. For example, the attributable costs of patient safety targets ranged from $2000 to $200,000. In general hospital populations, the cost per case of hospital-acquired infection ranged from $2132 to $15,018. Nosocomial bloodstream infections were associated with costs ranging from $2604 to $22,414. We also found no adequate economic burden data for important patient safety, such as wrong site surgery, retained surgical foreign bodies, contrast-induced nephropathy, and acute care-acquired pressure ulcers.
Our results are consistent with the few prior reviews of the economic burden of patient safety in the acute care setting. A 2005 review identified 165 articles that included an economic analysis as an objective, but 35% of these articles provided no economic analysis, and 25% provided no primary economic data. The remaining studies had significant gaps in their costing methodology, and only 16% conducted sensitivity analyses that could address these limitations.75 Another review of comparative economic evaluations of patient safety programs identified 40 studies published between 2001 and 2004, none of which provided sufficient information about both the cost of the prevention program and the cost of the patient safety being targeted.76 A 1999 study estimated the economic burden of patient safety in Utah and Colorado at $1,442,024 per event (1996 US dollars). This early estimate is much higher than estimates in our systematic review, because the 1999 study evaluated not just direct acute care costs, but also outpatient direct health care costs after the event, as well as indirect (societal) costs such as lost workforce productivity up to age 75 years. None of the adverse event studies in our review considered this broad range of costs for a prolonged time horizon.77
Our findings, in conjunction with these prior reviews, indicate that greater attention is needed to the methodologic standards for evaluating the economic burden of patient safety in the acute care setting. Better knowledge of the economic burden of patient safety will inform decisions about health policy, patient safety research programs, and improvement priorities. High quality economic burden studies are an essential component of comparative economic analyses, such as cost effectiveness studies. Most of the studies we identified considered only the acute care hospital economic perspective, but the economic perspective should extend beyond the acute care hospital, as it has been estimated that 22%–66% of the economic burden of patient safety in acute care are borne by the hospital.78,79 Economic burden studies for patient safety should explicitly consider cost categories, legal, marketing and operational perspectives (direct or indirect), and time frames (including short- and long-term effects). There are also important methodologic considerations when attributing costs to patient safety, rather than the patient’s underlying condition. These considerations include the reliability of data sources used to identify patient safety, the adequacy of methods to control for confounding factors such as comorbidity and severity of illness, and the appropriateness of estimation methods including the incorporation of adverse event timing, matching methods, and regression modeling.80 Differential timing of the occurrence of patient safety can lead to wide estimates of attributable costs.81–83
Our review has several important limitations. First, we focused on studies published between 2000 and 2010 and indexed in MEDLINE. Studies outside of our search strategy may contain potentially useful data. For example, we did not include a 2010 study by the Society of American Actuaries because it was not indexed in MEDLINE.84 However, our finding that 61% of studies provide no or limited costing methodology would be unchanged by the inclusion of a few additional studies. Second, we focused only on patient safety targets in the acute care setting. We did not include studies of patient safety targets from other settings, such as community or chronic care. Third, we did not evaluate the interrater reliability of our methodologic reviews. Our review method was designed to yield higher methodologic ratings, as we always took the higher rating of the two reviewers, yet we still identified a significant lack of methodologic features. Fourth, we arbitrarily assigned one point for each methodologic feature, so that we could report a simple summary measure of methodologic features. However, we recognize that methodologic features are not necessarily equally weighted. Finally, there was heterogeneity in study methods. Variability in costing and methodologic features made it impossible to generate summary estimates of economic burden for all patient safety targets.
In summary, the burden of patient safety targets ranged from as little as $2000 to $200,000 in hospitalized individuals to $600 million at a population level. These results are dependent on the resources and costs included in the analysis. We found that the majority of published studies on the economic burden of patient safety targets in acute care described no costing methodology. The methodologic quality of the remaining studies was moderate, but there were wide variations in methodologic quality and methods for attributing costs. Greater attention is needed to the methodologic standards for evaluation of economic burden. This study highlights the limitations in the methods required to conduct economic evaluations in patient safety. Such limitations make decision-making regarding the adoption of patient safety initiatives difficult. The identification of limitations will allow for focused work on their improvement and will allow for the development of guidelines for future economic evaluation in patient safety.
Acknowledgments
The authors acknowledge Ms Peggy Kee and Ms Evelyn Worthington for their administrative and data analysis, and Dr William Geerts, Dr Damon Scales, and Dr Andrew Simor for their assistance in the study design. This work was funded by an unrestricted grant from the Canadian Patient Safety Institute.
Footnotes
Contributions
Dr Nicole Mittmann, Ms Marika Koo, Dr Nick Daneman, Dr Andrew McDonald, Dr Michael Baker, Dr Anne Matlow, Dr Murray Krahn, Dr Kaveh Shojania, and Dr Edward Etchells all had substantial contributions to the concept and design, acquisition of data, or analysis and interpretation of data. All authors contributed to the draft or revision of the article in a critical manner and they all gave final approval of the version being submitted.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Landrigan CP, Parry GJ, Bones CB, Hackbarth AD, Goldmann DA, Sharek PJ. Temporal trends in rates of patient harm resulting from medical care. N Engl J Med. 2010;363(22):2124–2134. doi: 10.1056/NEJMsa1004404. [DOI] [PubMed] [Google Scholar]
- 2.Classen DC, Resar R, Griffin F, et al. “Global trigger tool” shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood) 2011;30(6):581–589. doi: 10.1377/hlthaff.2011.0190. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada [Internet] 3rd ed. vii. Ottawa, ON: Canadian Agency for Drugs and Technologies in Health; 2006. [Accessed September 30, 2011]. p. 46.p. A17. [cited Oct 20]. Available from: http://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf. [Google Scholar]
- 4.Mittmann N, Evans WK, Rocchi A, et al. Addendum to CADTH’s guidelines for the economic evaluation of health technologies: specific guidance for oncology products. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 5.Gabriel S, Drummond M, Maetzel A, et al. OMERACT 6 Economics working group report: a proposal for a reference case for economic evaluation in rheumatoid arthritis. J Rheumatol. 2003;30(4):886–890. [PubMed] [Google Scholar]
- 6.Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ. 1996;313:275–283. doi: 10.1136/bmj.313.7052.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drummond MF, O’Brien B, Stoddart GL, et al. Methods for the Economic Evaluation of Health Care Programmes. 2nd ed. Oxford UK: Oxford Medical Publications; 1997. [Google Scholar]
- 8.Shojania KG, Duncan BW, McDonald KM, Wachter RM. Report No: Evidence Report/Technology Assessment No 43. 2001. Making health practices safer: a critical analysis of patient safety practices. [PMC free article] [PubMed] [Google Scholar]
- 9.National Surgical Quality Improvement Program. Program Specifics: Data Analysis and Reporting. 2006. [Accessed September 30, 2011]. Available from: http://www.acsnsqip.org/main/programspecs/program_reportingjsp.
- 10.World Health Organization. Patient Safety: Implementing Change. 2011. [Accessed September 30, 2011]. Available from: http://www.who.int/patientsafety/implementation/en/
- 11.Bank of Canada. Daily currency converter. 2011. [Accessed September 30, 2011]. Available from: http://www.bankofcanada.ca/rates/exchange/daily-converter/
- 12.United States Department of Labor BoLS. CPI Inflation Calculator. 2011. [Accessed September 30, 2011]. Available from: http://www.bls.gov/data/inflation_calculator.htm.
- 13.Hoonhout LH, de Bruijne MC, Wagner C, et al. Direct medical costs of adverse events in Dutch hospitals. BMC Health Serv Res. 2009;9:27–37. doi: 10.1186/1472-6963-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehsani JP, Jackson T, Duckett SJ. The incidence and cost of adverse events in Victorian hospitals 2003–2004. Med J Aust. 2006;184(11):551–555. doi: 10.5694/j.1326-5377.2006.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 15.New PW, Jackson T. The costs and adverse events associated with hospitalization of patients with spinal cord injury in Victoria, Australia. Spine. 2010;35(7):796–802. doi: 10.1097/BRS.0b013e3181be76f5. [DOI] [PubMed] [Google Scholar]
- 16.Pappas SH. The cost of nurse-sensitive adverse events. J Nurs Adm. 2008;38(5):230–236. doi: 10.1097/01.NNA.0000312770.19481.ce. [DOI] [PubMed] [Google Scholar]
- 17.Morris JA, Carrillo Y, Jenkins JM, et al. Surgical adverse events, risk management, and malpractice outcome: Morbidity and mortality review in not enough. Ann Surg. 2003;237(6):844–852. doi: 10.1097/01.SLA.0000072267.19263.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoki N, Uda K, Ohta S, Kiuchi T, Fukui T. Impact of miscommunication in medical dispute cases in Japan. Int J Qual Health Care. 2008;20(5):358–362. doi: 10.1093/intqhc/mzn028. [DOI] [PubMed] [Google Scholar]
- 19.Kaushal R, Bates DW, Franz C, Soukup JR, Rothschild JM. Costs of adverse events in intensive care units. Crit Care Med. 2007;35(11):2479–2483. doi: 10.1097/01.CCM.0000284510.04248.66. [DOI] [PubMed] [Google Scholar]
- 20.Senst BL, Achusim LE, Genest RP, et al. Practical approach to determining costs and frequency of adverse drug events in a health care network. Am J Health-Syst Pharm. 2001;58(12):1126–1132. doi: 10.1093/ajhp/58.12.1126. [DOI] [PubMed] [Google Scholar]
- 21.Chen YY, Chou YC, Chou P. Impact of nosocomial infection on cost of illness and length of stay in intensive care units. Infect Control Hosp Epidemiol. 2005;26(3):281–287. doi: 10.1086/502540. [DOI] [PubMed] [Google Scholar]
- 22.Chen YY, Wang FD, Liu CY, Chou P. Incidence rate and variable cost of nosocomial infections in different types of intensive care units. Infect Control Hosp Epidemiol. 2009;30(1):39–46. doi: 10.1086/592984. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RR, Scott D, Cordell R, et al. The use of economic modeling to determine the hospital costs associated with nosocomial infections. Clin Infect Dis. 2003;36(11):1424–1432. doi: 10.1086/375061. [DOI] [PubMed] [Google Scholar]
- 24.Kilgore ML, Ghosh K, Beavers M, Wong DY, Hymel PA, Brossette SE. The costs of nosocomial infections. Med Care. 2008;46(1):101–104. doi: 10.1097/MLR.0b013e3181468991. [DOI] [PubMed] [Google Scholar]
- 25.Esatoglu AE, Agirbas I, Onder OR, Celik Y. Additional cost of hospital-acquired infection to the patient: a case study in Turkey. Health Serv Manage Res. 2006;19(3):137–143. doi: 10.1258/095148406777888062. [DOI] [PubMed] [Google Scholar]
- 26.Sheng WH, Wang JT, Lu DCT, Chie WC, Chen YC, Chang SC. Comparative impact of hospital-acquired infections on medical costs, length of hospital stay and outcome between community hospitals and medical centres. J Hosp Infect. 2005;59(3):205–214. doi: 10.1016/j.jhin.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Plowman R, Graves N, Griffin MAS, et al. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a distract general hosptial in England and the national burden imposed. J Hosp Infect. 2001;47(198):209. doi: 10.1053/jhin.2000.0881. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Imanaka Y, Sekimoto M, et al. Risk-adjusted increases in medical resource utilization associated with health care-associated infections in gastrectomy patients. J Eval Clin Pract. 2010;16:100–106. doi: 10.1111/j.1365-2753.2009.01121.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahieu LM, Buitenweg N, Beutels P, De Dooy JJ. Additional hospital stay and charges due to hospital-acquired infections in a neonatal intensive care unit. J Hosp Infect. 2001;47(3):223–229. doi: 10.1053/jhin.2000.0852. [DOI] [PubMed] [Google Scholar]
- 30.Graves N, Nicholls TM, Morris AJ. Modeling the costs of hospital-acquired infections in New Zealand. Infect Control Hosp Epidemiol. 2003;24(3):214–223. doi: 10.1086/502192. [DOI] [PubMed] [Google Scholar]
- 31.Defez C, Fabbro-Peray P, Cazaban M, Boudemaghe T, Sotto A, Daures JP. Additional direct medical costs of nosocomial infections: an estimation from a cohort of patients in a French university hospital. J Hosp Infect. 2008;68(2):130–136. doi: 10.1016/j.jhin.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Weber WP, Zwahlen M, Reck S, Feder-Mengus C, Widmer AF, Marti WR. Economic burden of surgical site infections at a European university hospital. Infect Control Hosp Epidemiol. 2008;29(7):623–629. doi: 10.1086/589331. [DOI] [PubMed] [Google Scholar]
- 33.Whitehouse JD, Friedman D, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–189. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]
- 34.Mahmoud NN, Turpin RS, Yang G, Saunders WB. Impact of surgical site infections on length of stay and costs in selected colorectal procedures. Surg Infect. 2009;10(6):539–544. doi: 10.1089/sur.2009.006. [DOI] [PubMed] [Google Scholar]
- 35.Penel N, Lefebvre JL, Cazin JL, et al. Additional direct medical costs associated with mosocomial infections after head and neck cancer surgery: a hosptial-perspective analysis. Int J Oral Maxillofac Surg. 2008;37:135–139. doi: 10.1016/j.ijom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Jenney AW, Harrington GA, Russo PL, Spelman DW. Cost of surgical site infections following coronary artery bypass surgery. ANZ J Surg. 2001;71(11):662–664. doi: 10.1046/j.1445-1433.2001.02225.x. [DOI] [PubMed] [Google Scholar]
- 37.Olsen MA, Butler AM, Willers DM, Gross GA, Hamilton BH, Fraser VJ. Attributable costs of surgical site infection and endometritis after low transverse cesarean delivery. Infect Control Hosp Epidemiol. 2010;31(3):276–282. doi: 10.1086/650755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blot SI, Depuydt P, Annemans L, et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005;41(11):1591–1598. doi: 10.1086/497833. [DOI] [PubMed] [Google Scholar]
- 39.Orsi BG, Di Stefano L, Noah N. Hospital-acquired, laboratory-confirmed bloodstream infection: Increased hospital stay and direct costs. Infect Control Hosp Epidemiol. 2002;23(4):190–197. doi: 10.1086/502034. [DOI] [PubMed] [Google Scholar]
- 40.Pirson M, Dramaix M, Struelens M, Riley TV, Leclercq P. Costs associated with hospital-acquired bacteraemia in a Belgian hospital. J Hosp Infect. 2005;59(1):33–40. doi: 10.1016/j.jhin.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Pirson M, Leclercq P, Jackson T, Leclercq M, Garrino M, Sion C. Financial consequences of hospital-acquired bacteraemia in three Belgian hospitals in 2003 and 2004. J Hosp Infect. 2008;68(1):9–16. doi: 10.1016/j.jhin.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Kilgore M, Brossette SE. Cost of bloodstream infections. Am J Infect Control. 2008;36:S172. e1–S172. e3. doi: 10.1016/j.ajic.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Elward AM, Hollenbeak CS, Warren DK, Fraser VJ. Attributable cost of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics. 2005;115(4):868–872. doi: 10.1542/peds.2004-0256. [DOI] [PubMed] [Google Scholar]
- 44.Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics. 2004;114(2):348–355. doi: 10.1542/peds.114.2.348. [DOI] [PubMed] [Google Scholar]
- 45.Chu VH, Crosslin DR, Friedman JY, et al. Staphylococcus aureus bateremia in patients with prosthetic devices: costs and outcomes. Am J Med. 2005;118:1416. e19–1416. e24. doi: 10.1016/j.amjmed.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Adrie C, Alberti C, Chaix-Couturier C, et al. Epidemiology and economic evaluation of severe sepsis in France: age, severity, infection site, and palce of acquisition (community, hospital, or intensive care unit) as determinants of workload and cost. J Crit Care. 2005;20(1):46–58. doi: 10.1016/j.jcrc.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Brun-Buisson C, Roudot-Thoraval F, Girou E, Grenier-Sennelier C, Durand-Zaleski I. The costs of septic syndromes in the intensive care unit and influence of hospital-acquired sepsis. Intensive Care Med. 2003;29(9):1464–1471. doi: 10.1007/s00134-003-1877-x. [DOI] [PubMed] [Google Scholar]
- 48.Festini F, Cocchi P, Mambretti D, et al. Nosocomial rotavirus gastroenteritis in pediatric patients: a multi-centre prospective cohort study. BMC Infect Dis. 2010;10:235–242. doi: 10.1186/1471-2334-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fruhwirth M, Berger K, Ehlken B, Moll-Schuler I, Brosl S, Mutz I. Economic impact of community- and nosocomially acquired rotavirus gastroenteritis in Austria. Pediatr Infect Dis J. 2001;20(2):184–188. doi: 10.1097/00006454-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Piednoir E, Bessaci K, Bureau-Chalot F, et al. Economic impact of healthcare-associated rotavirus infection in a paediatric hospital. J Hosp Infect. 2003;55(3):190–195. doi: 10.1016/j.jhin.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Tambyah PA, Knasinski V, Maki DG. The direct costs of nosocomial catheter-associated urinary tract infection in the era of managed care. Infect Control Hosp Epidemiol. 2002;23(1):27–31. doi: 10.1086/501964. [DOI] [PubMed] [Google Scholar]
- 52.Morse BC, Boland BN, Blackhurst DW, Roettger RH. Analysis of centers for medicaid and medicare services ‘Never events’ in elderly patients undergoing bowel operations. Am Surg. 2010;76(8):841–845. [PubMed] [Google Scholar]
- 53.Rosenthal VD, Buzman S, Migone O, Safdar N. The attributable cost and length of hospital stay because of nosocomial pneumonia in intensive care units in 3 hospitals in Argentina: A prospective matched analysis. Am J Infect Control. 2005;33(3):157–161. doi: 10.1016/j.ajic.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Dietrich ES, Demmler M, Schulgen G, et al. Nosocomial pneumonia: a cost-of-illness analysis. Infection. 2002;30(2):61–67. doi: 10.1007/s15010-002-1083-8. [DOI] [PubMed] [Google Scholar]
- 55.Brilli RJ, Sparling KW, Lake MR, et al. The business case for preventing ventilator-associated pneumonia in pediatric intensive care unit patients. Jt Comm J Qual Saf. 2008;34(11):629–638. doi: 10.1016/s1553-7250(08)34080-x. [DOI] [PubMed] [Google Scholar]
- 56.Macartney KK, Gorelick MH, Manning ML, Hodinka RL, Bell LM. Nosocomial respiratory syncytial virus infections: the cost-effectiveness and cost-benefit of infection control. Pediatrics. 2000;106(3):520–526. doi: 10.1542/peds.106.3.520. [DOI] [PubMed] [Google Scholar]
- 57.Bou R, Lorente L, Aguilar A, et al. Hospital economic impact of an outbreak of Pseudomonas aeruginosa infections. J Hosp Infect. 2009;71(2):138–142. doi: 10.1016/j.jhin.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 58.Fretz R, Schmid D, Jelovcan S, et al. An outbreak of norovirus gastroenteritis in an Austrian hospital, winter 2006–2007. Wien Klin Wochenschr. 2009;121(3–4):137–143. doi: 10.1007/s00508-008-1135-x. [DOI] [PubMed] [Google Scholar]
- 59.Zingg W, Colombo C, Jucker T, Bossart W, Ruef C. Impact of an outbreak of norovirus infection on hospital resources. Infect Control Hosp Epidemiol. 2005;26(3):263–267. doi: 10.1086/502537. [DOI] [PubMed] [Google Scholar]
- 60.Anil M, Helvaci M, Ozkalay N, et al. Salmonella typhimurium outbreak in a neonatal unit in Turkey. Indian J Pediatr. 2009;76(6):629–633. doi: 10.1007/s12098-009-0083-4. [DOI] [PubMed] [Google Scholar]
- 61.Baggett HC, Duchin JS, Shelton W, et al. Two nosocomial pertussis outbreaks and their associated costs – King County, Washington, 2004. Infect Control Hosp Epidemiol. 2007;28(5):537–543. doi: 10.1086/513497. [DOI] [PubMed] [Google Scholar]
- 62.Spearing NM, Jensen A, McCall BJ, Neill AS, McCormack JG. Direct costs associated with a nosocomial outbreak of Salmonella infection: An ounce of prevention is worth a pound of cure. Am J Infect Control. 2000;28(1):54–57. doi: 10.1016/s0196-6553(00)90012-9. [DOI] [PubMed] [Google Scholar]
- 63.Wilson SJ, Knipe CJ, Zieger MJ, et al. Direct costs of multidrug-resistance acinetobater baumannii in the burn unit of a public teaching hospital. Am J Infect Control. 2004;32:342–344. doi: 10.1016/j.ajic.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Watters K, O’Dwyer TP, Rowley H. Cost and morbidity of the MRSA in head and neck cancer patients: what are the consequences? J Laryngol Otol. 2004;118(9):694–699. doi: 10.1258/0022215042244732. [DOI] [PubMed] [Google Scholar]
- 65.Mauldin PD, Salgado CD, Durkalski VL, et al. Nosocomial infections due to mechicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: relationships with antibiotic use and cost drivers. Ann Pharmacother. 2008;42(3):317–326. doi: 10.1345/aph.1K501. [DOI] [PubMed] [Google Scholar]
- 66.Vonberg RP, Reichardt C, Behnke M, Schwab F, Zindler S, Gastmeier P. Cost of nosocomial Clostridium difficile-associated diarrhoea. J Hosp Infect. 2008;70(1):15–20. doi: 10.1016/j.jhin.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 67.Puzniak LA, Gillespie KN, Leet T, Kollef M, Mundy LM. A cost-benefit analysis of gown use in controlling vancomycin-resistant enterococcus transmission: Is it worth the price? Infect Control Hosp Epidemiol. 2004;25(5):418–424. doi: 10.1086/502416. [DOI] [PubMed] [Google Scholar]
- 68.Fuller RL, McCullough EC, Bao MZ, Averill RF. Estimating the costs of potentially preventable hospital acquired complications. Health Care Financ Rev. 2009;30(4):17–32. [PMC free article] [PubMed] [Google Scholar]
- 69.Caprini JA, Botteman MF, Stephens JM, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health. 2003;6(1):59–74. doi: 10.1046/j.1524-4733.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 70.MacDougall DA, Feliu AL, Boccuzzi SJ, Lin J. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thombotic syndrome. Am J Health-Syst Pharm. 2006;63(Suppl 6):S5–S15. doi: 10.2146/ajhp060388. [DOI] [PubMed] [Google Scholar]
- 71.Nadkarni JB, Iyengar KP, Dussa C, Watve S, Vishwanath K. Orthopaedic injuries foloowing falls by hospital in-patients. Gerontology. 2005;51:329–333. doi: 10.1159/000086370. [DOI] [PubMed] [Google Scholar]
- 72.Oliver D, Killick S, Even T, Willmott M. Do falls and falls-injuries in hospital indicate negligent care – and how big is the risk? A retrospective analysis of hte NHS litigation authority database of clinical negligence claims, resulting from falls in hospitals in England 1995 to 2006. Qual Saf Health Care. 2008;17:431–436. doi: 10.1136/qshc.2007.024703. [DOI] [PubMed] [Google Scholar]
- 73.Nurmi I, Luthje P. Incidence and costs of falls and fall injuries among elderly in institutional care. Scand J Prim Health Care. 2002;20(2):118–122. [PubMed] [Google Scholar]
- 74.Hill KD, Vu M, Walsh W. Falls in the acute hospital setting – impact on resource utilisation. Aus Health Rev. 2007;31(3):471–477. doi: 10.1071/ah070471. [DOI] [PubMed] [Google Scholar]
- 75.Schmidek JM, Weeks WB. What do we know about financial returns on investments in patient safety? A literature review. Jt Comm J Qual Patient Saf. 2005;31(12):690–699. doi: 10.1016/s1553-7250(05)31090-7. [DOI] [PubMed] [Google Scholar]
- 76.Fukuda H, Imanaka Y. Assessment of transparency of cost estimates in economic evaluations of patient safety programmes. J Eval Clin Pract. 2009;15(3):451–459. doi: 10.1111/j.1365-2753.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 77.Thomas EJ, Studdert DM, Newhouse JP, et al. Cost of medical injuries in Utah and Colorado. Inquiry. 1999;36(3):255–264. [PubMed] [Google Scholar]
- 78.Zhan C, Friedman B, Mosso A, Pronovost P. Medicare payment for selected adverse events: building the business case for investing in patient safety. Health Aff (Millwood) 2006;25(5):1386–1393. doi: 10.1377/hlthaff.25.5.1386. [DOI] [PubMed] [Google Scholar]
- 79.Mello MM, Studdert DM, Thomas EJ, Yoon CS, Brennan TA. Who pays for medical errors? An analysis of adverse events costs, the medical liabililty system, and incentives for patient safety improvement. J Empir Leg Stud. 2011;4(4):835–860. [Google Scholar]
- 80.Graves N, Harbarth S, Beyersmann J, Barnett A, Halton K, Copper B. Estimating the cost of health care-associated infecitons: mind your p’s and q’s. Clin Infect Dis. 2010;50(7):1017–1021. doi: 10.1086/651110. [DOI] [PubMed] [Google Scholar]
- 81.Barnett AG, Batra R, Graves N, Edgeworth J, Robotham J, Cooper B. Using a longitudinal model to estimate the effect of methicillin-resistant Staphylococcus aureus infection on length of stay in an intensive care unit. Am J Epidemiol. 2009;170(9):1186–1194. doi: 10.1093/aje/kwp249. [DOI] [PubMed] [Google Scholar]
- 82.Graves N, Weinhold D, Roberts JA. Correcting for bias when estimating the cost of hospital-acquired infection: an analysis of lower repiratory tract infections in non-surgical patients. Health Econ. 2005;14(7):755–761. doi: 10.1002/hec.967. [DOI] [PubMed] [Google Scholar]
- 83.Beyersmann J, Kneib T, Schumacher M, Gastmeier P. Nosocomial infection, length of stay, and time-dependent bias. Infect Control Hosp Epidemiol. 2009;30(3):273–276. doi: 10.1086/596020. [DOI] [PubMed] [Google Scholar]
- 84.Shreve J, Van Den Bos J, Gray T, Halford M, Rustagi K, Ziemkiewicz E. The economic measurement of medical errors. 2010. [Accessed September 30, 2011]. Available from: http://www.soa.org/files/pdf/research-econ-measurement.pdf. [DOI] [PubMed]