Abstract
Background
Asthma is associated with oxidant stress and diminished antioxidant defenses. Yet, the mechanistic role of oxidant stress and antioxidant supplementation in human asthmatics remains uncertain. We determined the effect of high doses of the antioxidant natural-source d-α-tocopheryl acetate for 16 weeks on allergen-induced airway oxidant stress, inflammation, and bronchial responsiveness to methacholine and allergen in atopic asthmatics in vivo.
Methods
Thirty three mild atopic asthmatics underwent bronchoscopy with baseline bronchoalveolar lavage and segmental allergen challenge. The allergen-challenged airway was lavaged 24h later. At least 3 weeks later patients underwent inhaled challenges with methacholine and specific allergen. Volunteers took 1500 IU of natural-source d-α-tocopheryl acetate daily for at least 16 weeks. At the end of the treatment the two bronchoscopies and inhaled methacholine and allergen challenges were repeated. F2-isoprostanes, specific markers of oxidant stress, and selected Th1 and Th2 cytokines were analyzed in the lavage fluid.
Results
Following supplementation of natural-source d-α-tocopheryl acetate plasma concentrations of α-tocopherol increased and γ-tocopherol decreased. Both baseline and allergen-induced F2-isoprostanes significantly decreased, providing biochemical evidence for an antioxidant effect. Natural-source d-α-tocopheryl acetate reduced allergen-provoked concentrations of interleukin 3 and interleukin 4, and augmented levels of interleukin 12 in bronchoalveolar lavage fluid. Natural-source d-α-tocopheryl acetate improved airway responsiveness to methacholine but did not alter airway reactivity to specific allergen.
Conclusions
Inhibition of oxidant stress by natural-source d-α-tocopheryl acetate modulates allergic inflammation and airway hyperresponsiveness in human atopic asthmatics in vivo. These results need to be confirmed by a randomized placebo controlled trial.
Keywords: Atopic asthma, antioxidants, oxidant stress
INTRODUCTION
Oxidant stress is thought to play pathophysiologic role in asthma (1). We and others have provided evidence for oxidant stress in asthma by showing an increased generation of F2-isoprostanes after allergen challenge in asthmatic lungs in vivo (2). F2-isoprostanes are non-cyclooxygenase prostanoid-like compounds produced from arachidonic acid by reactive oxygen species (ROS) that proved reliable markers of oxidant stress in vivo (3). Epidemiological studies have linked decreased consumption of antioxidants to allergies and asthma (4,5). Yet, in contrast to animal studies (6), the mechanistic role of oxidant stress and antioxidants supplementation in human asthmatics is uncertain.
Vitamin E is a potent lipid soluble ROS scavenger and chain-breaking antioxidant that consists of numerous isoforms, including α-, β-, γ-, and δ-tocopherols, and α-, β-, γ-, and δ-tocotrienols (7). Recent studies showed that supplementation of the α- and γ-tocopherol isoforms of vitamin E inhibits and upregulates, respectively, allergic airway inflammation in mice (8,9). Clinical trials in human asthmatics were often conducted with doses and durations of vitamin E that may not be sufficient to alter oxidant stress (10). We have recently shown that high doses for a long period of natural-source d-α-tocopheryl acetate are required to reduce F2-isoprostanes in humans with atherosclerosis (11).
In order to provide evidence of an antioxidant treatment effect in atopic asthma, we carried out an unblinded non-randomized trial in 33 asthmatics, measuring F2-isoprostanes to show an antioxidant effect, together with measures of nonspecific airway reactivity and surrogate cytokine markers. We utilized at least 16 weeks of high dose (1500units) of natural-source d-α-tocopheryl acetate, based upon our earlier work. We hypothesized that prolonged use of high doses of natural-source d-α-tocopheryl acetate will reduce the allergen-induced oxidant stress and modify airway inflammation and reactivity in human atopic asthmatics in vivo. The primary outcome of our study was the effect of natural-source d-α-tocopheryl acetate on the baseline and allergen-induced levels of F2-isoprostanes in the bronchoalveolar lavage fluid (BAL). The secondary outcomes were airway responsiveness to methacholine and specific allergen, and allergen-provoked concentrations of Th1 and Th2 cytokines in BAL. We found prolonged use of high doses of natural-source d-α-tocopheryl acetate reduced allergen-induced F2-isoprostanes formation, modified airway inflammation, and lessened bronchial reactivity in atopic asthmatics.
METHODS
Patients
Thirty three non-smoking mild atopic asthmatics (29 Caucasians and 4 African Americans) by NAEPP guidelines (12) were recruited from Middle Tennessee (Table 1). Patients had positive allergy skin tests, methacholine challenges, and inhaled allergen challenges. All asthma medications were discontinued at least 48h and antihistamines more than 10 days prior to procedures. Pregnancy was excluded by urine HCG testing. No volunteer had a history of a respiratory infection in the 6 weeks preceding the study. Volunteers consented the study protocol that was approved by the Vanderbilt University Committee for the Protection of Human Subjects. The use of allergens for challenges was approved by the U.S. Food and Drug Administration prior to initiation of the study.
Table 1.
Demographic findings, history of atopy and asthma, asthma medication use, and allergens used for challenges.
| Variable | Count or mean±SD |
|---|---|
| Gender, F/M | 22/11 |
| Average age (years), F/M | 31.3±7.5/27.6±4.7 |
| Race | |
| Caucasian | 29 |
| African American | 4 |
| Family history of atopy | 33 |
| Frequency of daily asthma symptoms | |
| ≤ 2 days/week | 33 |
| > 2 days/week but not daily | 0 |
| Nighttime awakenings | |
| ≤ 2 days/week | 33 |
| > 2 days/week but not daily | 0 |
| Baseline spirometry (%predicted) | |
| FVC | 110.4±16.4 |
| FEV1 | 97.2±15.4 |
| FEV1/FVC | 88±9 |
| Medications (daily or as needed) | |
| H1-antihistamine | 33 |
| Albuterol inhaler ≤ 2 days/week | 33 |
| Budesonide inhaler | 1 |
| Fluticasone + Salmeterol discuss | 4 |
| Montelukast | 5 |
| Allergen used for challenge (extracts' producer's labels) | |
| Dermatophagoides farinae | 4 |
| Dermatophagoides pteronyssinus | 3 |
| Cat hair | 16 |
| Ragweed, short | 3 |
| Bermuda Grass | 2 |
| Kentucky Blue | 2 |
| Ryegrass | 2 |
| Timothy | 1 |
Experimental methods
The study was a non-randomized pre-post interventional trial. Two screening visits comprised medical history, physical examination, spirometry, allergy skin testing, methacholine challenge, and inhaled allergen challenge with the allergen which showed the strongest wheal on skin test and correlated with clinical history. Participants underwent bronchoscopy with baseline (before allergen challenge) BAL in the lingula of the left upper lobe followed by segmental allergen challenge (SAC) in a subsegment of the right middle lobe. The allergen-challenged subsegment was lavaged 24h later. At least 3 weeks later patients underwent inhaled challenges with methacholine and allergen on two consecutive days. For at least 16 weeks volunteers took 1500 IU of natural-source d-α-tocopheryl acetate (RRR-α-tocopheryl acetate, Carlson Lab, Arlington Heights, IL) daily with meals to ensure optimal absorption. At the end of the treatment the two bronchoscopies and inhaled methacholine and allergen challenges were repeated.
Prick skin tests were done with diluent and histamine controls, and standardized aeroallergens (Greer Laboratories, Lenoir, NC) according to ACAAI/AAAAI guidelines (13). The dose of methacholine (Methapharm, Inc., Coral Springs, FL) causing 20% decrease in Forced Expiratory Volume in one second (FEV1) (Met-PC20) was determined according to the ATS guidelines using a Salter dosimeter (Salter Labs, Arvin, CA) (14). To complete statistical analysis of the response to methacholine an arbitrary value of 21 was assigned to three patients who failed developing 20% drop in FEV1 after inhalation of 20mg/ml methacholine following treatment. Inhaled allergen challenge was performed according to Cockcroft and colleagues (15). Spirometry was measured using Flow Screen computerized spirometer (VIASYS Healthcare GmbH, Hoechberg, Germany). The greatest fall in FEV1 between 0–3h post allergen was taken as the early airway response (EAR), and at 3–8h was the late airway response (LAR). Bronchoscopy was performed in fasted volunteers. Midazolam 1–2 mg and fentanyl 50–100mcg were administered intravenously as needed. Airways were anesthetized using topical lidocaine (≤ 7 mg/kg). BAL was accomplished using three 50 ml aliquots of normal saline. Allergen challenge was done employing two incremental doses of the allergen that showed positive skin and inhaled reaction, each dose in a 5 ml aliquot of normal saline. The first dose of the allergen was 10-fold higher than the concentration that gave 2+ skin reaction on intradermal test (5–8 mm wheal, 20–30 mm erythema). The subsegment was watched for 2–3 minutes. None of the volunteers developed a visible inflammatory reaction after the first dose of allergen. Thus, in each subject the second dose of the same allergen was instilled at concentration 100-fold higher than the 2+ skin reaction (16).
Laboratory methods
BAL cells were counted in Reichert Brightline Metallized Phase Hemacytometer. F2-isoprostanes were analyzed by stable isotope dilution method in conjunction with gas chromatography-negative ion chemical ionization-mass spectrometry (GC/NICI/MS) using 2H4-15-F2t-isoprostane as an internal standard (3). Interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-13, interferon (IFN)-γ, TNF-α, and CXCL8 were measured using human Th1/Th2 10-Plex Multi-Spot assay (Meso Scale Discovery, Gaithersburg, MD). IL-3, IL-6, IL-9, CCL5 (RANTES), CCL11 (eotaxin), and immunoglobulin E (IgE) were analyzed by Cytometric Bead Array (BD Biosciences, San Jose, CA). α- and γ-tocopherols were quantitated according to Gross and colleagues (17).
Statistical analysis
The analysis of the effect of inhaled allergen challenge on FEV1 was performed using a mixed-effects model to take into account correlation due to repeated measurements over time within the same subject. The model included the natural-source d-α-tocopheryl acetate treatment as the main effect, and the baseline FEV1 and logarithm of all-PC20 were included as covariates. A natural cubic spline function of time with 3 degrees of freedom was also included in the model in order to adjust for a non-linear time trend. The analyses of all other outcomes were conducted using nonparametric statistical analysis and Wilcoxon signed rank tests for changes after allergen testing and for changes after 16 weeks of treatment since their distributions were highly skewed. Likewise, the Met-PC20 thresholds before and after natural-source d-α-tocopheryl acetate treatment were analyzed using Wilcoxon signed rank test. Analyses were performed using STATA 11.0 (StataCorp, College Station, TX). Data were expressed as means ± standard deviation (SD), median (interquartile range), or ranges.
RESULTS
Clinical and demographic characteristics of participating asthmatics are presented in Table 1.
After supplementation of natural-source d-α-tocopheryl acetate plasma concentrations of α-tocopherol significantly increased (0.83±0.34 (0.2–1.7) vs. 1.6±0.6 (0.7–3.2) mg/dL, before vs. after treatment, mean±SD, range, p<0.0001) and γ-tocopherol decreased (0.15±0.056 (0.04–0.3) vs. 0.076±0.016 (0.05–0.1) mg/dL, before vs. after treatment, mean±SD, range, p<0.0001). The baseline airway oxidant stress, as measured by BAL levels of F2-isoprostanes, was significantly augmented by allergen. Sixteen or more weeks of natural-source d-α-tocopheryl acetate significantly reduced both baseline and allergen-induced F2-isoprostanes, providing biochemical evidence for an antioxidant effect (Figure 1).
Figure 1.
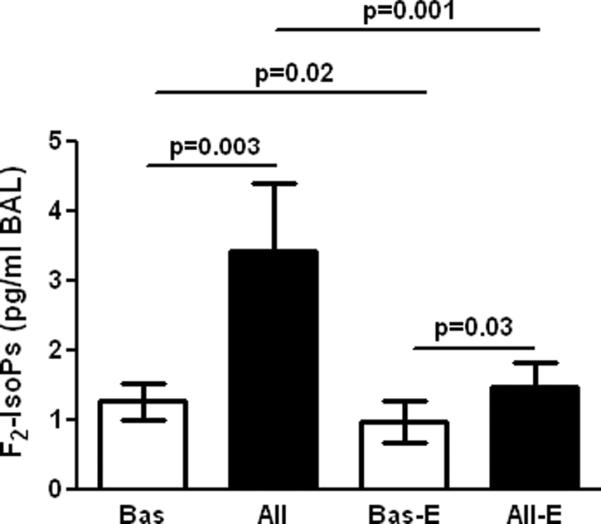
Comparison of F2-isoprostanes (F2-IsoPs) in BAL from the control lung segment and at 24 h after allergen challenge, before (Bas and All, respectively), and after supplementation of α-tocopherol (Bas-E and All-E, respectively) in atopic asthmatics in vivo.
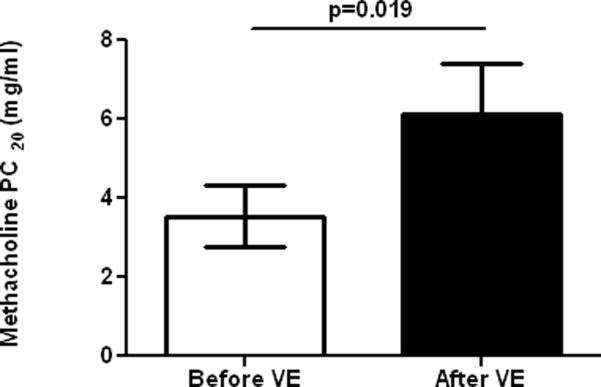
Supplementation of natural-source d-α-tocopheryl acetate caused a modest though significant improvement in Met-PC20 (Figure 2). In contrast, natural-source d-α-tocopheryl acetate did not alter airway reactivity to allergen (81.7±117.6 vs. 93.0±195.1, dilution fold of the allergen stock solution, before vs. after natural-source d-α-tocopheryl acetate, p=0.52). Additionally, there was no statistical difference in the FEV1 during either EAR or LAR between before- and after supplementation of natural-source d-α-tocopheryl acetate (p=0.14) after adjusting for baseline FEV1, logarithm of all-PC20, and a non-linear time trend. Unsurprisingly, only a trivial non-significant increase in baseline spirometric values after natural-source d-α-tocopheryl acetate was seen in this group of asthmatics who had normal baseline lung function.
Figure 2.
Effect of α-tocopherol on cumulative doses of methacholine (mg/ml) causing 20% decrease in FEV1 (PD20 FEV1).
The percentages of eosinophils and neutrophils in BAL cells increased both before and after treatment with natural-source d-α-tocopheryl acetate. A small but significant reduction in the percentage of allergen-provoked lymphocytes occurred after natural-source d-α-tocopheryl acetate.
Allergen increased BAL fluid concentrations of the Th2 cytokines IL-4, IL-5 and IL-13, and the Th1 cytokine IFN-γ. After natural-source d-α-tocopheryl acetate, allergen failed to increase IL-4 levels in BAL. There was a significant difference between the allergen-stimulated IL-4 levels before and after natural-source d-α-tocopheryl acetate. A non-statistically significant trend toward diminution of allergen-stimulated IL-5 and IL-13 levels was also found. In addition, natural-source d-α-tocopheryl acetate significantly reduced allergen-provoked concentrations of IL-3. In contrast, natural-source d-α-tocopheryl acetate increased BAL levels of the Th1 cytokine, IL-12. Non-statistically significant elevation of IFN-γ was noted (Table 3). BAL concentration of IL-1β, IL-2, IL-6, IL-10, TNF-α, CXCL8 and CCL-11were also augmented by allergen, but they were not modulated by supplementation of natural-source d-α-tocopheryl acetate (Table 3). Natural-source d-α-tocopheryl acetate had no effect on the allergen-stimulated concentrations of total IgE in BAL fluid (Table 3).
Table 3.
Baseline and allergen-induced levels of cytokines, chemokines, and IgE in BAL fluid, before and after natural-source d-α-tocopheryl acetate treatment (median (interquartile range)).
| Before treatment | After treatment | Allergen-induced before vs. after treatment | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Allergen | p | Baseline | Allergen | p | ||
| IL-1β | 0.49 (0.17–0.95) | 0.97 (0.3–1.69) | 0.01* | 0.28 (0.12–0.6) | 1.21 (0.52–1.98) | 0.016* | 0.22 |
| IL-2 | 0.17 (0.04–0.24) | 0.48 (0.2–0.99) | 0.0001* | 0.11 (0.03–0.26) | 0.69 (0.23–1.48) | 0.0001* | 0.53 |
| IL-3 | 1.7 (0.0–11.3) | 1.6 (0.0–7.7) | 0.12 | 1.01 (0.0–8.85) | 1.4 (0.0–4.5) | 0.18 | 0.0079* |
| IL-4 | 0.07 (0.005–0.12) | 0.17 (0.03–0.57) | 0.01* | 0.06 (0.0–0.18) | 0.045 (0.0–0.2) | 0.7 | 0.001* |
| IL-5 | 0.09 (0.25–0.17) | 1.0 (0.33–2.9) | 0.0001* | 0.09 (0.04–0.16) | 0.8 (0.41–13.6) | 0.0001* | 0.8 |
| IL-6 | 1.4 (0.0–3.3) | 21.5 (5.55–111.9) | 0.0001* | 1.4 (0.0–3.17) | 17.0 (4.35–94.6) | 0.000*1 | 0.49 |
| IL-9 | 0.0 (0.0–4.8) | 0.0 (0–6.25) | 0.39 | 0.0 (0.0–0.0) | 0.0 (0.0–5.6) | 0.03* | 0.96 |
| IL-10 | 0.14 (0.03–0.31) | 0.53 (0.29–1.09) | 0.0002* | 0.09 (0.0–0.26) | 0.7 (0.25–1.18) | 0.0001* | 0.9 |
| IL-12 | 0.045 (0.0–0.15) | 0.05 (0.002–0.21) | 0.06 | 0.0 (0.0–0.07) | 0.165 (0.78–0.41) | 0.0001* | 0.02* |
| IL-13 | 0.0 (0.0–0.43) | 0.6 (0.04–1.96) | 0.0003* | 0.02 (0.0–0.35) | 1.0 (0.075–3.6) | 0.0001* | 0.8 |
| TNF-α | 0.5 (0.26–0.6) | 2.76 (0.65–11.6) | 0.0001* | 0.45 (0.32–0.6) | 3.02 (0.96–14.3) | 0.0001* | 0.62 |
| IFN-Y | 0.34 (0.14–0.55) | 0.49 (0.23–0.96) | 0.006* | 0.34 (0.0–0.71) | 0.7 (0.3–1.65) | 0.0002* | 0.09 |
| CXCL8 | 11.34 (6.74–15.2) | 44.0 (25.2–93–5) | 0.0001* | 12.04 (6.2–18.14) | 53.2 (31.0–92.3) | 0.0001* | 0.28 |
| CCL5 | 2.44 (1.77–5.76) | 3.0 (1.36–7.11) | 0.3 | 1.83 (1.42–4.05) | 2.3 (1.71–8.34) | 0.21 | 0.7 |
| CCL11 | 0.0 (0.0–1.15) | 1.0 (0.0–4.02) | 0.009* | 0.0 (0–2.1) | 2.7 (0–5.45) | 0.003* | 0.34 |
| IgE | 2.73 (1.73–4.15) | 4.05 (2.81–10.8) | 0.004* | 2.44 (1.51–3.71) | 3.65 (2.87–12.9) | 0.0017* | 0.55 |
Discussion
We demonstrate here that long-term supplementation of the antioxidant natural-source d-α-tocopheryl acetate in high doses inhibits airway oxidant stress, modulates allergic inflammation, and improves airway hyperresponsiveness to methacholine in mild atopic asthmatics in vivo. These data suggest a mechanistic role of oxidant stress in the IgE-mediated airway inflammation and nonspecific airway hyperresponsiveness in human asthmatics in vivo. To our knowledge these findings are novel.
Plasma concentrations of α-tocopherol increased and γ-tocopherol decreased after supplementation of natural-source d-α-tocopheryl acetate (18). Using measurements of F2- isoprostanes (3), we showed suppression of airway oxidant stress by natural-source d-α-tocopheryl acetate during experimental exacerbation of allergic asthma in vivo. Although baseline levels of F2-isoprostanes were also decreased, there was a residual level present. Given the necessary role of oxidant reactions in the production of energy by living organisms it is not surprising that F2-isoprostanes are always detectable.
Airway hyperresponsiveness to methacholine is an essential feature of asthma (14). Our patients required higher concentrations of methacholine to achieve a 20% drop in FEV1 after taking natural-source d-α-tocopheryl acetate, suggesting improvement in their nonspecific bronchial hyperresponsiveness. In contrast, the triggering dose of specific allergen did not change, and neither the allergen-provoked EAR nor LAR was significantly modulated by natural-source d-α-tocopheryl acetate. Although there is a relationship between nonspecific airway reactivity to methacholine and allergen (15), there are precedents for the two becoming dissociated as in our study. For example, a neurokinin receptor antagonist increased the responsiveness to allergen challenge but left methacholine values unchanged (19). Inhalation of lysine aspirin or treatment with a leukotriene receptor antagonist decreased allergen challenge responses without changing the response to methacholine (20). Our protocol dictated delivering increasing doses of allergen until a fall in FEV1 of 20% was obtained. Thus subtle changes in responsiveness to allergen could have been missed in this study. All participating asthmatics had mild and well controlled disease, thus instruments typically used to quantify asthma control (21) were not employed.
Significant upregulation of airway eosinophilia and neutrophilia in BAL was indicative of an induction of airway inflammation by instilled allergens. The influx of inflammatory cells was associated with greater concentrations of the signature Th2 cytokines, IL-4, IL-5 and IL-13 and pro-eosinophilic chemokines such as CCL11 as well as other immunoregulatory cytokines including IL-1, IL-2, IL-6, IL-10, TNF-α, and CXCL8 (22). Enhancement of IFN-γ by allergen is not easily explainable. While some authors found an increased capacity of asthmatic airway T cells to produce IFN-γ (23), others reported decreased IFN-γ concentrations in BAL-fluid after allergen challenge (22). Specific IgE levels were not measured, but BAL concentrations of total IgE were augmented by allergen (24). Natural-source d-α-tocopheryl acetate appears to have relatively narrow modulatory effect on the IgE-mediated responses as suggested by a significant alleviation of the Th2 cytokine IL-4 and up-regulation of the Th1 cytokine IL-12. Non-significant trends towards reduction in IL-5 and IL-13, and an increase in IFN-γ were also observed. Nevertheless, our study shows a potential ability of natural-source d-α-tocopheryl acetate to modulate the imbalance in Th1 and Th2 cytokines that is linked to atopic disorders. Indeed, this important observation in vivo is supported by earlier investigations showing modulatory roles of ROS and intracellular glutathione on the Th1/Th2 cytokine balance in T cells and antigen presenting cells in vitro (25,26). Concentrations of IL-3, a growth factor for basophils, were also reduced by natural-source d-α-tocopheryl acetate in concordance with previous studies demonstrating ROS-dependency of IL-3 production in vitro (27). Because a great proportion of asthmatics develop Th2-driven inflammation (28), regulation of the Th1/Th2 balance by natural-source d-α-tocopheryl acetate is likely to be physiologically relevant in atopic asthma. Future investigations should determine if natural-source d-α-tocopheryl acetate affects influx or differentiation of a particular cell type(s), especially Th2 lymphocytes, intracellular ROS-signaling (25, 26) or ROS-dependent interaction among cells participating in allergic inflammation (29). The influx of eosinophils and neutrophils in BAL fluid was unaffected by natural-source d-α-tocopheryl acetate suggesting that the critical mechanisms regulating transepithelial migration of these cells is ROS-independent. Importantly, tissue infiltration by inflammatory cells was not assessed in this study. Given recent animal data showing a different regulation of the cellular inflammation in lung lavage compared to airway tissue by tocopherols, it would be important to determine if supplementation of natural-source d-α-tocopheryl acetate alters functional status of effector cells migrating to the human asthmatic airways in vivo (8,9).
Several limitations of our study deserve mention. Because of the invasive research measures and the relatively long treatment period, the study was not randomized, and involved only mild asthmatics. In as much as all participants had well controlled asthma, instruments typically used to quantify asthma control such as total asthma symptom severity score (TASS) or Asthma Quality of Life Questionnaire (AQLQ) score (21) were not employed. Certainly, future research should determine efficacy of antioxidants as a means to improve asthma management and reduce related morbidity in sicker asthmatics with moderate and severe disease. In addition, to obtain definitive data, placebo-controlled studies are necessary to determine efficacy of prolonged supplementation of high dose of natural-source d-α-tocopheryl acetate as a means to improve asthma management and reduce related morbidity in moderate and severe asthmatics. It needs also to be established if asthmatics with functional polymorphisms of antioxidant genes (30) could particularly benefit from d-α-tocopheryl acetate supplementation. Presently, the optimal antioxidant regimen in asthma is unknown. “α-Tocopherol is a weak antioxidant which poorly penetrates to subcellular compartments (7), although recent animal studies clearly highlighted its attenuating function on allergic inflammation (8,9). Certainly, better methods restoring disturbed redox balance in asthmatic airway cells should be sought. For example, mitochondrially targeted antioxidants might be more beneficial than natural-source d-α-tocopheryl acetate because mitochondrial dysfunction is associated with asthma, although currently these compounds are not available for human research (31). Nuclear factor (erythroid-derived 2)-like 2 (NRF2), the master transcription factor regulating several antioxidants has been strongly suggested as a therapeutic target in asthma. However, we have showed that allergen rendered the asthmatic alveolar macrophages incapable of responding to NRF2 enhancers in vivo, including the most potent natural NRF2 inducer, sulforaphane (32). Noteworthy, it is possible that the modulatory action of natural-source d-α-tocopheryl acetate on atopic asthma does not solely depend on oxidant stress-reduction, though the notion that tocopherols possess non-antioxidant properties is controversial (7).
Volunteers in this study did not suffer any adverse effects. However, there are some safety concerns associated with prolonged administration of high doses of α-tocopheryl including coagulopathy and an increased risk of malignancy. Nonetheless, in the Women's Health Study, 10 years of vitamin E administration was associated with much lower bleeding risks than those observed for aspirin use (33). No statistically significant associations between 10-year lung cancer incidence and vitamin E has been reported (34), and a recent report has suggested an risk of developing prostate cancer among healthy men participated in The Selenium and Vitamin E Cancer Prevention Trial (35). Noteworthy in the context of our investigation is the recent work demonstrating 10% reduction in the risk of chronic lung disease among women assigned to 600 IU vitamin E for 10 years (36).
Our study does not provide definitive answers to many important questions concerning the role of oxidative stress and antioxidants in human asthmatics. However, our data clearly and for the first time show that prolonged use of high doses of natural d-α-tocopherol has antioxidant properties, and improves airway reactivity perhaps by modulating allergic inflammation in human atopic asthmatics in vivo.
In summary, we describe a successful inhibition of airway oxidant stress by a new treatment method with natural-source d-α-tocopheryl acetate that results in modulation of allergic inflammation and bronchial hyperresponsiveness. These data provide novel evidence for a pathophysiological role of ROS and modulatory effects of the antioxidant natural-source d-α-tocopheryl acetate on atopic asthma in human in vivo. Our results need to be confirmed by a randomized placebo controlled trial. We hope that our study will restore the enthusiasm for exploring the role of oxidative stress and the potential benefits of antioxidant therapies in human asthma.
Table 2.
Cell differentiation in BAL fluid at baseline and after segmental allergen challenge, before and after treatment with natural-source d-α-tocopheryl acetate (percentage, mean±SD, range).
| Cell type | Before treatment | After treatment | Allergen-induced, before vs. after treatment | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Allergen | p | Baseline | Allergen | p | ||
| Macrophages | 83±11.1 (56–97) | 46.9±26 (2–92) | 0.0001 | 87.1±6.4 (73–97) | 47.2±28.8 (3–95) | 0.0001 | 0.6 |
| Eosinophils | 2.1±3.05 (0–11) | 17.6±23.4 (1–80) | <0.0001 | 1.7±2.4 (0–10) | 20±24.7 (0–95) | <0.0001 | 0.27 |
| Neutrophils | 2.9±2.9 (0–11) | 26±22.6 (0–68) | <0.0001 | 2±2.6 (0–12) | 26.5±25.2 (0–70) | <0.0001 | 0.38 |
| Lymphocytes | 11±9.3 (0–37) | 9.6±7.6 (0–32) | 0.74 | 8.7±6.5 (1–24) | 6±4.6 (90–19) | 0.11 | 0.02* |
Acknowledgements
The authors thank Dr. Myron D. Gross for performing analysis of α- and γ-tocopherols, Stephanie Sanchez and Holly Borntrager for help with measurement of F2-isoprostanes, and Dr. James Sheller, MD for helpful suggestions and reviewing the manuscript.
Funding: The study was supported by grants from the NIH (K23 HL080030, M01 RR-00095, and NIH P30 ES000267).
Footnotes
Authors Contributions Aimee Hoskins, RN, BRN had a major contribution to successful recruitment of volunteers and performing human experiments, collection and interpretation of data, and preparing the manuscript. Jackson L. Roberts, II, MD contributed to interpretation of data and preparing the manuscript. Ginger Milne, PhD contributed to analysis of biological samples and preparing the manuscript. Leena Choi, PhD performed statistical analysis of the data, contributed to interpretation of the results, and preparing the manuscript. Ryszard Dworski, MD, PhD was the principal investigator of the project. He coorditated all efforts associated with this investigation, performed bronchoscopy experiments, and had a major contribution to designing the study, interpretation of the results, and preparing the manuscript.
Conflicts of Interest: None of the aforementioned authors has any `Conflicts of interest' relevant to the work of this publication.
References
- 1.Dworski R. Oxidant stress and asthma. Thorax. 2000;55:S51–53. doi: 10.1136/thorax.55.suppl_2.S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dworski R, Murray JJ, Roberts LJ, II, Oates JA, Morrow JD, Fisher L, et al. Allergen-induced synthesis of F2-isoprostanes in atopic asthmatics. Evidence for oxidative stress. Am J Respir Crit Care Med. 1999;160:1947–1951. doi: 10.1164/ajrccm.160.6.9903064. [DOI] [PubMed] [Google Scholar]
- 3.Roberts LJ, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 4.Patel S, Murray CS, Woodcock A, Simpson A, Custovic A. Dietary antioxidant intake, allergic sensitization and allergic diseases in young children. Allergy. 2009;64:1766–1772. doi: 10.1111/j.1398-9995.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 5.Wood LG, Gibson PG. Reduced circulating antioxidant defenses are associated with airway hyperresponsiveness, poor control and severe disease pattern in asthma. Br J Nutr. 2010;103:735–741. doi: 10.1017/S0007114509992376. [DOI] [PubMed] [Google Scholar]
- 6.Talati M, Meyrick B, Peebles RS, Jr, Davies SS, Dworski R, Mernaugh R, et al. Oxidant stress modulates murine allergic airway responses. Free Radic Biol Med. 2006;40:1210–1219. doi: 10.1016/j.freeradbiomed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radical Biol & Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, et al. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCary CA, Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Supplemental and highly elevated tocopherol doses differentially regulate allergic inflammation: reversibility of α-tocopherol and γ-tocopherol's effects. J Immunol. 2011;186:3674–3685. doi: 10.4049/jimmunol.1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson PJK, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomized placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts LJ, II, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, et al. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radical Biol & Med. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Asthma Education and Prevention Program . Experts Panel Rapport 3: Guidelines for the Diagnosis and Management of Asthma. National Institute of Health, National Heart, Lung, and Blood Institute; Bethesda, MD: 2007. Pub no. 08-5846. [Google Scholar]
- 13.American College of Allergy, Asthma and Immunology, American Academy of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Practice Parameters for Allergy Diagnostic Testing. Ann Allergy Asthma Immunol. 1995;75:543–625. [PubMed] [Google Scholar]
- 14.American Thoracic Society Guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 15.Cockcroft DW, Davis BE, Boulet L-P, Deschesnes F, Gauvreau GM, O'Byrne P, et al. The links between allergen skin test sensitivity, airway responsiveness and airway response to allergen. Allergy. 2005;60:56–59. doi: 10.1111/j.1398-9995.2004.00612.x. [DOI] [PubMed] [Google Scholar]
- 16.Dworski R, Simons HU, Hoskins A, Yousefi S. Eosinophil and neutrophil extracellular DNA traps in human allergic asthmatic airways. J Allergy Clin Immunol. 2011;127:1260–1266. doi: 10.1016/j.jaci.2010.12.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross MD, Yu X, Hannan P, Prouty C, Jacobs Dr., Jr. Lipid standardization of serum fat-soluble antioxidant concentrations; the YALTA study. Am J Clin Nutr. 2003;77:458–466. doi: 10.1093/ajcn/77.2.458. [DOI] [PubMed] [Google Scholar]
- 18.Handelman GJ, Machlin LJ, Fitch K, Weiter JJ, Dratz EA. Oral alpha-tocopherol supplements decrease plasma gamma-tocopherol levels in humans. J Nutr. 1985;115:807–813. doi: 10.1093/jn/115.6.807. [DOI] [PubMed] [Google Scholar]
- 19.Boot JD, de Haas S, Tarasevych S, Roy C, Wang L, Amin D, et al. Effect of an NK1/NK2 receptor antagonist on airway responses and inflammation to allergen in asthma. Am J Respir Crit Care Med. 2007;175:450–457. doi: 10.1164/rccm.200608-1186OC. [DOI] [PubMed] [Google Scholar]
- 20.Boulet LP, Turcotte H, Laviolette M, Côté J, Wyile G, Bologa M. Inhibitory effects of BAY × 7195, a CYS leukotriene 1 receptor antagonist, on allergen-induced asthmatic responses. Ann Allergy Asthma Immunol. 1997;79:155–161. doi: 10.1016/s1081-1206(10)63103-1. [DOI] [PubMed] [Google Scholar]
- 21.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest. 1999;115:1265–1270. doi: 10.1378/chest.115.5.1265. [DOI] [PubMed] [Google Scholar]
- 22.Virchow JC, Jr, Walker C, Hafner D, Kortsik C, Werner P, Matthys H, et al. T cell and cytokines in bronchoalveolar lavage fluid after segmental allergen provocation in atopic asthma. Am J Respir Crit Care Med. 1995;151:960–968. doi: 10.1164/ajrccm/151.4.960. [DOI] [PubMed] [Google Scholar]
- 23.Krug NJ, Madden AE, Redington P, Lackie P, Djukanovic R, Schauer U, et al. T cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Crit Care Med. 1996;14:319–326. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 24.Wilson DR, Merrett TG, Varga EM, Smurthwaite L, Gould HJ, Kemp M, et al. Increases in allergen-specific IgE in BAL after segmental allergen challenge in atopic asthmatics. Am J Respir Crit Care Med. 2002;165:22–26. doi: 10.1164/ajrccm.165.1.2010112. [DOI] [PubMed] [Google Scholar]
- 25.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci USA. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nature Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 27.Sly LM, Kalesnikoff J, Lam V, Wong D, Song C, Omeis S, et al. IgE-induced mast cell survival requires the prolonged generation of reactive oxygen species. J Immunol. 2008;181:3850–3860. doi: 10.4049/jimmunol.181.6.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang H, Cao W, Kasturi SP, Rawindran R, Nakaya HI, Kundu K, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiteri MA, Bianco A, Strange SC, Fryer AA. Polymorphisms at the glutathione S-transferase, GSTP1 locus: a novel mechanism for susceptibility and development of atopic airway inflammation. Allergy. 2000;55:15–20. doi: 10.1034/j.1398-9995.2000.00502.x. [DOI] [PubMed] [Google Scholar]
- 31.Reddy PH. Mitochondrial dysfunction and oxidative stress in asthma: Implications for mitochondria-targeted antioxidant therapeutics. Pharmaceuticals. 2011;4:429–456. doi: 10.3390/ph4030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dworski R, Han W, Blackwell TS, Hoskins A, Freeman ML. Vitamin E prevents NRF2 suppression by allergens in asthmatic alveolar macrophages in vivo. Free Radic Biol Med. 2011;51:516–521. doi: 10.1016/j.freeradbiomed.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glynn RJ, Ridker PM, Goldhaber SZ, Zee RYL, Buring JE. Effect of random allocation to vitamin E supplementation on the occurance of venous thromboembolism. Report from the Womans' Health Study. Circulation. 116:1497–1503. doi: 10.1161/CIRCULATIONAHA.107.716407. [DOI] [PubMed] [Google Scholar]
- 34.Slatore CG, Littman AJ, Au DH, Satia JA, White E. Long-term use of supplemental vitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. Am J Respir Crit Care med. 2008;177:524–530. doi: 10.1164/rccm.200709-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein EA, Thompson IM, Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, et al. Vitamin E and the risk of prostate cancer. The selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agler AH, Kurth T, Gaziano JM, Buring JE, Cassano PA. Randomised vitamin E supplementation and risk of chronic lung disease in the Women's Health Study. Thorax. 2011;66:320–325. doi: 10.1136/thx.2010.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]