Abstract
Previous studies showed that treatment with 17-β-estradiol-3-benzoate (EB) reduces isoproterenol (ISOP) stimulated water intake by ovariectomized rats. This effect was observed 48 h after the second of two EB injections, suggesting that the attenuation is attributable to classic EB actions to alter gene expression. However, in addition to classic, slowly-occurring, genomic effects, estrogens have more rapidly-occurring effects that may be nongenomic or ‘nonclassical’ genomic effects. Thus, it is possible that the EB attenuation of water intake stimulated by ISOP is genomic, nongenomic, or both. Accordingly, we measured ISOP-induced water intake by OVX rats at different times after EB injections, using time points likely to indicate classic genomic effects (48 h or 24 h) or nonclassical genomic or nongenomic effects (90 min). We also examined EB effects on body weight, uterine weight, and plasma volume and Na+ concentration in the same animals using the same time points and EB dose. EB treatment decreased water intake stimulated by ISOP in both the 24-h and 48-h groups; however, water intake in the 90-min group was not affected by EB. Uterine weight was unchanged 90 min after EB, but was increased 24 h after the first injection of EB. In contrast, body weight decreased after EB, but not until 48 h after the second EB injection. Finally, EB did not alter plasma Na+ concentration or hematocrit, though plasma protein concentration increased transiently 24 h after EB treatment. Taken together, these findings suggest that the behavioral, morphological, and physiological effects of EB likely are attributable to slowly-occurring, classic genomic actions of estrogens. Moreover, the time course of the observed effects varied, suggesting tissue-specific differences in estrogen receptor density or subtype, or in co-activators or co-repressors that, ultimately, determine the timing and direction of EB effects.
Keywords: Thirst, Isoproterenol, Body weight, Plasma volume, Plasma Na+ concentration
1. Introduction
With increasing experimental focus on the non-reproductive effects of estrogens has come better understanding of both the range and the mechanisms of estrogenic actions. One striking example is body fluid balance, in which estrogens have been shown to affect renal, cardiovascular, and hormonal processes that subserve body fluid homeostasis [44,46,52,64]. Sodium intake and water intake, behaviors associated with body fluid balance, also are influenced by estrogens [11]. In fact, accumulating evidence supports the idea that estrogens reduce ‘volemic thirst’ — fluid intake stimulated by activation of the renin–angiotensin system. We and others have reported that, in rats, estrogens attenuate water intake stimulated by isoproterenol [19,20,30,32,61], a β-adrenergic agonist that produces a robust increase in circulating levels of angiotensin II (AngII). Importantly, the reduction of isoproterenol-induced drinking by estradiol-treated ovariectomized rats occurs despite comparable elevations in AngII [30]. Chronic treatment with estrogens increases both urine volume and urinary Na+ excretion in response to AngII [19], raising the possibility that the estrogenic attenuation of drinking stimulated by isoproterenol results from enhanced sensitivity to the osmotic dilution associated with rapid ingestion of large volumes of water. However, we recently showed that consumption of 0.15 M NaCl by ovariectomized rats in response to isoproterenol also is reduced by treatment with estradiol benzoate [25]. Therefore, CNS involvement in the effect of estrogens on drinking stimulated by AngII seems likely.
Neuronal activation in response to isoproterenol differs in specific central nuclei, depending on whether ovariectomized rats are treated with estradiol [26,31,32]. In this regard, estrogens are highly lipophilic and can easily access estrogen receptors (ERs) located throughout the CNS, particularly in areas involved in body fluid balance [2,24,45,50,51,53,54,59,60,62]. Like ERs located in the periphery, ERs within the CNS are members of the Nuclear Receptor Superfamily [12]; thus, estrogenic modulation of volemic thirst may be attributable to transcriptional changes associated with the activation of CNS ERs. Consistent with this idea, Kisley and colleagues showed that pharmacological disruption of the genomic effects of estrogens reverses the attenuation of water intake stimulated by central administration of AngII [28]. Moreover, estradiol treatment reduces AngII receptor binding [27] and AngII receptor gene expression [32] in the subfornical organ (SFO), a forebrain circumventricular organ strongly implicated in drinking elicited by AngII [17,33,38]. Estradiol treatment also decreases isoproterenol-induced neuronal activation in the SFO [32]. These observations, in conjunction with findings of ER and AngII receptor colocalization within the SFO [45], suggest that the estradiol attenuation of drinking stimulated by AngII involves decreased neuronal activation that occurs as a result of ER-mediated reduction in the expression of AngII receptors in the SFO.
The idea that ERs act primarily via classic, slowly-occurring steroid mechanisms to alter gene expression is supported by an abundance of data, and the most common ER subtypes within the CNS, ERα and ERβ, are well-known to operate via classic steroid mechanisms to regulate transcriptional activity in cells throughout the body [12]. However, the identification of other ERs in the CNS, including the g-protein coupled ER (GPER) [7], raises the possibility of actions that occur in a comparatively short time due to nongenomic effects [34,43,48], or to estrogen response element (ERE)-independent, ‘nonclassical’ genomic effects [1,3,37,39], rather than the hours to days necessary to detect changes in gene expression due to classic steroid hormone mechanisms. Although previous studies showing reduced AngII receptor expression [27,32] support the hypothesis that classic genomic effects of estrogens modulate drinking stimulated by isoproterenol, the time course of the protocol employed in these studies (two consecutive days of treatment with estradiol, with testing 48 h after the second day of treatment) does not rule out the possibility of more rapidly-occurring, ‘nonclassical’ genomic or nongenomic effects. Thus, the goal of the present study was to determine whether rapidly-occurring, nonclassical genomic or nongenomic effects of estradiol also might alter water intake stimulated by isoproterenol. To do so, we evaluated the time course of the effects, incorporating time points likely to indicate classic genomic effects (48 h) or nonclassical genomic or nongenomic effects (90 min) e.g., [1,3,34,37,39,43,48]; see also [10], as well as an intermediate time point. The second goal of these studies was to compare the time course of centrally-mediated behavioral effects of estradiol with those related to morphological and physiological effects [5,8,21,32,40]. Accordingly, in addition to evaluating estradiol effects on isoproterenol-induced water intake, we assessed the time course of estradiol effects on uterine size and body weight, as well as on plasma volume and plasma Na+ concentration.
2. Methods
2.1. Animals, ovariectomy, and estradiol treatment
Adult female Sprague–Dawley rats (Charles River) were used in these studies. Rats were 3–6 months old and weighed 250–350 g at the beginning of the experiments. Rats were individually housed in plastic cages in a temperature controlled (22±2 °C) room on a 12:12 h light–dark cycle (lights on at 7:00 am) with ad libitum access to water and Harlan rodent diet (no. 2018). Experimental protocols were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Oklahoma State University Center for Health Sciences Animal Care and Use Committee. All procedures were conducted during the light phase.
Rats were anesthetized with sodium pentobarbital (50 mg/kg body weight, i.p.; Sigma-Aldrich), bilaterally ovariectomized (OVX) using a ventral approach, and allowed 7–10 days to recover. OVX rats then were given either 17-β-estradiol-3-benzoate (EB; 10 μg in 0.1 ml sesame oil, s.c.; Fisher Scientific) or the oil vehicle (OIL; 0.1 ml, s.c.) on Day 1 and Day 2 of a 4-day protocol to approximate the pattern of estrogen fluctuations during the estrous cycle. Although this dose of EB likely produces comparatively high peaks in circulating estradiol and persistent (4–6 days) elevations in circulating estradiol levels [63], this protocol has been used effectively in other studies [15,25,28] including our previous studies of EB effects on water intake stimulated by isoproterenol [30,32]. Rats were weighed on Days 1, 2, and 4.
2.2. Water intake induced by isoproterenol
OVX rats were assigned to one of three groups in which isoproterenol (ISOP; 30 μg/kg body weight; Sigma-Aldrich) or the 0.15 M NaCl vehicle (Saline; 1.0 ml/kg body weight) was injected s.c. before a 2-h water intake test was conducted. All rats were tested in four hormone-drug conditions (OIL-Saline, EB-Saline, OIL-ISOP, and EB-ISOP), with testing conducted at weekly intervals. To control for order effects, one of two testing sequences was used: 1) week 1 — EB-ISOP, week 2 — OIL-Saline, week 3 — EB-Saline, and week 4 — OIL-ISOP; or 2) week 1 — OIL-ISOP, week 2 — EB-Saline, week 3 — OIL-Saline, and week 4 — EB-ISOP.
2.2.1. 90-min group
In one group of OVX rats (n=6), food and water were removed from the cages on Day 1 prior to EB or OIL treatment. Ninety minutes after EB or OIL treatment, rats were injected with ISOP or Saline and, 10–15 min later, were given deionized water in graduated cylinders. Water intake was recorded to the nearest 0.5 ml after 15, 30, 45, 60, and 120 min. At the end of the test, food and water were returned to the cages; rats were given EB or OIL on Day 2.
2.2.2. 24-h group
In the second group, OVX rats (n=6) were given EB or OIL on Day 1. Twenty-four hours later (i.e., on Day 2), food and water were removed from the cages, and rats were injected with ISOP or Saline. A 2-h water intake test was conducted as described, after which rats were given the second EB or OIL treatment, and food and water then were returned to the cages.
2.2.3. 48-h group
In the third group, OVX rats (n=7) were given EB or OIL on Day 1 and Day 2. Forty-eight hours after the second EB or OIL treatment (i.e., on Day 4), food and water were removed from the cages, and rats were injected with ISOP or Saline. A 2-h water intake test was conducted as described, after which food and water were returned to the cages.
Rats were weight-matched within both hormone and time groups.
2.3. Blood work and uterine weights
One week after the completion of behavioral testing, OVX rats in each of the 3 groups again were given EB or OIL on the schedules described, then deeply anesthetized with 0.5 ml of sodium pentobarbital (50 mg/ml) and decapitated to collect trunk blood and uterine tissue. Thus, uteri and trunk blood were collected on Day 1, 90 min after EB or OIL; on Day 2, 24 h after the first EB or OIL treatment; or on Day 4, 48 h after the second EB or OIL treatment. A 10-mm section of the uterus adjacent to the bifurcation was removed, stripped of fat and connective tissue, and weighed. Trunk blood was centrifuged for determination of hematocrit, plasma protein concentration (using a refractometer; Reichart), and plasma Na+ concentration (using an ion-sensitive electrode; EasyLyte).
Additional OVX rats were added to each of the three groups so that there were 5–6 rats in each condition for each of these measures. To ensure that results were not affected by repeated EB/OIL treatment weeks, these rats also were given EB and OIL in one of two sequences: 1) week 1 — EB, week 2 — OIL, week 3 — EB, and week 4 — OIL; or 2) week 1 — OIL, week 2 — EB, week 3 — OIL, and week 4 — EB prior to collection of the uterine tissue and trunk blood on week 5.
2.4. Statistics
All data are expressed as means±standard errors of the means (S.E.).
Water intake was analyzed using a 4-way repeated-measures analysis of variance (rm ANOVA; Statistica, StatSoft), with group (90 min, 24 h and 48 h), drug (ISOP and Saline), hormone treatment (EB and OIL), and time as factors, repeated for drug, hormone treatment, and time. Pair-wise comparisons of significant (p<0.05) main effects or interactions were conducted using Student–Newman–Keuls tests; specific planned comparisons were made using Bonferroni corrections.
Body weight at the beginning of behavioral testing, hematocrit, plasma protein concentration, plasma Na+ concentration, and uterine weight were analyzed using 2-way ANOVAs, with groups (90 min, 24 h, and 48 h) and hormone treatments (EB and OIL) as factors. Pairwise comparisons of significant (p<0.05) main effects or interactions were conducted using Student–Newman–Keuls tests.
Changes in body weight in EB- and OIL-treated OVX rats were calculated as percent change from Day 1 (100×[(Day 2 weight − Day 1 weight)/Day 1 weight]) or (100×[(Day 4 weight − Day 1 weight)/Day 1 weight]); these data were analyzed using 2-way ANOVA, with groups (24 h and 48 h) and hormone treatments (EB and OIL) as factors. Pair-wise comparisons of significant (p<0.05) main effects or interactions were conducted using Student–Newman–Keuls tests.
3. Results
3.1. Water intake
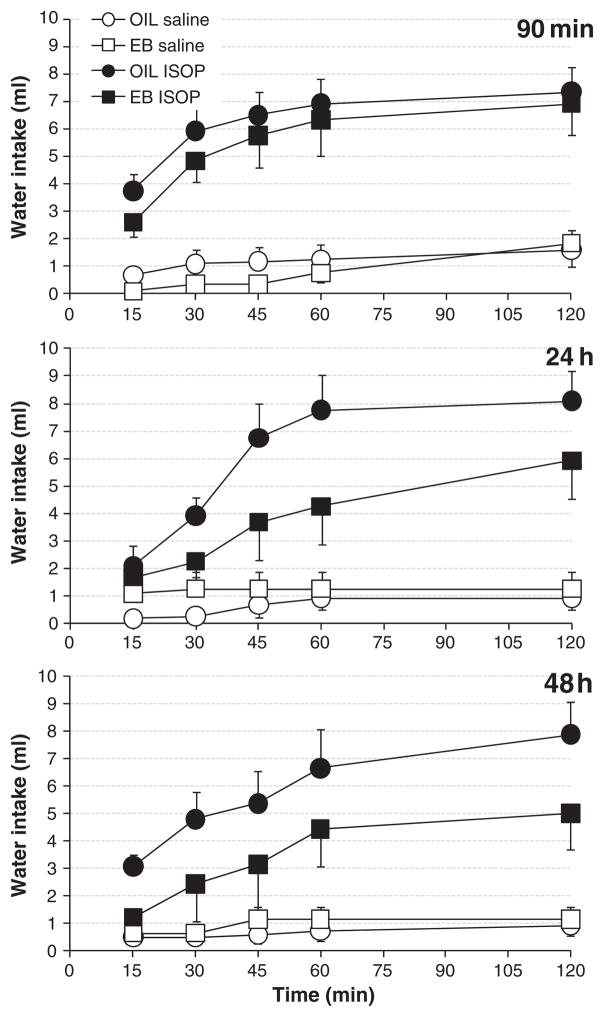
Water intake by EB- and OIL-treated OVX rats in the three groups is shown in Fig. 1. Four-way rm ANOVA revealed a main effect of drug (F(1,16) =66.563, p < 0.001), with water intake after ISOP significantly greater than that after Saline. In addition, there was a main effect of hormone treatment (F(1,16) =12.517, p < 0.01), with overall water intake by EB-treated OVX rats significantly less than that by OIL-treated rats. The interaction between drug and hormone treatment also affected water intake (F(1,16) =6.945, p < 0.05). Pair-wise comparisons of this interaction revealed that both EB-treated and OIL-treated OVX rats consumed significantly greater amounts of water after ISOP compared to that after Saline (both ps<0.001); however, EB-treated OVX rats drank significantly less water after ISOP than did OIL-treated OVX rats (p<0.01). In contrast, water intake by EB- and OIL-treated rats after Saline was comparable.
Fig. 1.
Cumulative water intake by OIL- (circles) and EB- (squares) treated OVX rats after s.c. injections with isoproterenol (ISOP; 30 μg/kg body weight; filled symbols) or 0.15 M NaCl vehicle (Saline; 1.0 ml/kg body weight; open symbols). Two-hour drinking tests were conducted 90 min after EB or OIL treatment (top), 24 h after the first EB or OIL treatment (middle), or 48 h after the second EB or OIL treatment (bottom). Planned comparisons of ISOP-induced water intake revealed no differences in the 90-min group (ns); however, in both the 24-h and 48-h groups, OIL-treated rats drank more water than did EB-treated rats (ps<0.001).
Water intake also was affected by time (F(4,64) =57.593, p < 0.001) and by the interaction between drug and time (F(4,64) =26.476, p<0.001). Further evaluation of this interaction revealed that, independent of group and hormone treatment, water intake after ISOP increased throughout the test, with intake at each time point after 15 min significantly greater than all preceding ones (all ps<0.01 and 0.001). Moreover, at each time point, water intake after ISOP was significantly greater than that after Saline (all ps<0.001), which did not change throughout the 2-h test.
Finally, planned comparisons of the total, 2-h water intake by EB-and OIL-treated OVX rats in each of the three groups after ISOP showed that EB-treated rats in both the 24-h and 48-h groups consumed significantly less water than did OIL-treated rats (both ps<0.001), whereas water intake by EB- and OIL-treated rats in the 90-min group was comparable.
3.2. Body weight
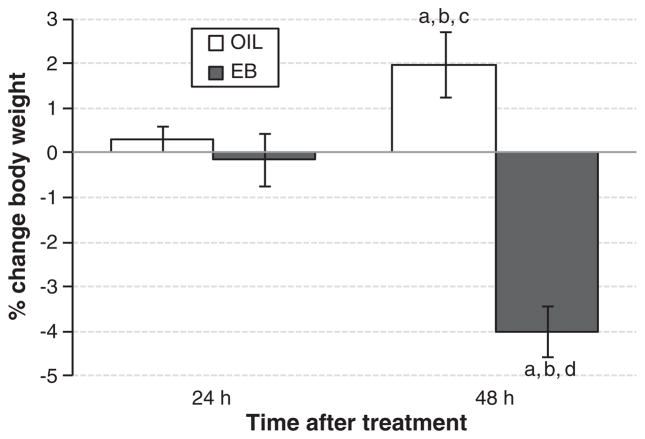
There were no differences in body weight at the beginning of behavioral testing (data not shown). Body weight changed in both EB- and OIL-treated rats during the 4-day protocol (Fig. 2). However, the direction of the change depended on hormone treatment (F(1,19) = 33.075, p<0.001), with weight loss in EB-treated rats and weight gain in OIL-treated rats. Body weight also was influenced by the interaction between group and hormone treatment (F(1,19) =24.340, p < 0.001). Pairwise comparisons of this interaction revealed that body weight changed little at 24 h regardless of hormone treatment, decreased significantly from 24-h to 48-h in EB-treated rats (p<0.001), and increased significantly from 24-h to 48-h in OIL-treated rats (p<0.05).
Fig. 2.
Change in body weight (% change from Day 1) in OIL- (open bars) and EB- (filled bars) treated OVX rats 24 h after the first EB or OIL treatment (left bars) or 48 h after the second EB or OIL treatment (right bars). a = significantly different from values 24 h after the first OIL treatment. b = significantly different from values 24 h after the first EB treatment. c = significantly different from values 48 h after the second EB treatment. d = significantly different from values 48 h after the second OIL treatment.
3.3. Uterine weight
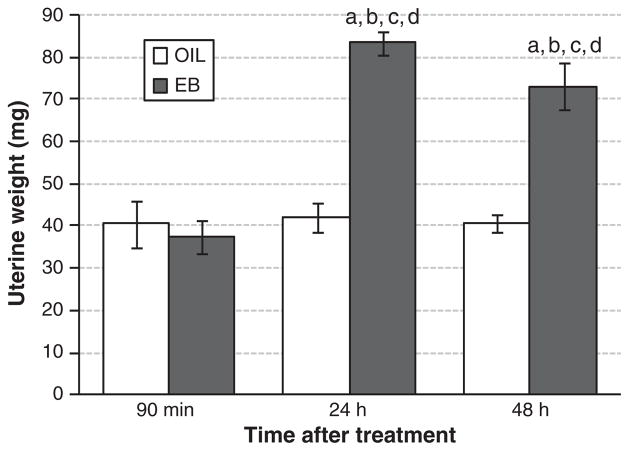
As shown in Fig. 3, uterine weight was affected by group (F(2,29) = 17.137, p<0.001), hormone treatment (F(1,29)=46.079, p < 0.001), and the interaction between group and hormone treatment (F(2,29) = 17.737, p<0.001). Pairwise comparisons of the interaction revealed that uterine weights in EB-treated OVX rats in the 24-h and 48-h groups were significantly greater than all others (all ps<0.001). In contrast, uterine weights in EB-treated rats in the 90-min group and in OIL-treated rats in all three groups were comparable.
Fig. 3.
Uterine weight (mg) in OIL-(open bars) and EB-(filled bars) treated OVX rats 90 min after EB or OIL treatment (left bars), 24 h after the first EB or OIL treatment (middle bars), or 48 h after the second EB or OIL treatment (right bars). a = significantly greater than values 90 min after the first OIL treatment. b = significantly greater than values 90 min after the first EB treatment. c = significantly greater than values 24 h after the first OIL treatment. d = significantly greater than values 48 h after the second OIL treatment.
3.4. Hematocrit, plasma protein concentration, and plasma Na+ concentration
As shown in Table 1, hematocrit was not affected by group or by hormone treatment. In contrast, there was a main effect of hormone treatment (F(1,29) =4.274, p < 0.05) on plasma protein concentration (Table 1). Overall, EB-treated rats had significantly greater plasma protein concentration than did OIL-treated rats. In addition, plasma protein concentration was affected by group (F(2,29) =6.748, p < 0.01). Pairwise comparisons revealed that, independent of hormone treatment, plasma protein concentration was significantly greater in the 24-h group compared to the 90-min (p<0.05) and the 48-h (p<0.01) groups. There was no interaction between group and hormone treatment. Plasma Na+ concentration (Table 1) was not affected by group or hormone treatment, or by the interaction between group and hormone treatment.
Table 1.
Hematocrit (%), plasma protein concentration (g/dl), and plasma Na+ concentration (mmol/l plasma water) in OIL- and EB-treated OVX rats. Independent of hormone treatment, plasma protein concentration 24 h after treatment was significantly greater than that after both 90 min (p<0.05) and 48 h (p<0.01).
| Hematocrit (%)
|
Plasma protein (g/dl)
|
Plasma Na+ (mmol/l plasma water)
|
||||||
|---|---|---|---|---|---|---|---|---|
| OIL | EB | OIL | EBa | OIL | EB | |||
| 90 min | 42.2±0.9 | 42.4±0.5 | 90 min | 6.6±0.2 | 6.8±0.2 | 90 min | 150.5±1.0 | 150.1±1.4 |
| 24 h | 42.8±0.7 | 45.0±2.0 | 24 hb | 6.7±0.2 | 7.3±0.1 | 24 h | 149.7±0.7 | 150.3±0.5 |
| 48 h | 40.3±1.3 | 42.5±0.6 | 48 h | 6.5±0.1 | 6.5±0.1 | 48 h | 149.6±0.5 | 151.0±0.6 |
Significant main effect of EB [F(1,29) =4.274, p < 0.05].
Significant main effect of group [F(2,29) =6.748, p < 0.01].
4. Discussion
The goal of the present study was twofold. First, we sought to evaluate the relative contributions of classic, slowly-occurring genomic effects vs. rapidly-occurring, nonclassical genomic or nongenomic effects of estradiol to the attenuation of volemic thirst in rats. Second, we compared the time course of these behavioral effects with those related to estradiol-induced changes in uterine weight, body weight, and measures of body fluid volume and Na+ concentration. To accomplish these goals, we first allowed sufficient time to ensure absorption of the injected EB [35], then selected time points based on generally accepted understanding of the time necessary for classic, slowly-occurring genomic effects, or more rapidly occurring, non-classical genomic or nongenomic effects. Thus, a 90-min delay after the first EB treatment was deemed likely to indicate nonclassical genomic or nongenomic effects e.g., [3,34,37]; see also [10], whereas classic genomic effects have been shown to occur 48 h after the second of two EB injections [27,32]. An additional time point 24 h after the first EB injection was included to allow better resolution of the time course of any observed changes. Our results show that full expression of the effects required time durations that likely indicate classic genomic effects; however, there were differences in the time after EB treatment at which individual effects were observed. The results also suggest tissue-specific effects related to the direction of EB changes in gene expression, with the striking uterine hypertrophy implying increased gene and protein expression [8,40,41], while the attenuated water intake in response to ISOP is consistent with reduced AngII receptor expression e.g., [32].
4.1. Water intake
As in our previous studies [30,32]; see also [25,61], ISOP-induced water intake was attenuated 48 h after the second EB treatment. Given the preponderance of evidence supporting a slowly-occurring, classic genomic effect that underlies the EB attenuation of water intake stimulated by ISOP, it is, perhaps, not surprising that there were no differences in water intake in response to ISOP 90 min after EB or OIL treatment. Nonetheless, this observation is instructive in regard to the time course of the development of the attenuation. Due to the specifics of the methods used, the frequency of the measurements, and the duration of the tests, we would have been able to detect EB effects on water intake that occurred as early as 100–105 min after EB treatment (90 min between EB and ISOP treatments, plus 10–15 min delay before the drinking test). On the other hand, since most of the water intake stimulated by ISOP occurs during the first hour of the test and little thereafter, it is unlikely that we would have been able to detect EB attenuation of ISOP-induced drinking that required more than ~3 hours to develop. Clearly, however, water intake stimulated by ISOP did not differ between EB- and OIL-treated rats in the 90-min groups at any time point. Thus, there is little, if any, evidence for nongenomic effects or for more rapidly-occurring nonclassical genomic effects during the first three hours after EB treatment that affect ISOP-induced water intake.
In contrast, EB attenuation of water intake in response to ISOP was readily apparent in the 24-h group. In regard to the attenuated ISOP-induced water intake 48 h after a second EB treatment, a likely explanation centers on reports of the colocalization of ERs and AngII receptors in the SFO [45], in conjunction with observations that AngII receptor binding [27], AngII receptor gene expression [32], and neuronal activation in response to ISOP [32] are all reduced in the SFO. Though it may initially seem somewhat surprising that similar changes could occur within 24 h after a single EB treatment, in fact, this is a sufficient time for classic steroidal regulation of transcription [34]. Thus, it is possible that the attenuation of water intake stimulated by ISOP that occurs 24 h after the first EB treatment also involves classic genomic effects to change protein expression within the CNS. More specifically, the attenuation of ISOP-induced water intake 24 h after the first EB treatment also may be attributable to decreased AngII receptors in the SFO. Accordingly, we propose that attenuation of drinking stimulated by AngII involves reduced expression of AngII receptors in the SFO that occurs at least 3 h after an initial increase in the circulating levels of estradiol, is well established by 24 h, and leads to blunted neuronal activation in response to ISOP. Clearly, additional studies will be necessary to test this hypothesis, to elucidate the time course of the changes, and to determine which ER subtype is important in the attenuation of ISOP-induced water intake by estrogens.
4.2. Body weight
Treatment with estradiol is known to reduce both food intake and body weight [22], effects are also seen in intact rats during the estrous phase of their cycle [13]. In addition, estrogens increase locomotor activity and metabolic rate [13,18] which, over time, undoubtedly contribute to body weight loss. In a previous study, Geary and Asarian [21] showed that food intake decreased slightly within 24 h after EB treatment, decreased further after a second EB treatment, and reached a nadir ~50 h after the second EB. Given that these investigators used a smaller dose of EB (2 μg), it seems likely that food intake was reduced as soon as 24 h after the 10 μg dose of EB used in our study, as well. Nevertheless, any reduction in feeding that may have occurred was insufficient to reduce body weight during that time. These findings suggest that the loss of body weight we observed was, therefore, attributable to more slowly developing EB effects to decrease food intake and/or to increase metabolism and/or locomotor activity.
It is possible that subtle changes in these factors, alone or in combination, occurred within 24 h after the first EB treatment, but not to degrees that were sufficient to reduce body weight until several days had elapsed. We have been unable to find reports detailing estrogenic effects on any of these factors at time points less than 24 h, with one exception. In that paper [36], it was reported that activity levels in diestrus rats were not affected by EB injections given 30 min prior to testing, suggesting that increased locomotor activity in EB-treated rats is attributable to classic genomic effects (though it should be noted that stereotypical behaviors related to drugs of abuse appear to involve rapid nongenomic actions of estrogens on central dopaminergic systems [6]). We did not monitor food intake, metabolic rate, or locomotor activity and, therefore, cannot say with any certainty which factor or combination of factors dictated the body weight loss we observed. Nonetheless, from the time course of the weight loss, it would appear that the effect involves slowly-occurring, classic genomic effects of EB. However, unlike EB effects to attenuate water intake stimulated by ISOP, EB-mediated effects on body weight did not occur within 24 h after the first EB treatment, but required a longer period of time.
4.3. Uterine weight
Estrogenic effects on the uterus result in hypertrophy attributable to endometrial proliferation, glandularization, and vascularization, along with an accompanying increase in blood flow, and the classic index of such changes is increased uterine weight. We saw no change in uterine weight 90 min after EB treatment, but uterine hypertrophy quite obviously occurred within 24 h after EB treatment. Moreover, EB effects on the uterus were maximal at that time, as uterine weight in the EB-treated 24-h group was indistinguishable from that in the EB-treated 48-h group. Thus, genomic effects that underlie uterine hypertrophy appear to be well-established and stable within 24 h after EB treatment. Interestingly, two previous studies separated by more than 30 years [40,41] detailed the time course of changes in uterine weight and reported increases well before 24 h. In 1971, Miura and colleagues found increased uterine weight within 2–4 h of EB treatment, along with changes in protein content 24 h after EB treatment. In 2004, Moggs and colleagues also found increased uterine weight (albeit far more modest) 2 h after EB treatment in a remarkable study that correlated gene expression with changes in uterine weight and morphology from 1 to 72 h after a single EB treatment. Both studies found uterine hypertrophy beginning 2 h after EB; thus, one might have expected an initial increase in uterine weight 90 min after EB. However, in the present study, 90 min was not sufficient for an EB-mediated increase in uterine weight.
Protein expression may lag behind the initiation of transcriptional activity by ~30 min, so that 2 h may be the threshold for detection of changes in gross uterine weight after EB. Alternatively, the discrepancy could be attributable to methodological differences, from how the uterus was weighed (the entire uterus [40,41] vs. a 10 mm segment; present study); to routes of administration (tail vein injection [40] vs. s.c. injection; present study [41]); to EB dose (400 μg [41] vs. 10 μg; present study [40]); and to species used (mice [41] vs. rats; present study [40]). In addition, differences in the time with low or no ovarian hormones before EB treatment was initiated also may be important. We alternated EB and OIL treatment weeks, so that rats sacrificed for analysis of uteri typically had been without EB for a maximum of 12 days. In contrast, rats in the study by Miura and colleagues were not tested until 21 days after OVX, while the study by Moggs and colleagues was designed to mimic puberty and gave immature mice (19–20 days old) a single large dose of EB. ER numbers or subtypes in the uterus may differ in prepubescent animals [16], and may change as the time without EB increases, both in young animals and in adults [14,16]. Thus, it is difficult to say with certainty whether the 30 min difference is critical for the cell differentiation and growth evidenced by increased uterine weight. Additional studies using our protocols will be necessary to more precisely determine the time point at which the uterine hypertrophy occurs, but it appears that this is a more slowly occurring genomic effect that is well-established and stable within 24 h after EB. Moreover, EB-mediated effects that underlie uterine hypertrophy appear to occur more rapidly than those associated with changes in body weight, and possibly earlier than those associated with ISOP-induced drinking, as well.
4.4. Hematocrit, plasma protein concentration, and plasma Na+ concentration
Ovarian hormones have been reported to influence both plasma volume and plasma Na+ concentration in humans and in rats [5,55]. These effects appear to be quite reliable in humans: plasma volume increases and plasma Na+ concentration decreases during the high estrogen phase of the menstrual cycle, as well as during the use of oral contraceptives, estrogen replacement therapy or estrogen supplementation [55–58]. In contrast, conflicting findings exist about the influence of estrogens on plasma volume and plasma Na+ concentration and/or plasma osmolality in rats; e.g., [5,9,23,25]. In the present study, we found no effect of EB treatment on plasma Na+ concentration or hematocrit, whereas EB treatment increased plasma protein concentration, which typically is an indication of reduced plasma volume. Certainly, methodological differences could account for the discrepancies in measurements of plasma volume and/or plasma Na+ concentration. Notably, the doses, duration, and delivery of EB used in these studies vary, from s.c. injections of ~3 μg/100 g body weight for two days; present study, to s.c. injections of 30 μg/100 g body weight for 14 days [5], to subcutaneous implantation of estrogen pellets, which produce continuously elevated hormone levels for 14 days prior to testing [23]. On the other hand, it seems unlikely that differences can be accounted for by the presence [5] or absence of progesterone; present study [23], as changes in plasma Na+concentration and hematocrit were comparable whether or not estradiol was supplemented with progesterone [5].
Another consideration in explaining the discrepant findings is that individual variability in plasma volume or plasma Na+ concentration may obscure more subtle differences due to hormone status. This possibility is further complicated by the increase in plasma protein concentrations in EB-treated rats, an increase that may be attributable to increased synthesis of clotting and inflammatory proteins [29], rather than to decreased plasma water, per se. To correct for increased circulating proteins after EB treatment, we expressed plasma Na+ concentration as mmol/l plasma water; however, doing so could make it more difficult to compare the present findings with those reported previously. Due to the effect of estrogens to increase plasma proteins [29], it seems likely that the better measure of volume status is hematocrit which, in our hands, indicates no effect of estrogens. In any case, although plasma protein concentration and hematocrit are commonly used to indicate changes in plasma volume, both are indirect measures of plasma volume. Thus, resolution to conflicting findings of EB effects on plasma volume — and on plasma Na+ concentration — will require direct methods, such as the dye dilution technique. Finally, it is important to remember that, under basal conditions, estrogens decrease consumption of food and water [13,21,22] and increase metabolism and locomotor activity [13,18]. These effects may lead to changes in total body water and, as a consequence, in total body Na+, that are secondary to those on food intake, water intake, and/or metabolism.
As we summarize, a brief discussion about the protocol and dose for estradiol treatment is warranted. We selected the 4-day protocol and 10 μg dose based on studies by McEwen and colleagues e.g., [22,42,63] and have employed both with reliable, replicable effects in a number of previous studies [15,25,30–32]; see also [22,26,28]. Nonetheless, our protocol likely produces comparatively high peaks in circulating estradiol and elevated levels that persist for 4–6 days [63]. Undoubtedly, greater amounts of circulating estradiol increase the rate at which ERs in target tissues are activated, be that to initiate slowly-occurring, classic genomic effects, or to trigger rapidly-occurring, nonclassical genomic or nongenomic effects. Despite the likelihood of high peak levels of EB, however, none of the changes we observed occurred within 90 min. Thus, our protocol allowed sufficient resolution to distinguish between slowly and rapidly-occurring effects and, importantly, none of the effects observed appeared to be attributable to rapidly-occurring genomic or nongenomic effects.
We also were able to detect differences in the timing of the classic, slowly-occurring genomic effects. EB effects on the uterus occurred within 24 h; see also [8,40,41], and appeared to be maximal at that time. In contrast, body weight changed little after 24 h but, instead, required additional time for the full effect. Lastly, the attenuation of ISOP-induced drinking was evident 24 h after EB treatment, but appeared to be more pronounced in the 48 h group. We should note that behavioral effects of steroids have been reported to occur within 15–30 min of administration [4,10,47], and may rapidly dissipate thereafter [4,47]. It is difficult to make comparisons between those studies and the present due to pronounced methodological differences that include species, sex, steroid, and route/site of administration, as well as the specifics of the behavioral testing; nonetheless, we cannot rule out the possibility that EB attenuation of water intake stimulated by ISOP also may involve transient, nongenomic effects. Thus, including additional time points and/or changing the number of treatment days may provide better resolution to more precisely determine the time course of the EB-mediated effects, not only to address the possibility of transient nongenomic effects of EB, but also to differentiate classic, slowly-occurring genomic effects from more rapidly-occurring nonclassical genomic effects. At present, however, in conjunction with findings from previous studies (e.g., [8,27,32,36,40,41,45]) our data suggest that the observed behavioral, morphological, and physiological changes are attributable to classic, slowly-occurring genomic effects of EB.
Importantly, our comparisons of multiple estrogenic effects at various time points were conducted in the same animals. Thus, our findings that the various effects follow different time courses are not due to methodological differences such as EB dose, treatment duration, or even the length of time without ovarian hormones before EB treatment was initiated. Rather, these findings may be attributable to differences in the number, sensitivity, and/or subtypes of EB receptors in various tissues [12], or to tissue-specific complements of coactivators and corepressors that determine the ‘direction’ of EB actions in transcriptional regulation; e.g., [49]. These obtained results may shed light on the mechanism(s) by which estrogens exert their effects, and better understanding of these mechanisms is critical, as evidenced by the fact that treatment of ER-positive breast with the SERM, tamoxifen, increases the risk of uterine cancer. Actions of estrogens are wide-ranging, with effects on both reproductive and non-reproductive organ systems in the periphery, as well as on neural systems in the brain [5,9,15,23,25,26,30–32,63]. Although estrogens have been implicated in pathological conditions ranging from breast cancer to Alzheimer’s disease, their role in physiological functions in addition to reproduction is no less important. Further investigation of the factors that determine differences in the time course and direction of the observed EB effects will be critical in understanding how EB influences gene and protein expression that underlie these physiological functions. More specifically, how does EB increase expression in the uterus [8,40,41] (as indicated by uterine hypertrophy), while concurrently decreasing AngII receptor expression in the SFO [32] (which likely underlies the attenuation of ISOP-induced water intake by EB-treated rats)? Clearly, additional studies will be required to better understand the diverse EB mechanisms which differentially affect gene and protein expression in various tissues and, consequently, the role of estrogens in normal, day-to-day functioning.
Acknowledgments
Grants from the National Institute on Deafness and Communication Disorders DC-06360 (KSC), the Oklahoma Center for the Advancement of Science and Technology Health Research Program HR 09-123 (KSC), and the Oklahoma IDeA Network of Biomedical Research Excellence (HH) supported this research.
Footnotes
Portions of these data were presented in preliminary form at the annual meeting of the Society for the Study of Ingestive Behaviors (Pittsburgh, PA; July, 2010).
References
- 1.Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–66. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- 2.Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proc Natl Acad Sci U S A. 1998;95:3281–6. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ascenzi P, Bocedi A, Marino M. Structure–function relationship of estrogen receptor alpha and beta: impact on human health. Mol Aspects Med. 2006;27:299–402. doi: 10.1016/j.mam.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Balthazart J, Cornil CA, Taziaux M, Charlier TD, Baillien M, Ball GF. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2006;138:783–91. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Barron WM, Schreiber J, Lindheimer MD. Effect of ovarian sex steroids on osmoregulation and vasopressin secretion in the rat. Am J Physiol. 1986;250:E352–61. doi: 10.1152/ajpendo.1986.250.4.E352. [DOI] [PubMed] [Google Scholar]
- 6.Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–7. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 7.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–21. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 8.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–40. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crofton JT, Share L. Sexual dimorphism in vasopressin and cardiovascular response to hemorrhage in the rat. Circ Res. 1990;66:1345–53. doi: 10.1161/01.res.66.5.1345. [DOI] [PubMed] [Google Scholar]
- 10.Cross E, Roselli CE. 17β-Estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1346–50. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- 11.Curtis KS. Estrogen and the central control of body fluid balance. Physiol Behav. 2009;97:180–92. doi: 10.1016/j.physbeh.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 12.Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, et al. International union of pharmacology. LXIV. Estrogen receptors. Pharmacol Rev. 2006;58:773–81. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 13.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson HA. Different regulation of the concentration of estrogen receptors in the rat liver and uterus following ovariectomy. FEBS Lett. 1982;149:91–5. doi: 10.1016/0014-5793(82)81078-8. [DOI] [PubMed] [Google Scholar]
- 15.Fan L, Smith CE, Curtis KS. Regional differences in estradiol effects on numbers of HSD2-containing neurons in the nucleus of the solitary tract of rats. Brain Res. 2010;1358:89–101. doi: 10.1016/j.brainres.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feherty P, Robertson DM, Waynforth HB, Kellie AE. Changes in the concentration of high-affinity oestradiol receptor in rat uterine supernatant preparations during the oestrous cycle, pseudopregnancy, pregnancy, maturation and after ovariectomy. Biochem J. 1970;120:837–44. doi: 10.1042/bj1200837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitts DA. Angiotensin II receptors in SFO but not in OVLT mediate isoproterenol-induced thirst. Am J Physiol. 1994;267:R7–15. doi: 10.1152/ajpregu.1994.267.1.R7. [DOI] [PubMed] [Google Scholar]
- 18.Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol. 2010;122:74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Fregly MJ. Effect of chronic treatment with estrogen on the dipsogenic response of rats to angiotensin. Pharmacol Biochem Behav. 1980;12:131–6. doi: 10.1016/0091-3057(80)90427-x. [DOI] [PubMed] [Google Scholar]
- 20.Fregly MJ, Thrasher TN. Attenuation of angiotensin-induced water intake in estrogen-treated rats. Pharmacol Biochem Behav. 1978;9:509–14. doi: 10.1016/0091-3057(78)90050-3. [DOI] [PubMed] [Google Scholar]
- 21.Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav. 1999;67:141–7. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 22.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56:281–9. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 23.Hartley DE, Dickson SL, Forsling ML. Plasma vasopressin concentrations and Fos protein expression in the supraoptic nucleus following osmotic stimulation or hypovolaemia in the ovariectomized rat: effect of oestradiol replacement. J Neuroendocrinol. 2004;16:191–7. doi: 10.1111/j.0953-8194.2004.01150.x. [DOI] [PubMed] [Google Scholar]
- 24.Hrabovszky E, Kallo I, Hajszan T, Shughrue PJ, Merchenthaler I, Liposits Z. Expression of estrogen receptor-beta messenger ribonucleic acid in oxytocin and vasopressin neurons of the rat supraoptic and paraventricular nuclei. Endocrinology. 1998;139:2600–4. doi: 10.1210/endo.139.5.6024. [DOI] [PubMed] [Google Scholar]
- 25.Jones AB, Curtis KS. Differential effects of estradiol on drinking by ovariectomized rats in response to hypertonic NaCl or isoproterenol: implications for hyper- vs. hypo-osmotic stimuli for water intake. Physiol Behav. 2009;98:421–6. doi: 10.1016/j.physbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisley LR, Sakai RR, Flanagan-Cato LM, Fluharty SJ. Estrogen increases angiotensin II-induced c-fos expression in the vasopressinergic neurons of the paraventricular nucleus in the female rat. Neuroendocrinology. 2000;72:306–17. doi: 10.1159/000054599. [DOI] [PubMed] [Google Scholar]
- 27.Kisley LR, Sakai RR, Fluharty SJ. Estrogen decreases hypothalamic angiotensin II AT1 receptor binding and mRNA in the female rat. Brain Res. 1999;844:34–42. doi: 10.1016/s0006-8993(99)01815-6. [DOI] [PubMed] [Google Scholar]
- 28.Kisley LR, Sakai RR, Ma LY, Fluharty SJ. Ovarian steroid regulation of angiotensin II-induced water intake in the rat. Am J Physiol. 1999;276:R90–6. doi: 10.1152/ajpregu.1999.276.1.R90. [DOI] [PubMed] [Google Scholar]
- 29.Koh KK, Yoon BK. Controversies regarding hormone therapy: insights from inflammation and hemostasis. Cardiovasc Res. 2006;70:22–30. doi: 10.1016/j.cardiores.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Krause EG, Curtis KS, Davis LM, Stowe JR, Contreras RJ. Estrogen influences stimulated water intake by ovariectomized female rats. Physiol Behav. 2003;79:267–74. doi: 10.1016/s0031-9384(03)00095-7. [DOI] [PubMed] [Google Scholar]
- 31.Krause EG, Curtis KS, Markle JP, Contreras RJ. Oestrogen affects the cardiovascular and central responses to isoproterenol of female rats. J Physiol. 2007;582:435–47. doi: 10.1113/jphysiol.2007.131151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krause EG, Curtis KS, Stincic TL, Markle JP, Contreras RJ. Oestrogen and weight loss decrease isoproterenol-induced Fos immunoreactivity and angiotensin type 1 mRNA in the subfornical organ of female rats. J Physiol. 2006;573:251–62. doi: 10.1113/jphysiol.2006.106740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause EG, Melhorn SJ, Davis JF, Scott KA, Ma LY, de Kloet AD, et al. Angiotensin type 1 receptors in the subfornical organ mediate the drinking and hypothalamic–pituitary–adrenal response to systemic isoproterenol. Endocrinology. 2008;149:6416–24. doi: 10.1210/en.2008-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–55. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 35.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–93. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 36.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–40. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 37.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7:497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masson DB, Fitts DA. Subfornical organ connectivity and drinking to captopril or carbachol in rats. Behav Neurosci. 1989;103:873–80. doi: 10.1037/h0092456. [DOI] [PubMed] [Google Scholar]
- 39.Mhyre AJ, Shapiro RA, Dorsa DM. Estradiol reduces nonclassical transcription at cyclic adenosine 3′,5′-monophosphate response elements in glioma cells expressing estrogen receptor alpha. Endocrinology. 2006;147:1796–804. doi: 10.1210/en.2005-1316. [DOI] [PubMed] [Google Scholar]
- 40.Miura S, Tsong YY, Koide SS. Hormonal effects of estrogen–protein conjugates on rat uterus. Biol Reprod. 1971;5:340–2. doi: 10.1093/biolreprod/5.3.340. [DOI] [PubMed] [Google Scholar]
- 41.Moggs JG, Tinwell H, Spurway T, Chang HS, Pate I, Lim FL, et al. Phenotypic anchoring of gene expression changes during estrogen-induced uterine growth. Environ Health Perspect. 2004;112:1589–606. doi: 10.1289/txg.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreines J, McEwen B, Pfaff D. Sex differences in response to discrete estradiol injections. Horm Behav. 1986;20:445–51. doi: 10.1016/0018-506x(86)90006-1. [DOI] [PubMed] [Google Scholar]
- 43.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, et al. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–40. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R794–9. doi: 10.1152/ajpregu.00424.2006. [DOI] [PubMed] [Google Scholar]
- 45.Rosas-Arellano MP, Solano-Flores LP, Ciriello J. Co-localization of estrogen and angiotensin receptors within subfornical organ neurons. Brain Res. 1999;837:254–62. doi: 10.1016/s0006-8993(99)01672-8. [DOI] [PubMed] [Google Scholar]
- 46.Rowland NE, Fregly MJ. Role of gonadal hormones in hypertension in the Dahl salt-sensitive rat. Clin Exp Hypertens A. 1992;14:367–75. doi: 10.3109/10641969209036195. [DOI] [PubMed] [Google Scholar]
- 47.Sakai RR, McEwen BS, Fluharty SJ, Ma LY. The amygdala: site of genomic and nongenomic arousal of aldosterone-induced sodium intake. Kidney Int. 2000;57:1337–45. doi: 10.1046/j.1523-1755.2000.00972.x. [DOI] [PubMed] [Google Scholar]
- 48.Sarkar SN, Huang R-Q, Logan SM, Yi KD, Dillon GH, Simpkins JW. Estrogens directly potentiate neuronal L-type Ca2+ channels. Proc Natl Acad Sci. 2008;105:15148–53. doi: 10.1073/pnas.0802379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–8. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 50.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 51.Simonian SX, Herbison AE. Differential expression of estrogen receptor and neuropeptide Y by brainstem A1 and A2 noradrenaline neurons. Neuroscience. 1997;76:517–29. doi: 10.1016/s0306-4522(96)00406-x. [DOI] [PubMed] [Google Scholar]
- 52.Somponpun SJ. Neuroendocrine regulation of fluid and electrolyte balance by ovarian steroids: contributions from central oestrogen receptors. J Neuroendocrinol. 2007;19:809–18. doi: 10.1111/j.1365-2826.2007.01587.x. [DOI] [PubMed] [Google Scholar]
- 53.Somponpun SJ, Johnson AK, Beltz T, Sladek CD. Estrogen receptor-alpha expression in osmosensitive elements of the lamina terminalis: regulation by hypertonicity. Am J Physiol Regul Integr Comp Physiol. 2004;287:R661–9. doi: 10.1152/ajpregu.00136.2004. [DOI] [PubMed] [Google Scholar]
- 54.Somponpun SJ, Sladek CD. Osmotic regulation of estrogen receptor-beta in rat vasopressin and oxytocin neurons. J Neurosci. 2003;23:4261–9. doi: 10.1523/JNEUROSCI.23-10-04261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stachenfeld NS, DiPietro L, Kokoszka CA, Silva C, Keefe DL, Nadel ER. Physiological variability of fluid-regulation hormones in young women. J Appl Physiol. 1999;86:1092–6. doi: 10.1152/jappl.1999.86.3.1092. [DOI] [PubMed] [Google Scholar]
- 56.Stachenfeld NS, DiPietro L, Palter SF, Nadel ER. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am J Physiol. 1998;274:R187–95. doi: 10.1152/ajpregu.1998.274.1.R187. [DOI] [PubMed] [Google Scholar]
- 57.Stachenfeld NS, Silva C, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol. 1999;87:1016–25. doi: 10.1152/jappl.1999.87.3.1016. [DOI] [PubMed] [Google Scholar]
- 58.Stachenfeld NS, Taylor HS. Effects of estrogen and progesterone administration on extracellular fluid. J Appl Physiol. 2004;96:1011–8. doi: 10.1152/japplphysiol.01032.2003. [DOI] [PubMed] [Google Scholar]
- 59.Stern JE, Zhang W. Preautonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor beta. Brain Res. 2003;975:99–109. doi: 10.1016/s0006-8993(03)02594-0. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology. 2004;145:3658–70. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- 61.Thrasher TN, Fregly MJ. Responsiveness to various dipsogenic stimuli in rats treated chronically with norethynodrel, ethinyl estradiol and both combined. J Pharmacol Exp Ther. 1977;201:84–91. [PubMed] [Google Scholar]
- 62.Voisin DL, Simonian SX, Herbison AE. Identification of estrogen receptor-containing neurons projecting to the rat supraoptic nucleus. Neuroscience. 1997;78:215–28. doi: 10.1016/s0306-4522(96)00551-9. [DOI] [PubMed] [Google Scholar]
- 63.Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- 64.Zheng W, Ji H, Maric C, Wu X, Sandberg K. Effect of dietary sodium on estrogen regulation of blood pressure in Dahl salt-sensitive rats. Am J Physiol Heart Circ Physiol. 2008;294:H1508–13. doi: 10.1152/ajpheart.01322.2007. [DOI] [PubMed] [Google Scholar]