Abstract
The alpha-glucosidase inhibitor acarbose has been used for more than 20 years in the management of hyperglycemia. Owing to its unique mode of action in the gastrointestinal tract, its properties are very different from other antidiabetic medications. Patients on long-term treatment to control a chronic disease are not only interested in good treatment efficacy, but are also even more interested in the safety and side effects of their medications. Significant aspects of acarbose predominantly regarding safety and tolerability in the management of type 2 diabetes and prediabetes are reviewed. It is concluded that acarbose is a convenient long-term treatment option, with benefits for both type 2 diabetics and patients in a prediabetic state.
Keywords: acarbose, prediabetes, type 2 diabetes, patient considerations, side effects
Introduction
Diabetes is certain to be among the most challenging health problems in the 21st century.1 According to prevalence estimates of the International Diabetes Federation, 366 million people have diabetes in 2011; by 2030 this will have risen to 552 million.1 The number of people with type 2 diabetes, which affects about 90% of all diabetic patients, is increasing in every country. The only positive aspect of this increase in new cases is that the disease is being diagnosed earlier and earlier. This is important for the patient, because it is the only way of minimizing or delaying complications.
When lifestyle interventions alone can no longer maintain good blood glucose control, pharmacological treatment options are introduced into the management of type 2 diabetes. One important treatment option is the alpha-glucosidase inhibitor acarbose which is licensed for the treatment of prediabetes in many countries and has been used worldwide for more than 20 years as treatment for type 2 diabetes, especially in its early stages. The present paper reviews the role of acarbose in the management of these conditions, with special regard to patient considerations.
Type 2 diabetes
A marked postprandial increase in blood glucose is the hallmark of diabetes, but is also observed in prediabetic individuals with impaired glucose tolerance. Individuals with both conditions lack the ability to regulate the release of insulin, which prevents this postprandial increase in healthy people. Therefore, a significant aspect of the pathogenesis of type 2 diabetes is an increased blood glucose concentration, with maximum, but metabolically inadequate, stimulation of insulin secretion. One reason for this is the reduced number of insulin-producing beta cells in the pancreas (caused by an increased rate of apoptosis) which results in relative dominance of secretion of glucagon, the insulin antagonist. Inadequate insulin secretion reduces the glucose flow from blood into peripheral organs. Simultaneously, glucagon increases the stimulation of gluconeogenesis in the liver, both fasting and postprandially between meals. Additionally, type 2 diabetics are insulin-resistant, which means that uptake of glucose into the cells of the peripheral organs is continuously deteriorating. In the long term, the continuously reducing number of beta cells and the decrease in peripheral glucose uptake causes an increase in glucose concentrations, and glucose toxicity intensifies. Hyperglycemia initiates a large number of regulatory processes which can exert harmful effects on the vascular system. Vessels are directly damaged, resulting in the increased morbidity and mortality associated with diabetes.2
The objective of any pharmacological and nonpharmacological therapeutic measures must therefore be a reduction in glucose toxicity, ie, in increased blood glucose concentrations. Other therapeutic objectives, such as blood pressure regulation, maintenance of normal blood lipid levels, and optimizing of body weight will not be reviewed here. The algorithms of national and international guidelines for the treatment of diabetes are based on the principle that patients should be established on suitable oral therapies for the reduction of blood glucose concentrations and glucose toxicity depending on their glycosylated hemoglobin (HbA1c) concentrations. These therapies should be extended as required and ultimately be supplemented by insulin substitution to compensate for lack of adapted insulin release.
Acarbose
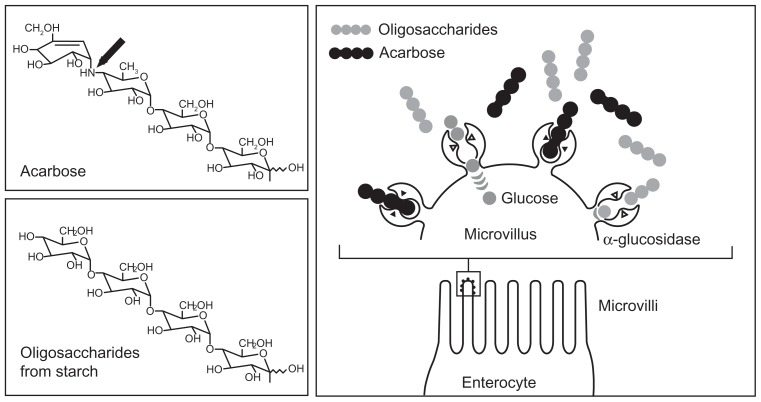
Acarbose belongs to the group of noninsulinotropic oral antidiabetic agents. Because of its unique mode of action, acarbose not only plays an essential and direct role in carbohydrate uptake from food into the blood, but also has an indirect role in the optimization of glucose metabolism over the whole day, as it contributes to the adaptation of insulin secretion. Figure 1 summarizes its mode of action.
Figure 1.
Acarbose mechanism of action: competitive inhibition of the intestinal enzymatic hydrolysis of oligosaccharides.
Copyright © 1991. Reprinted with permission Thieme Publishers. Bischoff H. Effect of acarbose on diabetic late complications and risk factors – new animal experimental results. Akt Endokr Stoffw. 1991;12:25–32.3
To enable glucose uptake and absorption by the body and availability as an energy source, intestinal cleavage of starch and oligosaccharides is necessary, because only monosaccharides can be taken up into the blood. Oligosaccharides are cleaved into monosaccharides by enzyme complexes called alpha-glucosidases, which are present in the brush border membrane of the small intestine.4 Acarbose is structurally similar to natural oligosaccharides, but has a 104 to 105 times higher affinity for alpha-glucosidases. This means that these enzyme complexes are competitively inhibited and that their availability to the oligosaccharides from dietary starch is reduced. Thus, monosaccharide formation decreases and less insulin is required for further metabolisation, leading to a reduction of food-induced postprandial increases in blood glucose and insulin.3,5
Therefore, the effect is not a classic reduction in blood glucose by increased insulin secretion as a reaction to an increase in blood glucose, but a reduction in blood glucose rise as an antihyperglycemic effect. Because reduced blood glucose concentrations result in markedly lower stimulation of insulin synthesis and insulin secretion, the hyperinsulinemia induced by insulin resistance is also decreased.6 Hyperinsulinemia is often found in patients with prediabetes or in the early stages of diabetes. However, in later disease stages, detectable beta cell stress is reduced by acarbose, and insulin synthesis and secretion are more efficient, which can for example be demonstrated by lower proinsulin concentrations on acarbose than on sulfonylurea treatment. Considering that proinsulin is regarded as an independent cardiovascular risk factor, this might also influence the prognosis regarding overall mortality of type 2 diabetics.7,8
The delayed digestion of carbohydrates and cleavage of oligosaccharides results in undigested carbohydrates reaching lower parts of the small intestine and stimulating glucagon-like peptide-1 (GLP-1) secretion there. This intestinal hormone delays emptying of the stomach, reduces glucagon secretion, and regulates insulin secretion, in fact depending on blood glucose concentrations. This might partly explain why longterm treatment with acarbose results not only in a reduction of postprandial but also of fasting blood glucose concentrations.9,10 Given that acarbose acts in the intestine, it can be combined in long-term treatment with all other antidiabetic agents to potentiate efficacy without potentiating adverse events.
Mode of action of acarbose and cardiovascular considerations
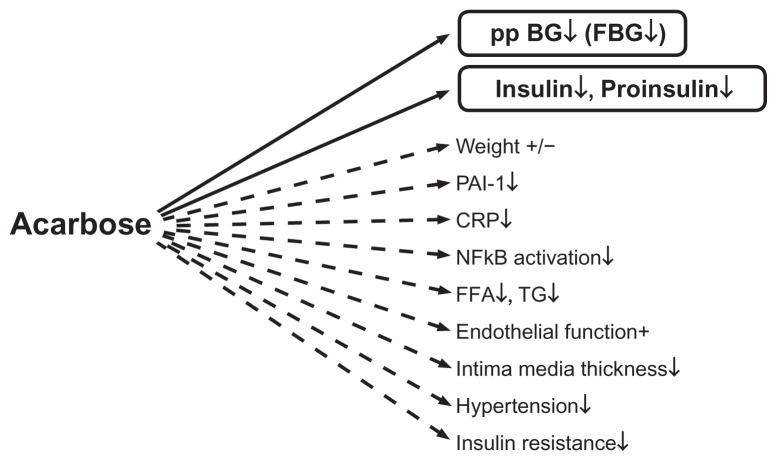
Lower blood glucose concentrations on acarbose have a positive effect by reducing formation of arteriosclerosis-enhancing factors.11–13 Figure 2 summarizes the direct and indirect effects of acarbose on several variables.
Figure 2.
Direct (−) and indirect (---) effects of acarbose on different hormonal, metabolic, and inflammatory variables. Copyright © 2009, Bentham Science Publishers. Adapted from Rosak C, Mertes G. Effects of acarbose on proinsulin and insulin secretion and their potential significance for the intermediary metabolism and cardiovascular system. Curr Diabetes Rev. 2009;5:157–164.7
Abbreviations: BG, blood glucose; CRP, C-reactive protein; FFA, free fatty acids; FBG, fasting blood glucose; NFκB, nuclear factor kappa B; PP, postprandial; PAI, PAI-1, plasminogen activator inhibitor-1; TG, triglycerides.
Via superoxide overproduction, high glucose concentrations result in reduced bioavailability of nitric oxide, which in turn decreases vascular response with subsequent reduced vasodilatation.14,15 Hyperglycemia also increases blood coagulation and platelet aggregation.16 The increased formation of adhesion molecules and increased adhesion of monocytes to endothelial cells leads to formation of thrombotic plaques.17,18 At the same time, hyperglycemia increases the formation of cytokines and C-reactive protein.19 Numerous studies have demonstrated the favorable effect of acarbose on endothelial function, intima media thickness, blood pressure, inflammatory variables such as C-reactive protein and nuclear factor kappa B, triglycerides, and coagulation.20–35
Because vasculotoxic processes occur at a very early disease stage, even patients with impaired glucose tolerance show increased arteriosclerotic changes and increased cardiovascular risk.36–38 Favorable effects of acarbose on cardiovascular clinical endpoints were demonstrated in the double-blind, placebo-controlled STOP-NIDDM (Study TO Prevent NonInsulin-Dependent Diabetes Mellitus).39,40 After at least 3 years of treatment, acarbose reduced the relative risk of cardiovascular events highly significantly in 1429 patients with impaired glucose tolerance by 49%, of myocardial infarction by 91% (one versus 12 cases in only 3 years), and of stroke by 44% in patients with impaired glucose tolerance. The relative risk of developing hypertension was reduced by 34%. This was the first study in diabetes research to demonstrate convincingly that the findings made in experimental investigations using surrogate parameters could be applied to patients in the clinical setting. A recent evaluation only of those individuals who were normotensive at the start of the STOP-NIDDM study showed a significantly reduced risk of developing hypertension on acarbose treatment (hazard ratio 0.59).41 To investigate if treatment of postprandial glycemia with acarbose can reduce cardiovascular morbidity and mortality in patients with impaired glucose tolerance and established cardiovascular disease or acute coronary syndrome, a large-scale, double-blind, multicenter study (ACE, Acarbose Cardiovascular Evaluation) in mainland China and Hong Kong is currently being conducted.42 It is planned to recruit 7500 patients for randomization to acarbose or placebo in addition to standard therapy with a minimum follow-up of 4 years.
Significance of acarbose properties for the patient
There are a number of acarbose properties arising from its unique mode of action which are of significance for the patient.
Hypoglycemia is rare
Hardly any cases of hypoglycemia occur in patients treated with acarbose, because the agent does not stimulate insulin release with hypersecretory effects. On the contrary, a tendency for insulin secretion to decrease has been demonstrated. Hypoglycemia is observed during acarbose treatment only when it is given in combination with insulinotropic substances or insulin itself. Even then, the risk of hypoglycemia is reduced because of combination with the antihyperinsulinemic agent, acarbose.43–47
No increase in body weight
An increase in body weight, observed with most oral antidiabetic agents and insulin, is rare. The compound is regarded as weight-neutral and appears to have a positive effect on body weight, especially in combination with insulinotropic diabetes treatments.43,45,48–51 The main reason is again the reduction of insulin secretion on acarbose treatment. Acarbose even seems to be superior in decreasing body weight when compared with the insulin-neutral treatments, metformin and vildagliptin.52,53 The weight-neutral or even slightly weight-reducing effect of acarbose is an important therapeutic consideration for many patients, because the majority of type 2 diabetic patients have a weight problem.
Physiologically favorable pleiotropic effects
Because acarbose reduces the increase in blood glucose via the intestine and, in doing so, modulates insulin secretion, it does not exert its effects via direct changes in intermediary metabolism. The result are basic physiologically favorable pleiotropic effects, and several risk factors are positively influenced, resulting in reduced morbidity and mortality for patients with impaired glucose tolerance and type 2 diabetes. 25,40 The practical consequences for patients are that they may have to take fewer concomitant medications, such as hypertensives or drugs for other risk factors. The opposite effect was observed in the RECORD (Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes) study, where the intake of statins and diuretics increased markedly during treatment with rosiglitazone.54
Long-term efficacy
Because most diabetic patients require lifelong treatment, the long-term efficacy of an antidiabetic agent is an important aspect. Unlike other classes of antidiabetic agent, the blood glucose-lowering activity of acarbose, with its subsequent metabolic effects, persists with long-term use. Benefits are maintained over several years without evidence of decreasing pharmacological activity. The United Kingdom Prospective Diabetes Study (UKPDS), for example, showed that the blood glucose- and HbA1c-lowering effect of acarbose persisted for at least 3 years, which was not the case with the oral agents, metformin and sulfonylureas, investigated in the same trial.55,56 Other studies confirmed the long-term efficacy of acarbose for up to 5 years.57–59 Therefore, acarbose is a reliable antidiabetic treatment option.
No reports of acarbose failure were found in the available literature. This may be due to the mode of action of acarbose. Other antidiabetic agents reduce blood glucose concentrations through their effect on beta cell function or insulin sensitivity. Both features deteriorate with age and with the duration of diabetes; therefore, the sites of action of these agents change over time. However, the site of action of acarbose is not affected by age. A comparative study of carbohydrate digestibility in elderly and young adults showed no loss of function in the elderly, leading to the conclusion that bowel function in the elderly and in the young does not differ.60 Further studies on aging and digestive function and on intestinal disaccharidase activity in relation to age showed that alpha-glucosidase activity remained constant from birth throughout adult life and was similar in all age groups.61,62 This is of great advantage for long-term and lifelong treatment with acarbose. In this context, it is important to remember that acarbose is suited to guideline-based treatment of patients not only as monotherapy but also in combination with any other oral therapy and insulin. Because of its long-lasting efficacy, acarbose should be of benefit in any stage of treatment for type 2 diabetes. Long-lasting efficacy might be a useful attribute for patients who have difficulty coping with frequent changes in regimen.
Gastrointestinal side effects
A huge number of controlled trials and surveillance studies as well as more than 20 years of worldwide clinical experience with acarbose have not shown significant toxicities or links with subsequent diseases.48 However, gastrointestinal side effects have been widely discussed as a limiting factor for treatment in some Western countries, eg, the US, UK, Northern Europe, and Germany. In many Asian countries and other European countries, such side effects do not appear to be so treatment-limiting or predominant.
Gastrointestinal symptoms associated with acarbose, mainly flatulence and sometimes soft stools or abdominal discomfort, might occur when undigested carbohydrates pass from the small intestine into the colon (malabsorption), resulting in fermentation by bacteria in the large bowel and intestinal gas production. Their incidence has varied widely in controlled trials and surveillance studies. Flatulence and/or meteorism has been reported for between less than 10% and more than 50% of patients in controlled trials, and depended more on investigational site than geographical region. There is an obvious trend to a higher incidence in the US trials (about 40%) than in German (about 25%) and Asian trials (about 17%), but the differences from trial to trial in each area are very wide.
Assuming that the treatment situations in surveillance studies are more comparable because of individualized therapy, the lack of fixed-dose regimens and the usual standard of care in the patient-doctor relationship, such studies may form a more reliable basis for comparison of intestinal tolerance of acarbose. The incidence of flatulence in the PROTECT (Precose Resolution of Optimal Titration to Enhance Current Therapies) surveillance study of 6142 patients in the US was 37%.63 In a German surveillance study with a fixed increasing dosage regimen in 27,803 patients, the incidence was 13%,64 and the incidence was 3.9% in 1996 German patients with free dosing of acarbose in a 5-year post-marketing surveillance study.59 Post-marketing surveillance studies in China and other Asian countries showed an incidence of 2% in 14,418 patients,51 and of only 0.6% in a Chinese post-marketing surveillance study in 2550 patients.65 There are several reasons for these differences in rates. Dose-ranging studies showed that the intestinal side effects of acarbose are dose-dependent,57,66 but did not conclusively show that 3 × 100 mg acarbose per day was more effective than 3 × 50 mg per day. It is interesting to note that most patients in the German 5-year post-marketing surveillance study59 and in the Asian post-marketing surveillance study51 were treated with 3 × 50 mg per day. An early study of acarbose in healthy volunteers clearly showed dose dependency of malabsorption on a single acarbose dose: on 50 mg, malabsorption occurred in only 6% of cases, but in 30% after 100 mg of acarbose.67 Slow titration from a low dosage to the maintenance dosage is recommended. In one study, gradually increasing the dosage up to 3 × 100 mg daily over 8 weeks reduced the frequency of adverse gastrointestinal events from 70% to 30% compared with the group starting with 3 × 100 mg (placebo 6%).68 A controlled trial with slow titration up to the nowadays widely used 3 × 50 mg per day regimen has never been done.
An important reason for the different incidences of gastrointestinal complaints is the great variability in α-glucosidase activity in the small intestine, largely due to the nutritional habits of the patient. The α-glucosidase content (sucrase, maltase) in the proximal and distal intestine was investigated with fiber-free and fiber-rich diets in the early research on acarbose (Figure 3).69 Interestingly, enzyme activity in the distal small intestine was poor on a fiber-free diet, therefore allowing carbohydrates not absorbed in the proximal small intestine to move into the colon. In contrast, a fiber-rich diet ensured high enzyme activity and thus carbohydrate digestion capacity, also in the distal part of the small intestine. Delaying the absorption of carbohydrates by adding acarbose resulted in a gradual increase in enzyme activity in the distal intestine in the fiber-free group, thus normalizing the digestive capacity. This observation explains why, at the beginning of therapy, the mode of action of acarbose in some patients results in a shift of carbohydrates into the colon, and why these side effects decrease with time. In contrast, under a fiber-rich diet, the already high enzyme activity in the distal intestine was not further enhanced by acarbose. Therefore, it can be assumed that patients with fiber-rich nutritional habits usually have at most only physiological malabsorption on acarbose, even at the beginning of therapy. It might further be assumed that traditional Asian and Chinese nutrition contains much more fiber than traditional Western nutrition.
Figure 3.
Sucrase and maltase content in small intestine under fiber-free or fiber-rich diet with or without addition of acarbose.
Data are from Creutzfeldt et al.69
A further study yielded possibly relevant information on nutritional habits: investigating the type of fiber-free carbohydrate, malabsorption was up to 200-fold higher after consuming sucrose than starch under acarbose.70 This was determined measuring breath hydrogen, and suggests a much higher incidence of gastrointestinal complaints after ingestion of soft drinks containing sugar.
Clinical studies and surveillance studies with acarbose show that most patients do not experience gastrointestinal complaints. The number who do can be minimized if their nutritional habits are taken into consideration and if the dose is slowly titrated from low doses to medium doses. This regimen also allows some patients to tolerate higher doses very well under long-term therapy.
Other antidiabetic medications
When assessing the significance of a medication in the treatment of a disorder, comparison with other substances used to treat the same disorder is of interest (Table 1). Insulinotropic agents of the sulfonylurea group are an important diabetes treatment option worldwide, not least because they are economically attractive. They will therefore be reviewed in detail. Sulfonylureas bind to a subunit known as the sulfonylurea receptor 1 of the ATP-regulating potassium channel (KIR 6.2, inward rectifier K channel) of the beta cell. This closes the K+ channel und depolarizes the cell membrane which in turn opens voltage-dependent calcium channels. Calcium ions can penetrate the cytoplasm and thereby induce the secretion of insulin.72 This is a dose-dependent process and occurs in the presence of hyperglycemia, normoglycemia, and hypoglycemia, ie, is not dependent on blood glucose concentrations.73 For this reason, hypoglycemia occurs on sulfonylureas. Patients with near-normal blood glucose profiles, the elderly, and those with impaired renal function are particularly susceptible. Because the blood glucose level is very important for the energy supply to the brain, a protective hormonal mechanism is triggered when hypoglycemia occurs, involving stimulation and high concentrations of adrenaline, noradrenaline, and glucagon. These hormones may themselves cause complications, such as cardiac arrhythmias, myocardial infarction, hypertensive crises, and stroke. It is difficult to recognize this life-threatening situation in elderly and long-term diabetics, which explains the high number of undetected cardiovascular deaths due to hypoglycemia on sulfonylureas, glinide, and insulin treatment. Sulfonylureas may also cause undesirable cardiovascular effects, such as cardiac arrhythmia, by binding to smooth muscles and the heart muscle. This represents a further aspect in the danger potential of this class of antidiabetic medications.74 So far, it has not been possible to demonstrate whether treatment with sulfonylureas decreases significant endpoints, such as cardiovascular events, in the long term. The only effect demonstrated in the UKPDS study was on retinopathy.75
Table 1.
Convenience of antidiabetic therapies
| Treatment | Site of action | Body weight | Hypoglycemia in monotherapy | Long-term efficacy | Gastrointestinal side effects | Safety (related diseases) | Mean reduction in HbA1c a |
|---|---|---|---|---|---|---|---|
| Acarbose | CH-digestive enzymes Small intestine |
−(↓) | − | + | + | − | −1% |
| Sulfonylureas | Beta cells | ↑ | ↑ | − | − | + | −1.25% |
| Glinides | Beta cells | ↑ | ↑ | − | − | ? | −0.75% |
| Metformin | Sensitivity to insulin Liver, peripheral tissues |
− | − | − | + | − | −1% |
| Gliptines | DPP-4 enzymes Plasma, peripheral tissues |
−(↓) | − | ? | (+) | (+) | −0.75% |
| Pioglitazone | PPAR-gamma receptor Cell nucleus |
↑ | − | (+) | − | + | −1% |
Note:
According to Sherifali et al.71
Abbreviations: CH, carbohydrate; PPAR, peroxisome proliferator-activated receptor; DPP-4, dipeptidyl peptidase 4.
Inadequately high insulin concentrations are often reached on sulfonylureas and this increases the anabolic effects of insulin and the appetite which, as was shown in the UKPDS study,75 leads to a marked increase in body weight. A higher incidence of malignant neoplasm and related mortality has been described on sulfonylurea and insulin therapy, at least in comparison with metformin treatment.76 This must be openly discussed and is becoming increasingly controversial. In the age of the World Wide Web, patients are also aware of these discussions. In addition to such discussions, patients might worry about attacks of hypoglycemia and undesired weight gain (also observed on insulin). Physicians should be mindful of these aspects when prescribing agents of this class.
Glinides act in a similar way to sulfonylureas.77,78 Their effectiveness and side effects are somewhat less marked because of a shorter half-life, but basically the same reservations apply.
Because of the disadvantages described for insulinotropic antidiabetic agents, noninsulinoptropic substances should preferably be used in the early and late stages of diabetes. Amongst such substances, guidelines worldwide recommend metformin as the oral antidiabetic of choice. Its mechanism of action has not been completely elucidated, but is mainly due to an improvement of peripheral and liver sensitivity to insulin.79,80 Patients taking metformin do not gain weight, and hypoglycemia does not occur on metformin monotherapy. However, metformin does frequently cause gastrointestinal side effects, and is contraindicated in the presence of impaired renal function. Regarding its effects on reducing the incidence of diabetes in Asian individuals with prediabetes, it seems to be less efficacious than in Western populations.81,82 Whether evidence-based findings with regard to long-term effects of metformin on endpoints, especially cardiovascular mortality, are adequate, has again become a point of controversy.
It is well known that one of the main reasons for noncompliance among patients is safety concerns. It should be mentioned that reports of severe and even fatal adverse events reach patients immediately nowadays, the principal channels being newspapers and the World Wide Web. In the case of antidiabetic medications, the past years have witnessed a clear decline in confidence in new drugs. Diabetes is not acutely life-threatening but is a chronic disease. Patients know that treatment is required lifelong. Negative news about different new drugs is therefore all the more worrying to them.
New drugs in the promising glitazone group were restricted to only a small minority of patients or even taken off the market after millions of prescriptions had been issued. The reasons were a clear increase in edema, cardiovascular morbidity, and even mortality,83–86 as well as a clear increase in bone fractures87,88 in macular edema,89 and an increased risk of bladder cancer.90 Even before these risks became apparent, treatment with glitazones had already proved disappointing for patients and physicians alike, and was also associated with weight gain and increasing low-density lipoprotein cholesterol,91,92 and a higher demand for statins and diuretics.54 All these issues were discussed in the public arena and were not restricted to expert circles.
Safety concerns have also been expressed about the new incretin-based therapies (glutides and gliptins) which modulate insulin secretion and blood glucose by increasing the gut hormone GLP-1. Glutides are associated with a high incidence of nausea and diarrhea, and gliptins with an increase in the incidence of infections and inflammation, predominantly of the respiratory system or as arthritis.93–95 These side effects are due to the proinflammatory and immune-modulating effects of their well described mode of action via dipeptidyl peptidase-4 inhibition. According to a systematic meta-analysis and review by the Cochrane Metabolic and Endocrine Disorder Group, the incidence of infections was 34% higher in patients treated with sitagliptin than in those not on a gliptin, and 5% higher on vildagliptin.96 This result weakened the positive image of these drugs as agents that do not increase hypoglycemia or cause weight gain. A large number of reports of severe pancreatitis have also been made available in the public media.93,97,98 Although consensus has not yet been achieved regarding the significance of these serious and possibly life-threatening side effects,99 precautionary restrictions were introduced in some countries, resulting in considerable worry amongst patients.
Safety of acarbose treatment
The lack of weight gain and hypoglycemia associated with administration of acarbose is not the only significant safety aspect when choosing appropriate therapy for the individual patient. No signs of potential cardiovascular harm were observed on acarbose therapy, and systemic adverse effects linked to acarbose are extremely rare, as the huge number of trials and the huge worldwide clinical experience show, which is an important fact to consider for both the physician and patient when prescribing antidiabetic medication. The positive safety profile of acarbose is consistent with its site of action in the gastrointestinal tract and its very low systemic availability.100,101 No severe or fatal adverse events were observed in a prospective, 5-year postmarketing surveillance study in 1996 patients.59 In a double-blind study in 1429 people with impaired glucose tolerance, half of them treated with acarbose for between 3 and 5 years, the incidence of adverse events was similar to that on placebo, and no serious adverse events occurred.39
Very rare cases of reversible increases in liver transaminases were reported in the first decade after acarbose was approved.102 No differences from placebo were observed in clinical studies. Only 19 individual cases were reported amongst 500,000 patients.103 Increases in transaminases have not been observed during treatment for impaired glucose tolerance. The use of acarbose in diabetic patients with elevated liver enzyme activity was investigated in several studies. All studies showed a beneficial effect of acarbose on chronic liver disease in such patients.104–106
The findings of large-scale, controlled trials and surveillance studies, as well as post-marketing experience, show that acarbose is one of the safest antidiabetic agents available, both alone and in combination.
Combination therapy
The natural course of type 2 diabetes involves a continuous increase in the rate of beta cell apoptosis. Therefore, it is necessary to combine agents early in the treatment to maintain HbA1c concentrations under 7% for overall good glycemic control. The combination of acarbose and metformin is a good example of how to achieve a positive complementary effect.7 The main effect of metformin is reduction of fasting blood glucose by decreasing gluconeogenesis in the liver. Adding acarbose decreases still elevated postprandial blood glucose concentrations without stimulating insulin secretion. In contrast with the beneficial effects of a metformin-acarbose combination, a combination of sulfonylurea and metformin was not successful. The UKPDS study already showed a higher mortality under this combination therapy.55 This finding was consolidated by subsequent studies, and such combinations are now regarded as obsolete.107
However, combinations of sulfonylureas or glinides with acarbose are effective. An interesting aspect of the combined use of these agents is not only markedly lower postprandial blood glucose concentrations but also accompanying lower insulin secretion. Therefore, acarbose modifies the insulin secretion stimulated by sulfonylureas, thereby reducing hypoglycemia rate, weight gain, insulin resistance, and glucose toxicity.7
Conclusion
Acarbose is a useful treatment option for the management of hyperglycemia. Favorable treatment aspects for the patients are, in particular, a high degree of safety with regard to severe side effects and complications, and the absence of hypoglycemia and effects on body weight. A further positive aspect of acarbose treatment is the reliable long-term effect because its site of action is maintained.
There are several practical points to consider:
Because of its unique antihyperglycemic mode of action, its safety and confirmed improvement of clinical endpoints in large-scale, long-term studies in individuals with impaired glucose tolerance, acarbose is approved in many countries for prediabetic conditions.
In type 2 diabetic patients, acarbose should be the preferred monotherapy for early disease stages with high postprandial blood glucose levels to enable patients to benefit from its pronounced effect on postprandial blood glucose.
In advanced type 2 diabetes, acarbose can be combined with all other antidiabetic agents and has favorable effects on the side effects of other drugs, such as body weight increase or hypoglycemic episodes. Antidiabetic therapies with a strong impact on fasting blood glucose should be preferred in combination.
To ensure maximum efficacy, acarbose must be taken directly at onset or during meals;108 administration, eg, 15 minutes before a meal reduces its efficacy by 50%. It is essential that the patient is made aware of this.
When initiating acarbose treatment, the rule should be “start low, go slow”. The patient’s nutrition habits must be elucidated, and in the case of a previous preference for a fiber-free diet, a very low starting dose should be chosen. The patient should be told to avoid sugar-containing drinks or drinks with other sugars such as fructose.
The positive safety profile of acarbose can be stressed, in particular with patients worrying about safety and who are known not to be compliant as a result. Unlike the physician, for whom the efficacy of a drug, especially its effects on mortality, are the prime consideration, patients are mainly concerned with the incidence and severity of side effects, possible safety risks in long-term use, impairment of quality of life, and ease of administration. These are the factors that determine patient compliance.
Numerous studies have shown that, ultimately, all oral antidiabetic agents have a similar efficacy on HbA1c. Therefore, it is important to take account of personal factors in treatment that are of consequence for the patient. The positive aspects of acarbose treatment for the patient mean that it is often a reliable choice.
Footnotes
Disclosure
CR has received speaking honoraria from Bayer Healthcare AG, Germany. GM is a former employee of Bayer Vital GmbH, Germany.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 5th ed. Brussels, Belgium: International Diabetes Federation; 2011. [Accessed May 16, 2012]. Available from: http://www.idf.org/diabetesatlas. [Google Scholar]
- 2.The DECODE Study Group. Glucose tolerance and cardiovascular mortality. Arch Intern Med. 2001;161:397–404. doi: 10.1001/archinte.161.3.397. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff H. Effect of acarbose on diabetic late complications and risk factors – new animal experimental results. Akt Endokr Stoffw. 1991;12:25–32. [Google Scholar]
- 4.Elsenhans B, Caspary WF. Absorption of carbohydrates. In: Caspary WF, editor. Structure and Function of the Small Intestine. Amsterdam, The Netherlands: Excerpta Medica; 1987. [Google Scholar]
- 5.Puls W. Pharmacology of glucosidase inhibitors. In: Kuhlmann J, Puls W, editors. Handbook of Experimental Pharmacology: Oral Antidiabetics. Vol. 119. Berlin, Germany: Springer; 1996. [Google Scholar]
- 6.Bischoff H. Alpha-glucosidase inhibition, a new therapeutic principle in the management of diabetes mellitus. In: Schwartz CJ, Born GVR, editors. New Horizons in Diabetes Mellitus and Cardiovascular Disease. London, UK: Current Science; 1995. [Google Scholar]
- 7.Rosak C, Mertes G. Effects of acarbose on proinsulin and insulin secretion and their potential significance for the intermediary metabolism and cardiovascular system. Curr Diabetes Rev. 2009;5:157–164. doi: 10.2174/157339909788920910. [DOI] [PubMed] [Google Scholar]
- 8.Alessema M, Dekker JM, Nijpels G, Stehouwer CDA, Bouter LM, Heine RJ. Proinsulin concentration is an independent predictor of all-cause and cardiovascular mortality: an 11-year follow-up of the Hoorn Study. Diabetes Care. 2005;28:860–865. doi: 10.2337/diacare.28.4.860. [DOI] [PubMed] [Google Scholar]
- 9.Qualmann C, Nauck MA, Holst JJ, Orskov C, Creutzfeldt W. Glucagon-like peptide 1 (GLP-1) [17–36 amide] secretion in response to luminal sucrose from the upper and lower gut: a study using alpha-glucosidase inhibition (acarbose) Scand J Gastroenterol. 1995;30:892–896. doi: 10.3109/00365529509101597. [DOI] [PubMed] [Google Scholar]
- 10.Göke B, Fuder H, Wieckhorst G, et al. Voglibose is an efficient alpha-glucosidase inhibitor and mobilizes the endogenous GLP-1 reserve. Digestion. 1995;56:493–501. doi: 10.1159/000201282. [DOI] [PubMed] [Google Scholar]
- 11.Hanefeld M, Temelkowa-Kurktschiev T. The postprandial state and the risk of atherosclerosis. Diab Med. 1997;14:S6–S11. doi: 10.1002/(sici)1096-9136(199708)14:3+<s6::aid-dia438>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Barrett-Connor E, Ferrara A. Isolated postprandial hyperglycemia and the risk of fatal cardiovascular disease in older woman and men. The Rancho Bernardo Study. Diabetes. 1998;21:1236–1240. doi: 10.2337/diacare.21.8.1236. [DOI] [PubMed] [Google Scholar]
- 13.de Vegt F, Dekker JM, Ruhè HG, Stehouwer CDA, Nijpels GBLM, Heine RJ. Hyperglycemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999;42:926–931. doi: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- 14.Giugliano D, Marfella R, Coppola L, et al. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation. 1997;95:1783–1790. doi: 10.1161/01.cir.95.7.1783. [DOI] [PubMed] [Google Scholar]
- 15.Kawano H, Motoyama T, Hirashima O, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol. 1999;34:146–154. doi: 10.1016/s0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 16.Ceriello A, Falleti E, Motz E, et al. Hyperglycemia-induced circulating ICAM-1 increase in diabetes mellitus: the possible role of oxidative stress. Horm Metab Res. 1998;30:146–149. doi: 10.1055/s-2007-978854. [DOI] [PubMed] [Google Scholar]
- 17.Marfella R, Esposito K, Giunta R, et al. Circulating adhesion molecules in humans: role of hyperglycemia and hyperinsulinemia. Circulation. 2000;101:2247–2251. doi: 10.1161/01.cir.101.19.2247. [DOI] [PubMed] [Google Scholar]
- 18.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 19.Festa A, D’Agostino R, Jr, Tracy RP, Haffner SM. C-reactive protein is more strongly related to post-glucose load glucose than to fasting glucose in non-diabetic subjects; the Insulin Resistance Atherosclerosis Study. Diab Med. 2002;19:939–943. doi: 10.1046/j.1464-5491.2002.00824.x. [DOI] [PubMed] [Google Scholar]
- 20.Shimabukuro M, Higa N, Chinen I, Yamakawa K, Takasu N. Effects of a single administration of acarbose on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients: a randomized crossover study. J Clin Endocrinol Metab. 2006;91:837–842. doi: 10.1210/jc.2005-1566. [DOI] [PubMed] [Google Scholar]
- 21.Wascher TC, Schmoelzer I, Wiegratz A, et al. Reduction of postchallenge hyperglycemia prevents acute endothelial dysfunction in subjects with impaired glucose tolerance. Eur J Clin Invest. 2005;35:551–557. doi: 10.1111/j.1365-2362.2005.01550.x. [DOI] [PubMed] [Google Scholar]
- 22.Kato T, Inoue T, Node K. Postprandial endothelial dysfunction in subjects with new-onset type 2 diabetes: an acarbose and nateglinide comparative study. Cardiovasc Diabetol. 2010;9:12–16. doi: 10.1186/1475-2840-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanefeld M, Chiasson JL, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschiev T. Acarbose slows progression of intima-media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004;35:1073–1078. doi: 10.1161/01.STR.0000125864.01546.f2. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal JH, Mauersberger H. Effects on blood pressure of the alpha-glucosidase- inhibitor acarbose compared with the insulin enhancer glibenclamide in patients with hypertension and type 2 diabetes mellitus. Clin Drug Investig. 2002;22:695–701. [Google Scholar]
- 25.Hanefeld M, Catagay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J. 2004;25:10–16. doi: 10.1016/s0195-668x(03)00468-8. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Lu J, Pan C. Comparison of serum C-reactive protein level in different glucose tolerant subjects and the change in serum CRP level in IGT subjects with acarbose. Chin J Endocrinol Metab. 2003;19:254–256. [Google Scholar]
- 27.Rudofski G, Reismann P, Schiekofer S. Reduction of postprandial hyperglycemia in patients with Type 2 diabetes reduces NF-kappaB activation in PBMCs. Horm Metab Res. 2004;36:630–638. doi: 10.1055/s-2004-825904. [DOI] [PubMed] [Google Scholar]
- 28.Baron AD, Eckel RH, Schmeiser L, Kolterman OG. The effect of short-term alpha-glucosidase inhibition on carbohydrate and lipid metabolism in type II diabetics. Metabolism. 1987;36:409–415. doi: 10.1016/0026-0495(87)90035-7. [DOI] [PubMed] [Google Scholar]
- 29.Kado S, Murakami T, Aoki A, et al. Effect of acarbose on postprandial lipid metabolism in type 2 diabetes mellitus. Diab Res Clin Pract. 1998;41:49–55. doi: 10.1016/s0168-8227(98)00062-x. [DOI] [PubMed] [Google Scholar]
- 30.Malaguarnera M, Giugno I, Ruello P, Rizzo M, Motto M, Mazzoleni G. Acarbose is an effective adjunct to dietary therapy in the treatment of hypertriglyceridemias. Br J Clin Pharmacol. 1999;48:605–609. doi: 10.1046/j.1365-2125.1999.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogawa S, Takeuchi K, Ito S. Acarbose lowers serum triglyceride and postprandial chylomicron levels in type 2 diabetes. Diab Obes Metab. 2004;6:384–390. doi: 10.1111/j.1462-8902.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 32.Tushuizen ME, Diamant M, Heine RJ. Postprandial dysmetabolism and cardiovascular disease in type 2 diabetes. Postgrad Med J. 2005;81:1–6. doi: 10.1136/pgmj.2004.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ceriello A, Taboga C, Tonutti L, et al. Post-meal coagulation activation in diabetes mellitus: the effect of acarbose. Diabetologia. 1996;39:469–473. doi: 10.1007/BF00400679. [DOI] [PubMed] [Google Scholar]
- 34.Tschoepe D. Decreased fibrinogen by treatment with the alpha-glucosidase inhibitor acarbose. Diabetes. 2004;53(Suppl 2):A189. [Google Scholar]
- 35.Shinoda Y, Inoue I, Nakano T, et al. Acarbose improves fibrinolytic activity in patients with impaired glucose tolerance. Metabolism. 2006;55:935–939. doi: 10.1016/j.metabol.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 36.Rathmann W, Giani G, Mielck A. Cardiovascular risk factors in newly diagnosed abnormal glucose tolerance: comparison of 1997 ADA and WHO criteria. Diabetologia. 1999;42:1268–1269. doi: 10.1007/s001250051305. [DOI] [PubMed] [Google Scholar]
- 37.Coutinho M, Gerstein HC, Wang Y, Yussuf S. The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 38.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes. 1999;48:937–942. doi: 10.2337/diabetes.48.5.937. [DOI] [PubMed] [Google Scholar]
- 39.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 40.Chiasson J-L, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance. The STOP-NIDDM Trial. JAMA. 2003;290:486–494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 41.Hanefeld M, Pistrosch F, Koehler C, Chiasson JL. Conversion of IGT to type 2 diabetes mellitus is associated with incident cases of hypertension: a post-hoc analysis of the STOP-NIDDM trial. J Hypertens. 2012;30:1440–1443. doi: 10.1097/HJH.0b013e328354663c. [DOI] [PubMed] [Google Scholar]
- 42.Acarbose Cardiovascular Evaluation (ACE study) [Accessed June 19, 2012]. Available from: http://www.dtu.ox.ac.uk/ace/
- 43.Coniff RF, Shapiro JA, Seaton TB, Bray GA. Multicenter, placebo-controlled trial comparing acarbose (Bay g 5421) with placebo, tolbutamide and tolbutamide-plus-acarbose in non-insulin-dependent diabetes mellitus. Am J Med. 1995;98:443–451. doi: 10.1016/S0002-9343(99)80343-X. [DOI] [PubMed] [Google Scholar]
- 44.Rosak C, Haupt E, Walter T, Werner J. The effect of combination treatment with acarbose and glibenclamide on postprandial glucose and insulin profiles: additive blood glucose lowering effect and decreased hypoglycaemia. Diabetes Nutr Metab. 2002;15:143–151. [PubMed] [Google Scholar]
- 45.Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410–1418. doi: 10.1001/jama.2010.405. [DOI] [PubMed] [Google Scholar]
- 46.Lin SD, Wang JS, Hsu SR, et al. The beneficial effect of α-glucosidase inhibitor on glucose variability compared with sulfonylurea in Taiwanese type 2 diabetic patients inadequately controlled with metformin: preliminary data. J Diabetes Complications. 2011;25:332–338. doi: 10.1016/j.jdiacomp.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Tschöpe D, Bramlage P, Binz C, et al. Antidiabetic pharmacotherapy and anamnestic hypoglycemia in a large cohort of type 2 diabetic patients – an analysis of the DiaRegis registry. Cardiovasc Diabetol. 2011;10:66. doi: 10.1186/1475-2840-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Laar FA, Lucassen PL, Akkermans LP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes. Results from a Cochrane systematic review and metaanalysis. Diabetes Care. 2005;28:154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 49.Bolen S, Feldmann L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147:386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 50.Schnell O, Mertes G, Standl E on behalf of the Acarbose-Insulin Combination Study Group. Acarbose and metabolic control in patients with type 2 diabetes with newly initiated insulin therapy. Diabetes Obes Metab. 2007;9:853–888. doi: 10.1111/j.1463-1326.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 51.Li C, Hung YJ, Quamruddin K, Aziz MFA, Stein H, Schmidt B. International noninterventional study of acarbose treatment in patients with type 2 diabetes mellitus. Diab Res Clin Pract. 2011;92:57–64. doi: 10.1016/j.diabres.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 52.Willms B, Ruge D. Comparison of acarbose and metformin in patients with type 2 diabetes mellitus insufficiently controlled with diet and sulphonylureas: a randomized, placebo-controlled study. Diabet Med. 1999;16:755–761. doi: 10.1046/j.1464-5491.1999.00149.x. [DOI] [PubMed] [Google Scholar]
- 53.Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med. 2008;25:435–441. doi: 10.1111/j.1464-5491.2008.02391.x. [DOI] [PubMed] [Google Scholar]
- 54.Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 55.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 56.Holman RR, Turner RC, Cull CA on behalf of the UKPDS Study Group. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (UKPDS 44) Diabetes Care. 1999;22:960–964. doi: 10.2337/diacare.22.6.960. [DOI] [PubMed] [Google Scholar]
- 57.Fischer S, Hanefeld M, Spengler M, Boehme K, Temelkowa-Kurktschiev T. European study on dose-response relationship of acarbose as a first-line drug in non-insulin-dependent diabetes mellitus. Acta Diabetol. 1998;35:34–40. doi: 10.1007/s005920050098. [DOI] [PubMed] [Google Scholar]
- 58.Hasche H, Mertes G, Bruns C, et al. Effects of acarbose treatment in type 2 diabetic patients under dietary training: a multicenter, double-blind, placebo-controlled, 2-year study. Diabetes Nutr Metab. 1999;12:277–285. [PubMed] [Google Scholar]
- 59.Mertes G. Safety and efficacy of acarbose in the treatment of type 2 diabetes: data from a 5-year surveillance study. Diabetes Res Clin Pract. 2001;52:193–204. doi: 10.1016/s0168-8227(01)00221-2. [DOI] [PubMed] [Google Scholar]
- 60.Brauer PM, Slavin JL, Marlett JA. Apparent digestibility of neutral detergent fiber in elderly and young adults. Am J Clin Nutr. 1981;34:1061–1070. doi: 10.1093/ajcn/34.6.1061. [DOI] [PubMed] [Google Scholar]
- 61.Bowman B, Rosenberg IH. Digestive function and aging. Hum Nutr Clin Nutr. 1983;37C:75–89. [PubMed] [Google Scholar]
- 62.Welsh JD, Poley JR, Bhatia M, Stevenson DE. Intestinal disaccharidase activities in relation to age, race and mucosal damage. Gastroenterology. 1978;75:847–855. [PubMed] [Google Scholar]
- 63.Buse J, Hart K, Minasi LA on behalf of the PROTECT Study Group. The PROTECT study: Final results of a large multicenter postmarketing study in patients with type 2 diabetes. Clin Ther. 1998;20:257–269. doi: 10.1016/s0149-2918(98)80089-1. [DOI] [PubMed] [Google Scholar]
- 64.Spengler M, Schmitz H, Landen H. Evaluation of the efficacy and tolerability of acarbose in patients with diabetes mellitus – a postmarketing surveillance study. Clin Drug Investig. 2005;25:651–659. doi: 10.2165/00044011-200525100-00004. [DOI] [PubMed] [Google Scholar]
- 65.Pan CY, Landen H. Post-marketing surveillance of acarbose treatment in patients with type 2 diabetes mellitus and subjects with IGT in China. Clin Drug Investig. 2007;27:397–405. doi: 10.2165/00044011-200727060-00003. [DOI] [PubMed] [Google Scholar]
- 66.Santeusiano F, Ventura MM, Contadini S, et al. Efficacy and safety of two different dosages of acarbose in non-insulin dependent diabetic patients treated by diet alone. Diabetes Nutr Metab. 1993;6:147–154. [Google Scholar]
- 67.Radziuk J, Kemmer F, Morishima T, Berchtold P, Vranic M. The effects of an alpha-glucosidase hydrolase inhibitor on glycemia and the absorption of sucrose in man determined using a tracer method. Diabetes. 1984;33:207–213. doi: 10.2337/diab.33.3.207. [DOI] [PubMed] [Google Scholar]
- 68.May C. Wirksamkeit und Verträglichkeit von einschleichend dosierter Acarbose bei Patienten mit nichtinsulinpflichtigem Diabetes mellitus unter Sulfonylharnstofftherapie. Diabetes Stoffwechsel. 1995;4:3–8. German. [Google Scholar]
- 69.Creutzfeldt W, Fölsch UR, Elsenhaus B, Ballmann M, Conlon M. Adaptation of the small intestine to induced maldigestion. Scand J Gastroenterol. 1985;20:45–53. doi: 10.3109/00365528509092212. [DOI] [PubMed] [Google Scholar]
- 70.Koytchev R, Richter W, Erkent U, et al. Influence of acarbose on blood glucose and breath hydrogen after carbohydrate load with sucrose or starch. Arzneimittelforschung. 2009;59:557–563. doi: 10.1055/s-0031-1296444. [DOI] [PubMed] [Google Scholar]
- 71.Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. 2010;33:1859–1864. doi: 10.2337/dc09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ashcroft FM. Mechanism of the glycemic effects of sulfonylureas. Horm Metab Res. 1996;28:456–463. doi: 10.1055/s-2007-979837. [DOI] [PubMed] [Google Scholar]
- 73.Panten U, Schwanstecher M, Schwanstecher C. Sulfonylurea receptors and mechanism of sulfonylurea action. Exp Clin Endocrinol Diabetes. 1996;104:1–9. doi: 10.1055/s-0029-1211414. [DOI] [PubMed] [Google Scholar]
- 74.Bijlstra PJ, Luttermann JA, Russel FGM, Thien T, Smits P. Interaction of sulphonylurea derivatives with vascular ATP-sensitive potassium channels in humans. Diabetologia. 1996;39:1083–1090. doi: 10.1007/BF00400658. [DOI] [PubMed] [Google Scholar]
- 75.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood- glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 76.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: A consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guay DRP. Repaglinide, a novel short-acting hypoglycemic agent for type 2 diabetes mellitus. Pharmacotherapy. 1998;18:1195–1204. [PubMed] [Google Scholar]
- 78.Marbury T, Huang WC, Strange P, Lobovitz H. Repaglinide versus glyburide: a one year comparison trial. Diab Res Clin Pract. 1999;43:155–165. doi: 10.1016/s0168-8227(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 79.Bloomgarden ZT. Metformin. Diabetes Care. 1995;18:1078–1092. doi: 10.2337/diacare.18.7.1078. [DOI] [PubMed] [Google Scholar]
- 80.Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 81.Li CL, Pan CY, Lu JM, et al. Effect of metformin on patients with impaired glucose tolerance. Diabet Med. 1999;16:477–481. doi: 10.1046/j.1464-5491.1999.00090.x. [DOI] [PubMed] [Google Scholar]
- 82.Yang W, Lin L, Qi J, et al. The preventive effect of acarbose and metformin on the progression to diabetes mellitus in the IGT population: a 3-year multicenter prospective study. Chin J Endocrinol Metab. 2001;17:131–136. [Google Scholar]
- 83.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 84.Graham DJ, Ouellet-Hellstrom R, MaCurdy TE, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 85.Wertz DA, Chang CL, Sarawate CA, Willey VJ, Cziraky MJ, Bohn RL. Risk of cardiovascular events and all-cause mortality in patients treated with thiazolidinediones in a managed-care population. Circ Cardiovasc Qual Outcomes. 2010;3:538–545. doi: 10.1161/CIRCOUTCOMES.109.911461. [DOI] [PubMed] [Google Scholar]
- 86.Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and metaanalysis of observational studies. BMJ. 2011;342:d1309. doi: 10.1136/bmj.d1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kahn SE, Zinman B, Lachin JM, et al. Rosiglitazone-associated fractures in type 2 diabetes. Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- 88.Dormuth CR, Carney G, Carleton B, Bassett K, Wright JM. Thiazolid-indiones and fractures in men and women. Arch Intern Med. 2009;169:1395–1402. doi: 10.1001/archinternmed.2009.214. [DOI] [PubMed] [Google Scholar]
- 89.Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med. 2012 Jun 11; doi: 10.1001/archinternmed.2012.1938. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 90.Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34:1369–1371. doi: 10.2337/dc10-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2008;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 92.Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeeley RJ. A role for central nervous system PPAR-γ in the regulation of energy balance. Nat Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cure P, Pileggi A, Alejandro R. Exenatide and adverse events. N Engl J Med. 2008;358:1969–1970. doi: 10.1056/NEJMc0707137. [DOI] [PubMed] [Google Scholar]
- 94.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes. Systematic review and metaanalysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 95.Williams-Herman D, Engel SS, Round E, et al. Safety and tolerability of sitagliptin in clinical studies: a pooled analysis of data from 10,246 patients with type 2 diabetes. BMC Endocr Disord. 2010;10:7–11. doi: 10.1186/1472-6823-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C. Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag. 2008;4:753–768. doi: 10.2147/vhrm.s1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tripathy NR, Basha S, Jain R, Shetty S, Ramachandran A. Exenatide and acute pancreatitis. J Assoc Physicians India. 2008;56:987–988. [PubMed] [Google Scholar]
- 98.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide- 1-based therapies. Gastroenterology. 2011;141:150–156. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: Evaluation of the risks and benefits. Diabetes Care. 2010;33:428–433. doi: 10.2337/dc09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Salvatore T, Giugliano D. Pharmacokinetic-pharmacodynamic relationships of acarbose. Clin Pharmacokinet. 1996;30:94–106. doi: 10.2165/00003088-199630020-00002. [DOI] [PubMed] [Google Scholar]
- 101.Ahr HJ, Krause HP, Siefert HM, Steinke W, Weber H. Pharmacokinetics of acarbose, Part II: Distribution to and elimination from tissues and organs following single or repeated administration of [14C] acarbose to rats, dogs and man. Arzneimittelforschung. 1989;39:1261–1267. [PubMed] [Google Scholar]
- 102.Coniff RF, Krol A. Acarbose: a review of US clinical experience. Clin Ther. 1997;19:16–26. doi: 10.1016/s0149-2918(97)80069-0. [DOI] [PubMed] [Google Scholar]
- 103.Lebovitz HE. Alpha-glucosidase-inhibitors as agents in the treatment of type 2 diabetes. Diabetes Reviews. 1998;6:132–145. [Google Scholar]
- 104.Haupt E, Hillebrand I, Pfeiffer H. Effectiveness and tolerability of the alpha-glucosidase-inhibitor acarbose in NIDDM patients with elevated liver enzyme activity. In: Creutzfeldt W, editor. Acarbose for the Treatment of Diabetes Mellitus. Berlin, Germany: Springer Verlag; 1988. [Google Scholar]
- 105.Zillikens MC, Swart GR, van den Berg JWO, Wilson JHP. Effects of the glucosidase-inhibitor acarbose in patients with liver cirrhosis. Aliment Pharmacol Ther. 1989;3:453–459. doi: 10.1111/j.1365-2036.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 106.Kihara Y, Ogami Y, Tabaru A, Unoki H, Otsuki M. Safe and effective treatment of diabetes mellitus associated with chronic liver diseases with an alpha-glucosidase-inhibitor, acarbose. Gastroenterology. 1997;6:777–782. doi: 10.1007/BF02936954. [DOI] [PubMed] [Google Scholar]
- 107.Rao AD, Reynolds K, Kuhadiya N, Fonsera VA. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality? A meta-analysis. Diabetes Care. 2008;31:1672–1678. doi: 10.2337/dc08-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosak C, Nitzsche G, Konig P, Hofmann U. The effect of timing and the administration of acarbose on postprandial hyperglycemia. Diab Med. 1995;12:979–984. doi: 10.1111/j.1464-5491.1995.tb00409.x. [DOI] [PubMed] [Google Scholar]