Abstract
The regulation of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity by 2-carboxyarabinitol 1-phosphate (CA1P) was investigated using gas-exchange analysis of antisense tobacco (Nicotiana tabacum) plants containing reduced levels of Rubisco activase. When an increase in light flux from darkness to 1200 μmol quanta m−2 s−1 was followed, the slow increase in CO2 assimilation by antisense leaves contained two phases: one represented the activation of the noncarbamylated form of Rubisco, which was described previously, and the other represented the activation of the CA1P-inhibited form of Rubisco. We present evidence supporting this conclusion, including the observation that this second phase, like CA1P, is only present following darkness or very low light flux. In addition, the second phase of CO2 assimilation was correlated with leaf CA1P content. When this novel phase was resolved from the CO2 assimilation trace, most of it was found to have kinetics similar to the activation of the noncarbamylated form of Rubisco. Additionally, kinetics of the novel phase indicated that the activation of the CA1P-inhibited form of Rubisco proceeds faster than the degradation of CA1P by CA1P phosphatase. These results may be significant with respect to current models of the regulation of Rubisco activity by Rubisco activase.
The proportion of active Rubisco, the enzyme responsible for CO2 fixation in photosynthetic cells, is modulated in response to changes in incident PPFD in parallel with changes in flux through photosynthesis. Cells exposed to high irradiance will have more Rubisco in the activated form than cells exposed to a lower irradiance. The regulation of Rubisco activity involves the reversible binding of CO2 and Mg2+ to the active site (Lorimer and Miziorko, 1980). In this carbamylated state the site is catalytically active; when it is not carbamylated the site is inactive. In the carbamylated state the active site can bind the substrate RuBP and catalyze either carboxylation or oxygenation. The noncarbamylated active site can also bind RuBP. However, this noncatalytic binding of RuBP, which is relatively tight because of the absence of catalytic release pathways, prevents access of other compounds to the active site, precluding carbamylation and maintaining the enzyme in an inactive form (Jordan and Chollet, 1983). Some plants have an additional mechanism for regulating Rubisco activity in response to light that does not involve carbamylation-decarbamylation. In these plants the inhibitor CA1P binds to the carbamylated active site, preventing RuBP binding and subsequent catalysis (Gutteridge et al., 1986; Berry et al., 1987; Moore and Seemann, 1994).
CA1P is present in darkened leaves of numerous species including tobacco (Nicotiana tabacum; Servaites et al., 1986), bean (Berry et al., 1987), potato (Gutteridge et al., 1986), and beet (Moore et al., 1991). In most species that contain CA1P, it accumulates in darkened leaves to concentrations approaching that of Rubisco active sites, but little if any is present in irradiated leaves. Because of its high affinity for the carbamylated active site (Kd = 32 nm; Berry et al., 1987), the presence of equimolar amounts of CA1P to active sites would almost completely inhibit Rubisco in leaves.
The stromal protein Rubisco activase activates both inactive forms of Rubisco, the noncarbamylated, RuBP-ligated form and the CA1P-inhibited form, by forcing the dissociation of the inactivating ligand (Robinson and Portis, 1988; Portis, 1992). Rubisco activase is required to maintain high Rubisco activity levels in leaves grown at ambient CO2 concentrations. Arabidopsis (Portis, 1992) and tobacco mutants (Mate et al., 1993, 1996) with undetectable or low amounts of activase can survive only when grown at elevated CO2 concentrations. Activase activity is also important in determining the rate of Rubisco activation following an increase in light flux. In experiments in which antisense tobacco plants contained reduced activase levels, the rate at which the noncarbamylated form of Rubisco was activated following an increase in light flux was proportional to the activase content (Hammond et al., 1998).
Although activase catalysis releases CA1P from the active site of Rubisco, it does not convert CA1P into an uninhibitory form. This is thought to be the role of specific phosphatases that have been isolated from the chloroplasts of tobacco (Salvucci et al., 1988) and bean (Moore et al., 1995). These enzymes hydrolyze CA1P to 2-carboxyarabinitol and Pi, neither of which are strong inhibitors of Rubisco. They do not, however, dephosphorylate the CA1P bound to Rubisco sites (Salvucci et al., 1988). Thus, phosphatases can affect activation only by influencing the amount of free CA1P. The activities of phosphatases are affected by a range of metabolites. Generally, those at relatively high concentrations in illuminated leaves activate CA1P phosphatases, whereas Pi, which would be at a higher concentration in darkness, is inhibitory (Gutteridge and Julien, 1989; Holbrook et al., 1991; Kingston-Smith et al., 1992; Charlet et al., 1997). The nature of the interactions between CA1P phosphatase and metabolites indicates a role for this enzyme in the light regulation of Rubisco activity through its effect on stromal CA1P concentration.
To date, most studies of the regulation of Rubisco by CA1P have focused on biochemical measurements of leaf CA1P content and Rubisco activity under different light conditions. Additionally, there has been considerable progress made in elucidating the CA1P biosynthetic and degradative pathways (Andralojc et al., 1994, 1996; Martindale et al., 1997). Here we describe an investigation of the regulation of Rubisco activity by CA1P in intact leaves, in which primarily a gas-exchange technique was used to analyze the kinetics of Rubisco activation (Woodrow and Mott, 1989; Mott and Woodrow, 1993). Using antisense tobacco plants containing reduced levels of Rubisco activase, we were able to discern a phase in the activation of Rubisco that represents the activation of the CA1P-inhibited form of Rubisco.
MATERIALS AND METHODS
Plant Material
Two genotypes of tobacco (Nicotiana tabacum L. cv Wisconsin 38) were used in these experiments: wild type and a type transformed with antisense DNA targeted at the Rubisco activase protein (Mate et al., 1993). The transgenic plants contained T-DNA from pαTACT having antisense genes containing the 3′ two-thirds of the Rca mRNA. The R1 progeny of the primary transformant A52, which had two T-DNA inserts (Mate et al., 1993), was used. Typically, the leaves of antisense plants had Rubisco activase levels approximately 10% to 20% of those of the wild type. The plants were grown from seed in potting medium consisting of 50% vermiculite, 25% sand, and 25% pine bark in a controlled-environment growth cabinet. They were supplied with one-quarter-strength Hoagland solution every 2 d and exposed to a PPFD of 350 μmol m−2 s−1 with a photoperiod of 12 h. Photoperiod and darkness air temperatures were 25°C and 20°C, respectively.
Gas Exchange
Gas-exchange measurements with a single-pass system similar to the one described by Mott (1988) were made to determine leaf photosynthetic and respiratory rates. A differential IR analyzer (model 225 Mark 3, Analytical Development Company, Hertfordshire, UK) was used to measure the CO2 concentration, and a dew-point hygrometer (Dew-10, General Eastern Instruments, Watertown, MA) was used to measure water vapor concentration. A type t thermocouple was used to measure leaf temperature. The upper surface of the leaf was illuminated using a 400-W metal halide lamp. The net CO2 assimilation rate (A), the stomatal conductance (gs), and the leaf intercellular CO2 concentration (ci) were calculated using the equations of von Caemmerer and Farquhar (1981). Before experiments were initiated the leaves were exposed to a PPFD of 1200 μmol m−2 s−1 for 60 min.
Typically, the rate of CO2 assimilation by leaves was measured continually following an increase in PPFD from darkness or 105 to 1200 μmol m−2 s−1. Before the PPFD was increased, the leaves were exposed to darkness or to the low light intensity for various periods ranging from 10 to 120 min. The RH of the air being supplied to the chamber was 70% during measurements. However, this was increased to 90% during the period of reduced illumination to reduce the decline in gs. Gas-exchange data were recorded at 5-s intervals until A had reached a steady state. During the gas-exchange measurements, leaf temperature was 25°C. A was normalized to a ci of 250 μL L−1 unless otherwise stated, to compensate for changes in the rate of assimilation resulting from changes in ci. Normalization assumes that the relationship between A and ci is linear and passes through the CO2-compensation point (Woodrow and Mott, 1989).
CA1P Analysis
Leaf discs (1.43 cm2) were excised from leaves exposed to various light environments and then rapidly frozen in liquid N2 within 2 s of excision. The CA1P content was then determined using a method similar to that of Moore et al. (1991). The frozen leaf tissue was ground to a fine powder with a mortar and pestle in liquid N2 and extracted rapidly with 300 μL of 0.46 n HClO4. This mixture was centrifuged for 3 min at 13,800g before a 150-μL aliquot of the supernatant was taken and neutralized with 47.5 μL of a solution containing 1.67 n KOH and 0.133 m Hepes. The neutralized extract was incubated at 4°C for 30 min to allow maximal precipitation of KClO4, and the solution was further clarified by centrifugation for 1 min at 13,800g.
The CA1P concentration in the extracts was determined by measuring its inhibition of purified and carbamylated Rubisco. Rubisco was purified from spinach according to the procedure of Edmondson et al. (1990) and was activated for 1 h at 4°C in a buffer comprising 200 mm Bicine-KOH, pH 8.2, 30 mm NaHCO3, 40 mm MgCl2, 5 mm DTT, and 32 μm Rubisco active sites. An equal volume of the CA1P extract was added to the activated Rubisco, making the final concentration 100 mm Bicine-KOH, pH 8.2, 15 mm NaHCO3, 20 mm MgCl2, 2.5 mm DTT, and 16 μm Rubisco active sites. The activated Rubisco was incubated with the CA1P extract for 20 min at 25°C before being added to an assay with a final volume of 0.5 mL consisting of 50 mm Bicine-KOH, pH 8.2, 15 mm NaH14CO3 (specific activity 3.7 GBq mol−1), 20 mm MgCl2, 0.5 mm RuBP, and 5 mm DTT. The assay was stopped after 30 s by adding 0.5 mL of 2 n HCl and evaporated to dryness with heating. Acid-stable 14C fixed by Rubisco was determined using liquid-scintillation counting. Inhibition of Rubisco by CA1P was quantified by comparing with assays containing identical amounts of Rubisco but no CA1P. It was assumed that each inactive Rubisco site was bound with one molecule of CA1P and that all CA1P was bound to Rubisco sites (i.e. negative cooperativity was negligible), because there was always at least a 3-fold excess of Rubisco sites compared with the CA1P concentration.
RESULTS
Rubisco Activation in the Wild Type
When a wild-type tobacco leaf was exposed to a low light flux (105 μmol m−2 s−1) for 30 min and then to a sudden increase in PPFD to 1200 μmol m−2 s−1, there were two kinetically distinct phases during the subsequent increase in A. The first was a fast phase, presumably representing rapid RuBP production (Woodrow and Mott, 1992), and the second was a slower, exponential phase, representing the production of active Rubisco from inactive forms (Fig. 1A; Woodrow and Mott, 1992). The kinetics of this second Rubisco phase have been used in several studies to examine the activation of Rubisco in leaves of spinach, wild-type tobacco, and anti-activase tobacco (Woodrow and Mott, 1989, 1992; Mott and Woodrow, 1993; Hammond et al., 1998). The same kinetic analysis was used here. We first plotted the ln of the difference between the final A (Af) and A to confirm that this second phase was linear. It showed exponential kinetics from about 1.2 min on (Fig. 1B). We then used nonlinear regression analysis to fit a curve to the data points in this Rubisco phase. The equation of the curve is:
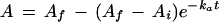 |
1 |
where Ai is the initial extrapolated assimilation rate at time 0 (when the PPFD was increased), ka is the apparent rate constant for the phase, and t is time after the increase in PPFD. The Af, Ai, and ka values for the experiment in Figure 1A were 20.45 μmol m−2 s−1, 11.35 μmol m−2 s−1, and 0.503 min−1, respectively. The initial velocity of Rubisco activation (vi) was calculated by first differentiating Equation 1, which yields the following equation:
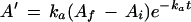 |
2 |
where A′ is the rate of increase in A. At time 0 this equation becomes
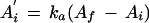 |
3 |
where A′i is the initial rate of increase in A. Using the rate equations and the kinetic constants for carboxylation and oxygenation by Rubisco (von Caemmerer et al., 1994), we converted A′i into vi and Af− Ai into the amount of Rubisco activated during the light-intensity transient. In calculating vi, we assumed that the RuBP concentration was saturating for Rubisco activity and that respiration did not change (Woodrow and Mott, 1989). For the experiment in Figure 1A, vi was 76.03 nmol active sites m−2 s−1. We also calculated the proportion of Rubisco active sites that were inactive before the PPFD was increased (Pr):
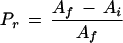 |
4 |
In the example shown in Figure 1A, the value of Pr was 0.45.
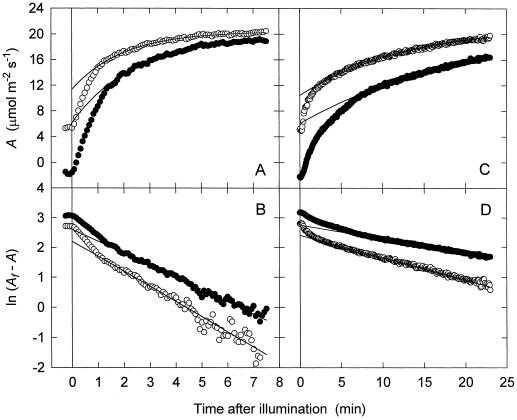
Figure 1.
A comparison of the increase in A upon illumination of a wild-type (A and B) and an antisense tobacco leaf (C and D) following exposure to either darkness or low light flux. The leaf was either illuminated at a PPFD of 105 μmol m−2 s−1 for 30 min (○) or darkened for 60 min (•) before the light flux was increased to 1200 μmol m−2 s−1 at time 0. A, A plotted over time. B, The ln of the difference between the final assimilation rate (Af) and the measured A plotted over time. The linear portions of the logged data are exponential and exponential curves were fitted to these portions (solid lines in A and B). C, Same as A except for an antisense plant. D, Same as B except for an antisense plant.
Another experiment was done with the same wild-type leaf that was darkened for 60 min before being exposed to a PPFD of 1200 μmol m−2 s−1. The kinetics of Rubisco activation were also exponential, but the rate constant was different (Fig. 1, A and B). Analysis of the exponential Rubisco phase revealed that both the Pr (0.66) and the vi (91.94 nmol sites m−2 s−1) were higher when the initial condition was darkness instead of low PPFD.
Novel Phase of Rubisco Activation in Anti-Activase Tobacco
When similar experiments were done using an anti-activase plant, the kinetics of the increase in CO2 assimilation from low PPFD were slower but still consisted of the two distinct phases present in the wild type (Fig. 1C). The assimilation kinetics from darkness, however, showed a significant qualitative difference from those of the same leaf when the initial PPFD was 105 μmol quanta m−2 s−1 and from those of the wild type (Fig. 1C). Instead of one slow phase (the Rubisco phase) after the initial fast phase, there were two. The first of these slower phases did not persist beyond 10 min after the increase in PPFD. The second slower phase dominated the time course from about 10 min after the increase in PPFD until a steady state was approached. This phase showed exponential kinetics typical of the Rubisco phase, as indicated by the linearity of a semilogarithmic plot of this portion of the data (Fig. 1D). The curve through the solid points in Figure 1, C and D, is an exponential function (Eq. 1) fitted to the linear portion of the log plot after the novel phase had ceased. From these curve analyses, the Af values were found to be similar for the two antisense experiments: from darkness (21.79 μmol m−2 s−1) and from low PPFD (21.58 μmol m−2 s−1). The Pr value, however, was higher following 60 min of darkness (0.72) than following 30 min of low PPFD (0.51). The vi from darkness (12.26 nmol sites m−2 s−1) was not appreciably different from that from low PPFD (13.75 nmol sites m−2 s−1).
To analyze the kinetics of the novel phase we subtracted the contribution of the Rubisco phase from the overall assimilation time course according to the following equation:
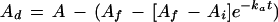 |
5 |
Therefore, the difference between the two curves (Ad) approaches 0 as t approaches infinity (i.e. steady state). The change in Ad over time was then used to describe the kinetics of the novel phase, which is clearly distinguishable from the fast RuBP phase in the antisense plants (Fig. 2, A and B). The latter phase was complete after approximately 2 min. In contrast to the antisense plant, no additional phase could be distinguished in the plot of Ad over time for the wild type (Fig. 2B).
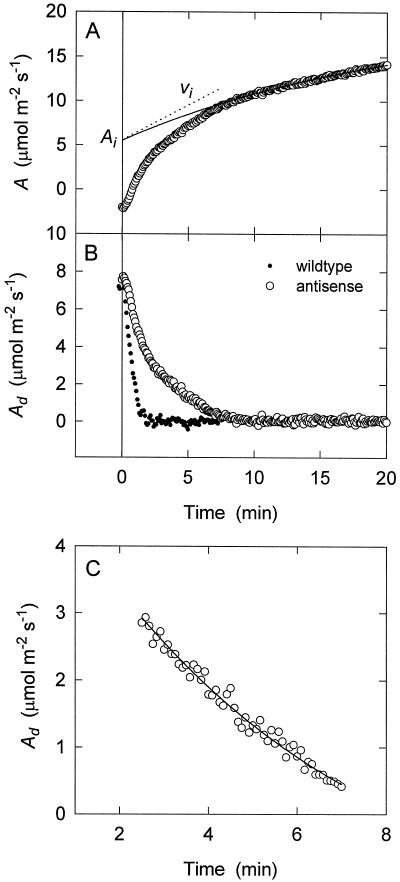
Figure 2.
Resolution of the various phases from the activation of A following illumination of a darkened antisense leaf. A, A trace (○) with an exponential curve fit to the exponential portion (solid line). The amount of Rubisco sites sequestered in the E*R form (Ai) and the vi were calculated from extrapolation of this exponential curve to time 0. B, Difference between the exponential fit and the measured A (Ad) plotted over time for an antisense plant (○) and a wild-type plant (•). C, Portion of the antisense data after the rapid RuBP-limiting phase and before the later exponential Rubisco phase (when Ad equals 0; see Results) plotted over time. To these data another exponential curve of the same form as that used to characterize the Rubisco phase was fitted (line).
To quantify the contribution of the novel phase to the overall increase in photosynthetic rate, we needed to extrapolate it to 0 time as we had done for the Rubisco phase. Unlike the latter phase, however, the kinetics of the novel phase are apparently more complex. After completion of the RuBP phase (at about 2 min), the velocity (i.e. the rate of change in Ad) decreases gradually with time, and then toward the end, it decreases relatively rapidly to 0 (Fig. 2B). Accordingly, we could not fit the entire novel phase adequately to an exponential function decaying to 0 at infinite time. However, by eliminating the last part of the curve (no more than 10% of the total change in Ad during the phase) and the initial RuBP phase (the first 2.5 min), we could model the kinetics of Ad extremely accurately using an exponential function (Eq. 2). In each case the exponential function was more highly correlated with the data than the linear function. Also, removal of more data at either the beginning or end of the time course had little if any effect on the shape of the fitted exponential curve. An example of the curve fitting is shown in Figure 2C. In this case, the time course is clearly curved, and in accordance with this, the exponential equation was more correlated with the data than the linear function.
We then used this exponential function to extrapolate Ad to 0 time. This extrapolated value (Aid) was used with the kinetic constants for Rubisco (see above) to calculate both the number and proportion (Pc) of Rubisco sites inactivated by CA1P (i.e. that which accounts for the novel phase) before the PPFD was increased. The latter is given by the following equation:
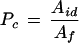 |
6 |
For the example plotted in Figure 2, the number of Rubisco sites in this form was determined to be 4.06 μmol m−2, compared with 10.63 μmol m−2 in the noncarbamylated form. The contribution of Pc was 0.28, whereas the value of Pr was 0.72. This means that the novel phase accounted for 28% of the total increase in Rubisco activity following the increase in PPFD. Differentiation of the exponential equation fitted to this phase allowed us to determine the initial velocity of Rubisco activation during this phase:
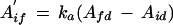 |
7 |
where A′if is the initial rate of increase of this resolved phase and Afd is the final Ad value determined by the exponential curve-fitting process. This value was negative and had no mechanistic significance. Again, this initial rate of assimilation increase (A′if) was converted into the initial rate of Rubisco site activation (vid) using the kinetic constants for Rubisco. The initial velocity of Rubisco activation in the Rubisco phase (vi) was 12.3 nmol sites m−2 s−1, whereas vid was 15.8 nmol sites m−2 s−1.
Correlation of CA1P Content with the Magnitude of the Novel Gas-Exchange Phase
The next experiments involved testing the hypothesis that the novel phase detectable in the antisense plants reflects removal of CA1P from ECM. We raised this hypothesis because it is known that CA1P regulates Rubisco activity to a degree in darkened tobacco leaves (Servaites et al., 1986; Moore et al., 1991). By varying the dark period before increasing the PPFD, we were able to vary the magnitude of the novel phase (Afd − Aid) and correlate this with leaf CA1P content. We found that the number of Rubisco sites sequestered in the form responsible for the novel phase (the dark-originating form of inactive Rubisco) indeed correlated linearly with the amount of CA1P extracted from the same leaf after being exposed to the same period of darkness (Fig. 3A). The biochemical assay for CA1P did not exclude the possibility that other naturally occurring, tight-binding inhibitors of the carbamylated Rubisco active site were contributing to the reduced in vitro Rubisco activity that we attributed to CA1P alone. When treated with alkaline phosphatase, however, the inhibitor(s) of Rubisco extracted from darkened tobacco leaves was found to dissociate with kinetics similar to those of purified CA1P (Berry et al., 1987).
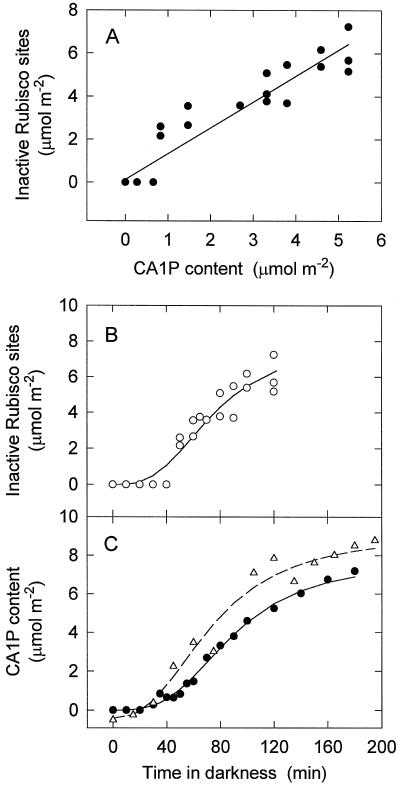
Figure 3.
A, The correlation of Rubisco sites in the inactive form responsible for the novel gas-exchange phase with leaf CA1P content (r2 = 0.85). The amount of CA1P and inactive sites was varied by altering the period for which the leaf was darkened. The data are also plotted separately as a function of time in darkness. B, Inactive Rubisco. C, CA1P content (•). CA1P content for the wild-type is also plotted (▵). Inactive Rubisco sites were quantified from gas-exchange measurements made on several leaves of one plant. Leaf CA1P content was determined biochemically using samples taken from one of the leaves used for gas exchange.
We also used the data presented in Figure 3A to examine the relationship between the time in darkness and the magnitude of the novel phase. We found that this phase had similar sigmoidal kinetics to the CA1P determined biochemically (Fig. 3, B compared with C). The formation of CA1P in darkened leaves was also determined for wild-type tobacco using the biochemical assay, and these data show that reduction in the activase concentration had a negligible effect on the rate of CA1P formation and the final steady-state concentration of CA1P (Fig. 3C).
Relationship between CA1P Disappearance and Gas-Exchange Kinetics
Apart from correlating the appearance of the novel phase (which we will now refer to as the CA1P phase) with CA1P concentration, experiments were also conducted to study the relationship between the activation of this form of Rubisco and the metabolism of CA1P in reilluminated leaves in the non-steady state. Following 90 min of darkness, the activation of Rubisco in an anti-activase leaf exposed to a PPFD of 1200 μmol m−2 s−1 was measured using gas exchange, and the kinetics of the CA1P phase were determined. The experiment was repeated with the same leaf, except that instead of making gas-exchange measurements leaf samples were taken at various times and the CA1P content was determined. From the gas-exchange data, the initial amount of inactive Rubisco sites in the dark-originating form was 4.20 μmol m−2, which correlated with the CA1P content of the leaf (3.57 μmol m−2; Fig. 4A). During the non-steady state, however, there was less correlation between inactive Rubisco sites and the CA1P content. The leaf CA1P content was higher than the number of inactive Rubisco sites except at low levels of CA1P (Fig. 4B). There was significant deviation from the proportional relationship between CA1P and inactive Rubisco sites that would be expected if CA1P binding was very tight and if CA1P degradation following release from Rubisco was very fast (Fig. 4B).
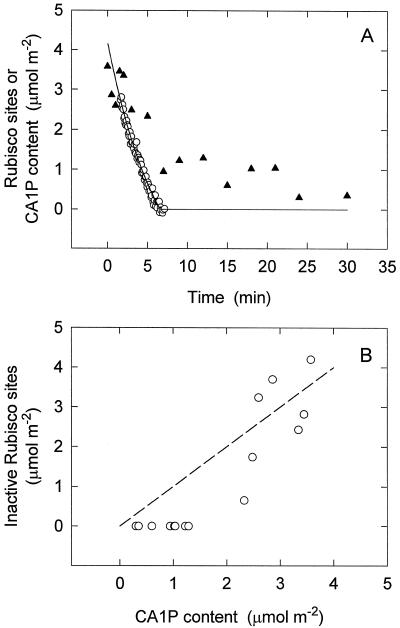
Figure 4.
A, The relationship between leaf CA1P content determined biochemically (▴) and the dark-originating form of inactive Rubisco sites (○) following an increase in PPFD from darkness to 1200 μmol m−2 s−1. B, Same data replotted to examine the correlation between leaf CA1P and inactive Rubisco. The dashed line is the relationship expected if CA1P binding to Rubisco sites is complete and CA1P degradation following release is very fast, i.e. a proportional relationship. The amount of CA1P in an antisense leaf predarkened for 90 min was determined for samples taken at various periods after increasing the PPFD to 1200 μmol m−2 s−1. The activation kinetics of the dark-originating form of inactive Rubisco was previously determined for the same leaf following 90 min of darkness.
DISCUSSION
The data presented here indicate that light-mediated Rubisco activation in previously darkened tobacco leaves can be substantially limited by two processes: the carbamylation of the inactive enzyme and removal of CA1P from the active enzyme. By slowing down the rate of Rubisco activation using anti-activase plants, we were able to resolve the kinetics of these two processes. Carbamylation proceeds exponentially, as has been described previously (Woodrow and Mott, 1992; Woodrow et al., 1996; Hammond et al., 1998), but the kinetics of CA1P removal are more complex. For much of the time the velocity (i.e. the rate of change of Ad) decreased relatively slowly, but at very low concentrations of the active enzyme-CA1P complex it declined rapidly to 0.
There is considerable evidence that the novel phase in the assimilation time course reflects the removal of CA1P from the carbamylated form of Rubisco. Like CA1P, the form of inactive Rubisco responsible for this phase appeared only in leaves exposed to very low light fluxes. The amount of extractable CA1P determined biochemically for anti-activase leaves that had been held in darkness for varying periods correlated with the amount of Rubisco in the form responsible for the novel phase (Fig. 3A). In view of the relatively high affinity of active Rubisco for CA1P (Kd = 32 nm; Berry et al., 1987), it is likely that almost all of the CA1P extracted was initially complexed with Rubisco. Thus, our data show a direct correlation between the concentration of the CA1P-inhibited form of Rubisco and the magnitude of the CA1P phase over a considerable range of CA1P concentrations. Moreover, the appearance of the inactive form of Rubisco when a leaf was removed to darkness followed kinetics similar to the those of CA1P determined biochemically (Fig. 3, B and C). It is noteworthy that these kinetics are consistent with published rates of CA1P synthesis in bean after darkening (Sage et al., 1993; Andralojc et al., 1994, 1996).
We did not expect a tight correlation between the total CA1P content and the calculated amount of CA1P-inhibited Rubisco at any point during the CA1P phase. We expected that a significant pool of free CA1P would accumulate in the stroma under these non-steady-state conditions until metabolized by CA1P phosphatase, and this was observed (Fig. 4). This relationship between total CA1P content and the amount of CA1P-inhibited Rubisco during these conditions is consistent with the small amount and low specific activity of CA1P phosphatase in tobacco (Gutteridge and Julien, 1989; Salvucci and Holbrook, 1989; Holbrook et al., 1991). The lag in degradation of CA1P following its removal from Rubisco sites is significant because it indicates that the activation of the CA1P-inactivated form of Rubisco is controlled by activase and not by CA1P phosphatase.
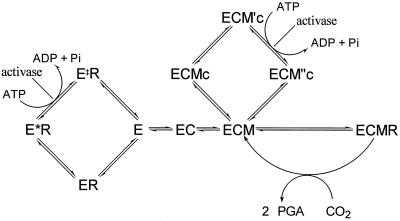
The activity of purified CA1P phosphatase is regulated by metabolites, including NADPH, RuBP, and Pi, in a way that would promote its activity in illuminated leaves and inhibit it in darkened leaves (Salvucci et al., 1988; Holbrook et al., 1991; Kingston-Smith et al., 1992). Despite this apparent regulation, which suggests a role for CA1P phosphatase in the regulation of Rubisco activity, our data indicate that the activity of this enzyme during the non-steady state would not be sufficient to affect the equilibrium between ECMc and ECM or between ECM“c and ECM by degrading free CA1P (see Fig. 5 for a description of these forms of Rubisco). The significant concentration of free CA1P present in the stroma during activation would not favor CA1P dissociation from Rubisco. Instead, it is likely that activase favors CA1P release through its catalysis of ECMc to ECM′c and the effect of this on the equilibrium between ECM′c and ECM“s (Fig. 5).
Figure 5.
The pathways involved in the regulation of Rubisco activity. Rubisco active sites (E) can bind sequentially an activator CO2 (C) and a Mg2+ ion (M) to form the active ternary complex ECM. This form can then bind substrate RuBP (R) and catalyze either carboxylation or oxygenation. Rubisco sites may be inactivated by either of two mechanisms: the binding of R to the noncarbamylated E and the binding of CA1P (c) to ECM. The E form binds R in two stages: an initial loose association occurs (ER), followed by a slow conformational change to produce the higher affinity form E*R. The binding of c to ECM also probably takes two steps, initially forming ECMc and finally forming ECM′c. Activase interacts with forms of Rubisco associated with sugar phosphates (such as RuBP and CA1P) in a manner that reduces the affinity of Rubisco for the ligand. ECM′c is converted into ECM“c, which readily dissociates to produce ECM, and E*R is converted into E‡R, which readily dissociates to produce E. There is no evidence that either E‡R is different from ER or ECM“c is different from ECMc. We differentiate between them because they arise from different processes and, therefore, may be different. The products of activase catalysis (E‡R and ECM“c) are not necessarily homologous. PGA, Phosphoglyceraldehyde.
We did not biochemically characterize the processes responsible for the Rubisco phase because this has been done previously (Hammond et al., 1998). What we have shown for tobacco is that the exponential Rubisco phase represents the activation of the noncarbamylated form of Rubisco chelated with RuBP (Fig. 5). This is most likely a sequential process in which activase first facilitates the dissociation of RuBP, allowing carbamylation of the site to then complete its activation (ECM). This process has been shown to be limited by activase activity when activation occurs from low PPFDs (Hammond et al., 1998). Hammond et al. (1998) also suggested that the production of active Rubisco is exponential because activase activity is down-regulated as activation proceeds. The prime candidates for such regulation are ATP and ADP. Activase activity is regulated by ATP positively and by ADP negatively (Robinson and Portis, 1989; Portis, 1992), enabling activase catalysis to be modulated downward as Rubisco activity and the Calvin cycle flux increases. If the exponential decline in Rubisco activation rate indeed reflects a proportional reduction in activase activity, then this change should also affect the kinetics of CA1P removal, assuming that activase is largely responsible for CA1P removal from active Rubisco (Robinson and Portis, 1988).
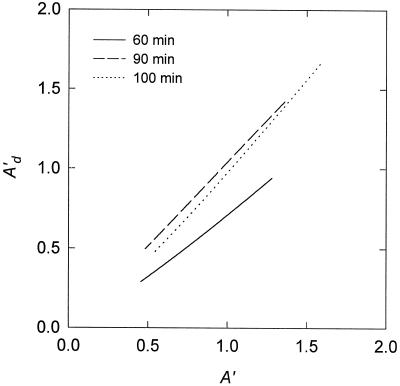
To test this hypothesis we compared A′ with the rate of change in A during the CA1P phase (i.e. the absolute slope of the Ad versus the time plot; Fig. 6). According to our hypotheses regarding the mechanisms underlying these two phases, this is equivalent to comparing the velocity of CA1P removal from ECM with the velocity of RuBP removal from the Rubisco active site during most of the CA1P phase. Although there was not a large change in either velocity for the three examples shown in Figure 6, we found that they were very highly correlated. Such a relationship is consistent with a model of Rubisco activation by activase involving simultaneous catalysis of the removal of RuBP (from the Rubisco active site) and CA1P (from ECM, Fig. 5) and progressive down-regulation of activase activity as A increases.
Figure 6.
Three examples of the relationship between the rate of increase in A (A′), as determined by the exponential fit to the Rubisco phase, and the rate of increase in Ad (A′d), as determined by the exponential fit to the CA1P phase. The different examples represent separate gas-exchange experiments in which antisense tobacco plants were darkened for different periods before increasing the PPFD (the times are indicated on the figure). The solid line indicates data transformed from the experiment shown in Figure 2.
The hypothesis that activase is subject to flux-related feedback inhibition can also be tested by measuring vi during the Rubisco phase. Because there was less active Rubisco after sustained darkness than after low PPFD in our experiments, vi should be faster from darkness than from low PPFD, assuming that factors (other than Rubisco activity) affecting the feedback mechanism are constant. This prediction also applies to wild-type tobacco. In the experiment in which darkness was used, the Pr was 0.66 and the vid was 91.94 nmol sites m−2 s−1. In the experiment in which low PPFD was used, the Pr was 0.45 and the vi was 76.03 nmol sites m−2 s−1 (Fig. 1A). The antisense plants, however, had a slightly lower rate of activation from darkness than low PPFD, despite there being a larger amount of inactive Rubisco after darkening. This discrepancy could reflect the difficulty of accurately measuring the initial rate of activation when an extrapolation of 7 min is required, or it could reflect a more complex regulatory mechanism than the one proposed.
The CA1P phase was not visible in the wild type during activation from darkness, which is not surprising in view of the speed of the process, even though CA1P was present in wild-type leaves at concentrations similar to those in the antisense plants when darkened for the same period (Fig. 3C). The reason for the absence of this phase is that the activation of the CA1P-inhibited form of Rubisco is complete before CO2 assimilation becomes limited by Rubisco in the wild-type plants. The antisense plants, which typically had approximately 10% to 20% of the activase of the wild type, activated the putative CA1P-inhibited form in about 7 to 8 min. If we assume that the rate of activation of this form is dependent on activase concentration (like the RuBP-inhibited form; Hammond et al., 1998), then it would take approximately 1 to 1.5 min for complete activation of the CA1P-inhibited form in the wild type. This is short enough for the activation of this form to be obscured by the non-Rubisco-limiting portion of the CO2-assimilation trace (i.e. the fast phase; Fig. 1A).
The kinetics of CA1P synthesis were unaffected by the reduced amounts of activase in the antisense plants compared with the wild type (Fig. 3C). This is compatible with the finding that reduced levels of activase have a negligible effect on the kinetics of formation of the other inactive form of Rubisco (E*R) under low PPFD (Hammond et al., 1998). If activase catalysis during darkness was significant, then the reduced levels of activase in the antisense plants may have affected CA1P formation. The lack of an effect indicates that activase does not interact significantly with Rubisco during darkness.
The regulation of Rubisco activity by CA1P has been enigmatic. The rate at which the form of Rubisco inhibited by CA1P is activated and the factors limiting this process have remained undefined. We were able to measure relatively accurately the kinetics of activation of a form of Rubisco in tobacco leaves that is probably inhibited by CA1P. When we compared these kinetics with the rate of CA1P degradation, it became apparent that significant concentrations of CA1P accumulate in the stroma during Rubisco activation. Such an accumulation of CA1P indicates that control of activation of the CA1P-inhibited form of Rubisco lies more with activase than with CA1P phosphatase. The rate of activation of the CA1P-inhibited form of inactive Rubisco, like the noncarbamylated form, appeared to be controlled by activase activity, which was down-regulated as the flux increased. This latter suggestion is supported by the finding that activase activity was regulated during the activation of the ECM′c form of Rubisco in a manner similar to the activation of the E*R form.
Abbreviations:
- CA1P
2-carboxyarabinitol 1-phosphate
- ECM
active ternary complex formed when Rubisco active sites (E) bind sequentially an activator CO2 (C) and a Mg2+ ion (M)
- RuBP
ribulose 1,5-bisphosphate
Footnotes
This work was supported by grants from the Australian Research Council and the University of Melbourne. E.T.H. received an Australian Postgraduate Award from the Australian Research Council and a Collaborative Research Scholarship from the Australian National University. I.E.W. was supported by a Senior Research Fellowship from the Australian Research Council.
LITERATURE CITED
- Andralojc PJ, Dawson GW, Parry MAJ, Keys AJ. Incorporation of carbon from photosynthetic products into 2-carboxyarabintol-1-phosphate and 2-carboxyarabinitol. Biochem J. 1994;304:781–786. doi: 10.1042/bj3040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andralojc PJ, Keys AJ, Martindale W, Dawson GW, Parry MAJ. Conversion of d-hamamelose into 2-carboxy-d-arabinitol and 2-carboxy-d-arabinitol 1-phosphate in leaves of Phaseolus vulgaris L. J Biol Chem. 1996;271:26803–26809. doi: 10.1074/jbc.271.43.26803. [DOI] [PubMed] [Google Scholar]
- Berry JA, Lorimer GH, Pierce J, Seemann JR, Meek J, Freas S. Isolation, identification, and synthesis of 2-carboxyarabinitol 1-phosphate, a diurnal regulator of ribulose-bisphosphate carboxylase activity. Proc Natl Acad Sci USA. 1987;84:734–738. doi: 10.1073/pnas.84.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet T, Moore Bd, Seemann JR. Carboxyarabinitol 1-phosphate phosphatase from leaves of Phaseolus vulgaris and other species. Plant Cell Physiol. 1997;38:511–517. [Google Scholar]
- Edmondson DL, Badger MR, Andrews TJ. A kinetic characterization of slow inactivation of ribulose bisphosphate carboxylase during catalysis. Plant Physiol. 1990;93:1376–1382. doi: 10.1104/pp.93.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S, Julien B. A phosphatase from chloroplast stroma of Nicotiana tabacum hydrolyses 2′-carboxyarabinitol 1-phosphate, the nocturnal inhibitor of Rubisco to 2′-carboxyarabinitol. FEBS Lett. 1989;254:225–230. [Google Scholar]
- Gutteridge S, Parry MAJ, Burton S, Keys AJ, Mudd A, Feeney J, Servaites JC, Pierce J. A nocturnal inhibitor of carboxylation in leaves. Nature. 1986;324:274–276. [Google Scholar]
- Hammond ET, Andrews TJ, Mott KA, Woodrow IE. Regulation of Rubisco activation in antisense plants of tobacco containing reduced levels of Rubisco activase. Plant J. 1998;14:101–110. doi: 10.1046/j.1365-313X.1998.00103.x. [DOI] [PubMed] [Google Scholar]
- Holbrook GP, Galasinski SC, Salvucci ME. Regulation of 2-carboxyarabinitol 1-phosphatase. Plant Physiol. 1991;97:894–899. doi: 10.1104/pp.97.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan DB, Chollet R. Inhibition of ribulose bisphosphate carboxylase by substrate ribulose 1,5-bisphosphate. J Biol Chem. 1983;258:13752–13758. [PubMed] [Google Scholar]
- Kingston-Smith AK, Major I, Parry MAJ, Keys AJ. Purification and properties of a phosphatase in French bean (Phaseolus vulgaris L.) leaves that hydrolyses 2′-carboxy-d-arabinitol 1-phosphate. Biochem J. 1992;287:821–825. doi: 10.1042/bj2870821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G, Miziorko H. Carbamate formation on the ε-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+ Biochemistry. 1980;19:5321–5328. doi: 10.1021/bi00564a027. [DOI] [PubMed] [Google Scholar]
- Martindale W, Parry MAJ, Andralojc PJ, Keys AJ. Synthesis of 2′-carboxy-d-arabinitol-1-phosphate in French bean (Phaseolus vulgaris L.): a search for precursors. J Exp Bot. 1997;48:9–14. [Google Scholar]
- Mate CJ, Hudson GS, von Caemmerer S, Evans JR, Andrews TJ. Reduction of ribulose bisphosphate carboxylase activase levels in tobacco (Nicotiana tabacum) by antisense RNA reduces ribulose bisphosphate carboxylase carbamylation and impairs photosynthesis. Plant Physiol. 1993;102:1119–1128. doi: 10.1104/pp.102.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mate CJ, von Caemmerer S, Evans JR, Hudson GS, Andrews TJ. The relationship between CO2-assimilation rate, Rubisco carbamylation and Rubisco activase content in activase-deficient transgenic tobacco suggests a simple model of activase action. Planta. 1996;198:604–613. doi: 10.1007/BF00262648. [DOI] [PubMed] [Google Scholar]
- Moore Bd, Kobza J, Seemann JR. Measurement of 2-carboxyarabinitol 1-phosphate in plant leaves by isotope dilution. Plant Physiol. 1991;96:208–213. doi: 10.1104/pp.96.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Bd, Seemann JR. Evidence that 2-carboxyarabinitol 1-phosphate binds to ribulose-1,5-bisphosphate carboxylase in vivo. Plant Physiol. 1994;105:731–737. doi: 10.1104/pp.105.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Bd, Sharkey TD, Seemann JR. Intracellular localization of CA1P and CA1P phosphatase activity in leaves of Phaseolus vulgaris L. Photosynth Res. 1995;45:219–224. doi: 10.1007/BF00015562. [DOI] [PubMed] [Google Scholar]
- Mott KA. Do stomata respond to CO2 concentration other than intercellular? Plant Physiol. 1988;86:200–203. doi: 10.1104/pp.86.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA, Woodrow IE. Effects of O2 and CO2 on non-steady-state photosynthesis. Further evidence of Rubisco limitation. Plant Physiol. 1993;102:859–866. doi: 10.1104/pp.102.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis AR. Regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase activity. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:415–437. [Google Scholar]
- Robinson SP, Portis AR. Release of the nocturnal inhibitor, carboxyarabinitol-1-phosphate, from ribulose bisphosphate carboxylase/oxygenase by Rubisco activase. FEBS Lett. 1988;233:413–416. [Google Scholar]
- Robinson SP, Portis AR. Adenosine triphosphate hydrolysis by purified Rubisco activase. Arch Biochem Biophys. 1989;268:93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- Sage RF, Reid CD, Moore Bd, Seemann JR. Long-term kinetics of the light-dependent regulation of ribulose-1,5-bisphosphate carboxylase/oxygenase activity in plants with and without 2-carboxyarabinitol 1-phosphate. Planta. 1993;191:222–230. [Google Scholar]
- Salvucci ME, Holbrook GP. Purification and properties of 2-carboxy-d-arabinitol 1-phosphatase. Plant Physiol. 1989;90:679–685. doi: 10.1104/pp.90.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci ME, Holbrook GP, Anderson JC, Bowes G. NADPH-dependent metabolism of the ribulose bisphosphate carboxylase/oxygenase inhibitor 2-carboxyarabinitol 1-phosphate by a chloroplast protein. FEBS Lett. 1988;231:197–201. [Google Scholar]
- Servaites JC, Parry MAJ, Gutteridge S, Keys AJ. Species variation in the predawn inhibition of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol. 1986;82:1161–1163. doi: 10.1104/pp.82.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR, Hudson GS, Andrews TJ. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta. 1994;195:88–97. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Woodrow IE, Kelly ME, Mott KA. Limitation of the rate of ribulosebisphosphate carboxylase activation by carbamylation and ribulose bisphosphate carboxylase activase activity: development and tests of a mechanistic model. Aust J Plant Physiol. 1996;23:141–149. [Google Scholar]
- Woodrow IE, Mott KA. Rate limitation of non-steady-state photosynthesis by ribulose-1,5-bisphosphate carboxylase in spinach. Aust J Plant Physiol. 1989;16:487–500. [Google Scholar]
- Woodrow IE, Mott KA. Biphasic activation of ribulose bisphosphate carboxylase in spinach leaves as determined from non-steady-state CO2 exchange. Plant Physiol. 1992;99:298–303. doi: 10.1104/pp.99.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]