Abstract
Monoamine releasers constitute one class of candidate medications for treatment of cocaine abuse, and concurrent cocaine-versus-food choice procedures are potentially valuable as experimental tools to evaluate the efficacy and safety of candidate medications. This study assessed choice between cocaine and food by rhesus monkeys during treatment with five monoamine releasers that varied in selectivity to promote release of dopamine and norepinephrine (DA/NE) vs. serotonin (5HT) [m-fluoroamphetamine, (+)-phenmetrazine, (+)-methamphetamine, napthylisopropylamine and (±)-fenfluramine]. Rhesus monkeys (n=8) responded under a concurrent-choice schedule of food delivery (1-g pellets, fixed-ratio 100 schedule) and cocaine injections (0 – 0.1 mg/kg/inj, fixed-ratio 10 schedule). Cocaine choice dose-effect curves were determined daily during continuous seven-day treatment with saline or each test compound dose. During saline treatment, cocaine maintained a dose-dependent increase in cocaine choice, and the highest cocaine doses (0.032 – 0.1 mg/kg/inj) maintained almost exclusive cocaine choice. Efficacy of monoamine releasers to decrease cocaine choice corresponded to their pharmacological selectivity to release DA/NE vs. 5HT. None of the releasers reduced cocaine choice or promoted reallocation of responding to food choice to the same extent as substituting saline for cocaine. These results extend the range of conditions across which DA/NE-selective releasers have been shown to reduce cocaine self-administration.
Keywords: Cocaine; choice; nonhuman primates; monoamine releasers; dopamine; serotonin, norepinephrine
INTRODUCTION
Drug self-administration procedures play a critical role in the preclinical evaluation of candidate medications for the treatment of drug abuse (Mello and Negus, 1996). Specifically, medications that safely decrease self-administration of an abused drug in animals may have therapeutic value for treating abuse of that drug by humans. However, medication effects on drug self-administration can be influenced by variables that include the schedule of reinforcement under which drug self-administration is maintained (Arnold and Roberts, 1997; Negus and Banks, 2011). Assessment of the range of schedules across which a candidate medication decreases drug self-administration may be useful for prediction of the range of conditions across which that medication might decrease drug use clinically.
Concurrent “choice” schedules have emerged as one potentially useful family of procedures for assessment of candidate medication effects on drug self-administration (Griffiths et al., 1975; Woolverton and Balster, 1981; Czoty et al., 2005b; Bergman, 2008; Haney and Spealman, 2008; Thomsen et al., 2008; Negus and Banks, 2011). Under these schedules, a target drug of abuse (e.g. cocaine) and an alternative reinforcer (e.g. food pellets in animals or money in humans) are simultaneously available under separate schedules of reinforcement, and subjects allocate their responding across the two schedules. There are three potential advantages to the use of concurrent schedules in medications development. First, these procedures generate measures of behavioral allocation in addition to measures of behavioral rate. These two dependent measures in turn permit dissociation of selective medication effects on relative reinforcing effects of the self-administered drug (reflected by changes in allocation) from nonselective medication effects on motor or cognitive competence (reflected by changes in rate) (Negus and Banks, 2011). Second, drug abuse typically occurs in environments that also include other commodities, and drug abuse treatment seeks not only to reduce drug-taking behavior but also to promote reallocation of behavior to activities maintained by more adaptive reinforcers (Vocci, 2007). Concurrent choice procedures provide an explicit, albeit simplified, model of this environmental context, and thereby permit assessment of the degree to which candidate medications can promote reallocation of drug-taking behavior. Finally, preclinical human laboratory studies provide a key step in drug development between animal studies and clinical trials, and human laboratory research increasingly relies on concurrent schedules to assess effects of candidate medications on choice between a target drug of abuse and an alternative non-drug reinforcer (e.g. money or vouchers) (Haney and Spealman, 2008). The parallel use of concurrent schedules in animal and human laboratory studies may facilitate animal-to-human translation of results at this critical juncture in drug development.
The purpose of the present study was to examine effects of monoamine releasers that ranged in selectivity for promoting dopamine/norepinephrine (DA/NE) vs. serotonin (5HT) release in an assay of cocaine vs. food choice in rhesus monkeys. Monoamine releasers constitute one class of compounds under consideration as candidate medications for the treatment of cocaine dependence (Grabowski et al., 2004; Rothman et al., 2007). For example, maintenance on the DA/NE-selective releaser amphetamine safely decreased cocaine use in a controlled clinical trial (Grabowski et al., 2001). Similarly, in preclinical laboratory studies using concurrent choice procedures, amphetamine maintenance decreased cocaine choice and increased choice of an alternative reinforcer in both humans and rhesus monkeys (Negus, 2003; Greenwald et al., 2010; Rush et al., 2010). Monoamine releasers with lower pharmacological selectivity than amphetamine to release DA/NE vs. 5HT are of interest because they produce fewer abuse-related effects than amphetamine (Wee et al., 2005; Kimmel et al., 2009). Previous studies have compared effects of amphetamine and a series of other releasers on responding for cocaine and food under a multiple, second-order schedule of sequential cocaine and food availability in rhesus monkeys (Negus and Mello, 2003b; Rothman et al., 2005; Negus et al., 2007, 2009). Under these conditions, the behavioral selectivity of monoamine releasers to decrease cocaine- vs. food-maintained responding varied as a function of pharmacological selectivity to release DA/NE vs. 5HT. The greatest behavioral selectivity was achieved with the releasers phenmetrazine and methamphetamine, which have 37- to 40-fold selectivity for releasing DA vs. 5HT and retain significant abuse liability. Releasers with more prominent serotonergic effects and lower abuse liability failed to produce selective decreases in cocaine- vs. food-maintained responding.
The present study was designed to evaluate the degree to which these effects obtained under a multiple second-order schedule would extend to a concurrent choice schedule of cocaine and food availability. We hypothesized that DA/NE-selective releasers that selectively decreased cocaine- vs. food-maintained responding under the multiple schedule would reduce cocaine choice and increase food choice under the choice schedule. Conversely, non-selective or 5HT-selective releasers that produced nonselective reductions in both cocaine- and food-maintained responding under the multiple schedule were predicted to have no effect on cocaine vs. food choice up to doses that decreased overall rates of responding. The effects of removing cocaine or food availability were also examined for comparison to effects of monoamine releasers.
METHODS
Subjects
Studies were conducted in 8 adult male rhesus monkeys (Macaca mulatta) that had been surgically implanted with double lumen catheters using aseptic procedures as described previously (Negus, 2003). Monkeys were fed a diet of fresh fruit and food biscuits (Lab Diet Monkey Biscuits, PMI Feeds, Inc., St. Louis, MO) provided in the afternoon after the operant session to maintain body weight. Food deprivation protocols were not used. In addition, monkeys could receive up to 50 1-gm banana-flavored pellets (Grain-based Precision Primate Pellets, Test Diets, Richmond, IL) during daily experimental sessions (see below). Water was continuously available. A 12 hr light-dark cycle was in effect (lights on from 07.00 to 19.00 h). Three monkeys had exposure to the cocaine-versus-food choice procedure and treatment with monoaminergic and/or opioid test compounds (unpublished results), two monkeys had prior cocaine and methadone self-administration histories, and three monkeys were experimentally naïve at the start of these studies. The facility was licensed by both the United States Department of Agriculture and the Association for Assessment and Accreditation of Laboratory Animal Care, and protocols were approved by the Institutional Animal Care and Use Committee.
Apparatus and Catheter Maintenance
Experimental sessions were conducted in each monkey's home cage. The front wall was equipped with an operant response panel that included three square response keys arranged horizontally. Each housing chamber was also equipped with a pellet dispenser (Med Associates, Model ENV-203-1000, St. Albans, VT) and two syringe pumps (Model PHM-108, Med Associates), one for each lumen of the double lumen catheter. One syringe pump (the “self-administration pump”) was used to deliver self-administered cocaine injections through one lumen of the double-lumen catheter. The second syringe pump (the “treatment pump”) was used to deliver saline or test compounds through the second lumen of the catheter. This second pump was programmed to deliver 0.1 mL infusions every 20 min from 12:30 h each day until 12:00 h the next afternoon. Operation of the operant response panels and data collection were accomplished with microprocessors and software purchased from Med Associates Inc. The intravenous catheter was protected by a tether and jacket system (Lomir Biomedical, Malone, NY) that permitted monkeys to move freely in the cage. Catheter patency was periodically evaluated by intravenous (IV) administration of ketamine (5 mg/kg) through the catheter lumen and after each drug treatment that produced a rightward shift in the cocaine choice curve. The catheter was considered to be patent if IV administration of ketamine produced a loss of muscle tone within 10 s.
Behavioral Procedures
Baseline Procedures
Choice sessions were conducted seven days a week during daily 2hr sessions from 10.00 to 12.00 h as described previously (Negus, 2003). The terminal choice schedule consisted of five 20-min components separated by 5-min timeout periods. During each component, the left, food-associated key was illuminated with red stimulus lights, and completion of the fixed-ratio (FR) requirement resulted in the delivery of a food pellet. The right, cocaine-associated key was illuminated with green stimulus lights, and completion of the FR requirement on this key resulted in the delivery of a cocaine dose. A different cocaine dose was available during each of the five successive components (0, 0.0032, 0.01, 0.032 and 0.1 mg/kg/inj during components 1–5, respectively), and dose was varied by changing the resulting volume (0, 0.01, 0.03, 0.1 and 0.3 mL/inj, respectively) of each injection. Stimulus conditions on the drug-associated key were also varied by flashing the stimulus lights on and off in 3 s cycles. (Component 1: 0 s on, 3 s off; Component 2: 0.1 s on, 2.9 s off; Component 3: 0.3 s on, 2.7 s off; Component 4: 1 s on, 2 s off; Component 5: 3 s on, 0 s off). Thus, longer flashes (and shorter inter-flash intervals) were associated with higher cocaine doses. The response requirements were set at FR 100 on the food-associated key and FR 10 on the cocaine-associated key for all monkeys, because our previous studies indicated that, under these response requirements, monkeys usually switched from the food-associated key to the drug-associated key during the fourth response period, when an intermediate unit dose of 0.032 mg/kg/inj cocaine was available (Negus, 2003). Consequently, with the procedures used in this study, it was possible to observe both leftward and rightward shifts in the cocaine choice dose-effect curves that might result from manipulation of experimental variables.
During each component, monkeys could complete up to 10 total ratio requirements on the food- and cocaine-associated keys. Responding on either key reset the ratio requirement on the other key. Completion of each ratio requirement initiated a 30 s timeout, during which all stimulus lights were turned off, and responding had no scheduled consequences. If all 10 ratio requirements were completed before the 20-min component had elapsed, then all stimulus lights were extinguished and responding had no scheduled consequences for the remainder of that component. Choice behavior was considered to be stable when the lowest cocaine dose maintaining at least 80% cocaine choice varied by ≤0.5 log units for three consecutive days.
Testing Procedures
Once cocaine choice was stable as defined above, testing began. The monoamine releasers were selected for study based on their previously reported in vitro range of functional selectivities to release DA/NE vs. 5HT and their behavioral effects in rhesus monkeys responding for cocaine and food under a multiple, second-order schedule (Negus et al., 2007; Negus et al., 2009). Specifically, the in vitro functional selectivities to release DA/NE vs. 5HT (expressed as the ratio of IC50 to release 5HT ÷ IC50 to release DA) were as follows: m-fluoroamphetamine (a.k.a. PAL-353; 80), (+)-phenmetrazine (40), (+)-methamphetamine (37), napthylisopropylamine (a.k.a. PAL-287; 0.27) and (±)-fenfluramine (<0.0079). (Note that potencies to release DA and NE were similar for each compound, with slightly higher potencies to release NE than DA). Each dose of each drug was tested for a period of seven consecutive days. The infusion rate for the test drug administered by the treatment pump was identical to that used in our previous studies with these compounds (Negus, 2003; Negus et al., 2007, 2009). The dose ranges for each test drug were as follows: m-fluoroamphetamine (0.032–0.32 mg/kg/h), (+)-phenmetrazine (0.032–0.32 mg/kg/h), (+)-methamphetamine (0.032–0.1 mg/kg/h), napthylisopropylamine (0.032–1.0 mg/kg/h) and (±)-fenfluramine (0.1–0.32 mg/kg/h). At the conclusion of each test period, a saline control treatment period was reinstated for at least four days until cocaine choice returned to pre-test levels and was stable as defined above. The effects of each test drug on cocaine choice were evaluated in groups of four monkeys. In general, all doses of one test drug were tested in a given monkey before initiation of studies with another test drug. Both the sequence of drug doses and the sequence of drugs were mixed across monkeys.
In a second set of experiments, availability of either cocaine or food was temporarily removed to establish boundary conditions for comparison to the effects of the test drugs. More specifically, these conditions were studied to examine effects of the extreme case wherein reinforcing consequences associated with either cocaine or food choice were eliminated. To remove cocaine availability, the cocaine solution was replaced with saline in the “self-administration” pump for seven days so that all other stimuli, including intravenous injections, were the same under both conditions. To remove food availability, food pellets were removed from the pellet dispenser so that all other stimuli, including the sound of the pellet dispenser motor, were the same under both conditions. At the end of each 7-day test period, standard conditions of cocaine and food availability were reinstated until choice behavior recovered to baseline levels.
Drugs
Cocaine HCl and (+)-methamphetamine HCl were provided by the National Institute on Drug Abuse Drug Supply Program, Bethesda, MD. m-Fluoroamphetamine hemi-fumarate, (+)-phenmetrazine fumarate, and napthylisopropylamine fumarate (also known as PAL-287; Rothman et al, 2005; Negus et al, 2007) were provided by BE Blough (Research Triangle Institute, Research Triangle Park, NC). (±)-Fenfluramine HCl was purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in sterile water and all solutions were filter-sterilized using a 0.22-μm Millipore filter (Millipore Corporation, Billerica, MA). Drug doses were calculated using the salt forms listed above.
Data Analysis
The primary dependent variables for each component were (1) percent cocaine choice, defined as (number of choices completed on the cocaine-associated key ÷ total number of choices completed on both the cocaine- and food-associated keys)*100, and (2) total number of choices completed obtained from the last 3 days of each 7-day treatment period. These variables were then plotted as a function of cocaine dose. Cocaine choice dose-effect curves were analyzed using a two-way repeated measures ANOVA with cocaine dose and treatment dose as the main factors. A significant ANOVA was followed by the Dunnett multiple comparisons post-hoc test to compare test conditions with baseline conditions. The ED50 value of the cocaine choice dose-effect curve was defined as the dose of cocaine that produced 50% cocaine choice. ED50 values were calculated by interpolation when only two data points were available (one below and one above 50% cocaine choice) or by linear regression when at least three data points were available on the linear portion of the dose-effect curve. Log ED50 values were calculated for each monkey during (a) the last three “baseline” days before introduction of each treatment manipulation and (b) the last three days of each 7-day treatment period. In cases where log ED50 values could not be determined because cocaine choice was greater or less than 50% for all cocaine doses, conservative estimates of the cocaine-choice ED50 were determined by assuming 100% cocaine choice at the next higher half-log dose (0.32 mg/kg/inj) or 0% cocaine choice at the next lower half-log dose (0.001 mg/kg/inj). Additional dependent variables collected during each session included total choices, total food choices, total cocaine choices, and total cocaine intake. Values were compared by repeated measures one-factor ANOVA, with treatment condition as a within-subjects factor. A significant ANOVA was followed by the Dunnett multiple comparisons post-hoc test to compare test conditions with baseline conditions. The criterion for significance was set a priori at the 95% level of confidence (p < 0.05).
RESULTS
Baseline choice between food and cocaine
During saline treatment, monkeys responded primarily for food when low cocaine doses were available (0–0.01 mg/kg/inj) and primarily for cocaine when higher cocaine doses were available (0.032–0.1 mg/kg/inj) (Figures 1–6, upper left panels, open circles). The baseline cocaine choice ED50 value is shown in Table 1. The value shown in Table 1 is the mean of all baseline determinations for each treatment condition and then averaged across all treatment conditions. These baseline data were averaged for analysis because baseline ED50 values were stable across all determinations. Specifically, baseline (± SEM) cocaine choice ED50 values (mg/kg/inj) were 0.017 (0.016 – 0.019) before m-fluoroamphetamine, 0.015 (0.013 – 0.017) before phenmetrazine, 0.015 (0.013 – 0.018) before methamphetamine, 0.016 (0.013 – 0.019) before napthylisopropylamine, 0.019 (0.015 – 0.023) before fenfluramine and 0.017 (0.015 – 0.019) before removing cocaine and food availability. The number of choices completed per component is also shown in Figures 1–6 (upper right panels, open circles). In general, monkeys usually completed the maximum number of choices available in each component under baseline conditions. Figures 1–6 (bottom panels, open bars) also show baseline numbers of total choices, food choices, cocaine choices and cocaine intake.
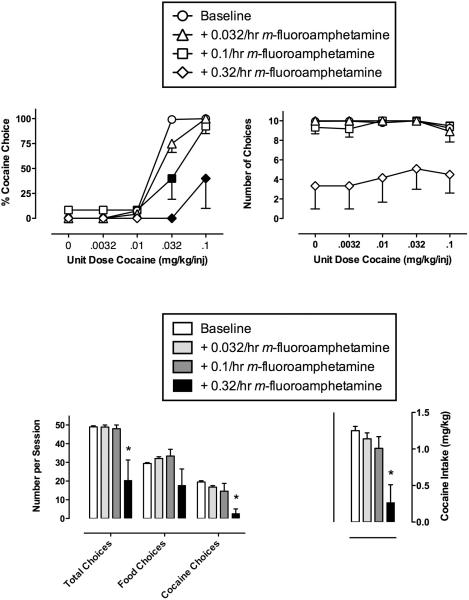
Figure 1.
Effects of continuous 7-day treatment with m-fluoroamphetamine (0.032–0.32 mg/kg/h) on choice between cocaine and food. Upper vertical axes: percent cocaine choice (left) or the number of choices completed per component (right) during treatment with saline (Baseline) or increasing doses of m-fluoroamphetamine. Upper horizontal axes: unit dose of cocaine in milligrams per kilogram per injection. Bottom panels show total choices, food choices, cocaine choices and cocaine intake during choice sessions before or during 7-day treatment with m-fluoroamphetamine. Lower left vertical axis: Number of choices per session. Lower right vertical axis: cocaine intake in mg/kg/day during choice sessions. Lower horizontal axes: experimental endpoint. All points and bars represent mean data ± SEM from four monkeys obtained during the last 3 days of each 7-day treatment. Filled symbols indicate significantly different (p < 0.05) from baseline conditions within a cocaine dose. Asterisk indicates significantly different (p < 0.05) from baseline conditions.
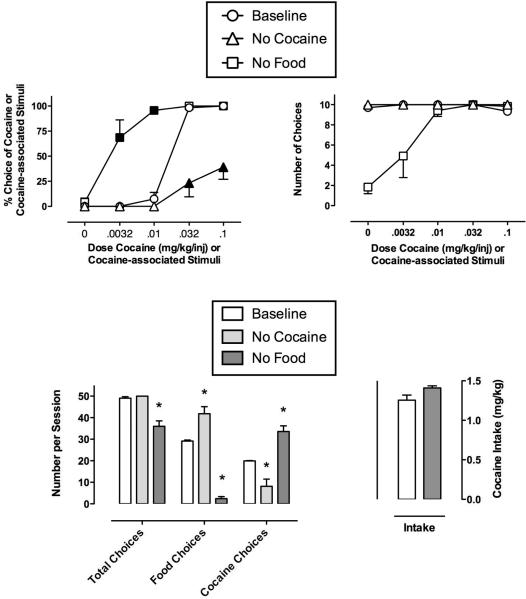
Figure 6.
Effects of removing cocaine or food availability on choice. Horizontal axes in the upper panels show unit dose of cocaine available (during “Baseline” and “No Food” conditions), or unit dose of cocaine associated with the prevailing discriminative stimuli (during the “No Cocaine” condition). Other details as in Fig. 1.
Table 1.
Mean cocaine choice ED50 value in mg/kg per injection (95% confidence limits; CL) under baseline (saline) conditions and during 7-day treatment with m-fluoroamphetamine, (+)-phenmetrazine, (+)-methamphetamine, napthylisopropylamine and (±)-fenfluramine, or during removal of cocaine or food availability.
| Treatment Condition | ED50 (95% CL) |
|---|---|
| Baseline (n=8) | 0.017 (0.016 – 0.017) |
| m-Fluoroamphetamine 0.032 mg/kg/h (n=4) | 0.024 (0.019 – 0.030)* |
| m-Fluoroamphetamine 0.1 mg/kg/h (n=4) | 0.032 (0.013 – 0.075) |
| m-Fluoroamphetamine 0.32 mg/kg/h (n=4) | 0.117 (0.057 – 0.237)A |
| Phenmetrazine 0.032 mg/kg/h (n=4) | 0.030 (0.016 – 0.056) |
| Phenmetrazine 0.1 mg/kg/h (n=4) | 0.024 (0.012 – 0.049) |
| Phenmetrazine 0.32 mg/kg/h (n=4) | 0.055 (0.053 – 0.058)B |
| Methamphetamine 0.032 mg/kg/h (n=4) | 0.013 (0.009 – 0.019) |
| Methamphetamine 0.056 mg/kg/h (n=4) | 0.024 (0.010 – 0.056) |
| Methamphetamine 0.1 mg/kg/h (n=4) | 0.021 (0.003 – 0.132)C |
| Napthylisopropylamine 0.1 mg/kg/h (n=4) | 0.013 (0.010 – 0.016) |
| Napthylisopropylamine 0.32 mg/kg/h (n=4) | 0.018 (0.012 – 0.029) |
| Napthylisopropylamine 1.0 mg/kg/h (n=3) | 0.0 11 (0.006 – 0.021)D |
| Fenfluramine 0.1 mg/kg/h (n=4) | 0.014 (0.01 – 0.034) |
| Fenfluramine 0.32 mg/kg/h (n=4) | 0.014 (0.006 – 0.034)B |
| No Cocaine (n=4) | 0.074 (0.048 – 0.115)*,E |
| No Food (n=4) | 0.003 (0.002 – 0.004)* |
Significantly different from baseline as defined by non-overlapping 95% confidence limits.
Mean ED50 value was estimated in one monkey because cocaine choice did not exceed 50% at any cocaine dose and treatment eliminated all responding in another monkey.
Mean ED50 value could not be determined because cocaine choice was 100% for some components and eliminated responding during other components of the choice session in one monkey.
Mean ED50 value was estimated because cocaine choice did not exceed 50% in one monkey and was not lower than 50% in another monkey at any cocaine dose.
Mean ED50 value could not be determined because treatment eliminated responding in one monkey.
ED50 value based on doses associated with prevailing discriminative stimuli.
Effects of m-fluoroamphetamine treatment on cocaine choice
Figure 1 (upper left panel) shows that m-fluoroamphetamine produced a dose-dependent rightward shift in the cocaine choice dose-effect curve with the lowest dose (0.032 mg/kg/h) producing a significant increase in the cocaine choice ED50 value (Table 1). The intermediate (0.1 mg/kg/h) dose increased the cocaine choice ED50 value in three of the four monkeys, but decreased the cocaine choice ED50 value in the fourth animal (See Figure 7 for individual cocaine choice ED50 values for each treatment condition). Furthermore, the highest dose also produced an increase in the cocaine choice ED50 value in three of the four monkeys, but eliminated responding in the fourth monkey (Fig. 7). Repeated measures two-way analysis of variance (ANOVA) demonstrated a significant effect of cocaine dose (F4,8=31.8, p<0.001), no significant effect of m-fluoroamphetamine treatment and a significant cocaine dose and m-fluoroamphetamine treatment interaction (F12,21=3.2, p<0.05). Post-hoc analysis revealed that treatment with 0.1/hr and 0.32/hr m-fluoroamphetamine significantly decreased choice of the 0.032 mg/kg/inj cocaine dose and treatment with 0.32/hr m-fluoroamphetamine also significantly decreased choice of the 0.1 mg/kg/inj cocaine dose (filled symbols, Figure 1, upper left panel). Figure 1 (lower left panel) also shows the number of total, food and cocaine choices completed during the session. Repeated-measures one-way ANOVA demonstrated a main effect of m-fluoroamphetamine treatment on total choices (F3,9=6.5, p<0.05) and cocaine choices (F3,9=9.4, p<0.01), but not food choices. Post-hoc analysis revealed that treatment with 0.32 mg/kg/h m-fluoroamphetamine significantly (p< 0.05) decreased total choices and cocaine choices compared to baseline. For cocaine intake (bottom right panel), repeated-measures one-way ANOVA demonstrated a significant main effect of m-fluoroamphetamine treatment on cocaine intake (F3,9=8.9, p<0.01) and post-hoc analysis revealed that treatment with 0.32 mg/kg/h m-fluoroamphetamine significantly decreased cocaine intake.
Figure 7.
Cocaine choice ED50 values in individual animals during each experimental manipulation. Horizontal axes: Experimental manipulation. Vertical axis: Cocaine choice ED50 value in mg/kg/inj (log scale). Different symbols represent individual animals. Horizontal line represents the mean for each condition. For the “No Cocaine” conditions, the ED50 value was based on the cocaine doses associated with the prevailing discriminative stimuli.
Effects of phenmetrazine treatment on cocaine choice
Figure 2 (upper left panel) shows that phenmetrazine did not significantly alter the cocaine choice dose-effect curve or cocaine ED50 values (Table 1; Figure 7). The highest dose (0.32 mg/kg/h) increased the cocaine choice ED50 value in three monkeys; however, in the fourth monkey during treatment with 0.32 mg/kg/h phenmetrazine, responding was eliminated during availability of low cocaine doses (0–0.01 mg/kg/inj), and 100% cocaine choice was observed during availability of higher cocaine doses (0.0320–0.1 mg/kg/inj). Repeated measures two-way ANOVA demonstrated a significant effect of cocaine dose (F4,12=52.2, p<0.001), no significant effect of phenmetrazine treatment and a significant cocaine dose and phenmetrazine treatment interaction (F12,33=2.3, p<0.05). Post-hoc analysis revealed that treatment with all phenmetrazine doses significantly decreased choice of the 0.032 mg/kg/inj cocaine dose. Figure 2 (lower left panel) also shows the number of total, food and cocaine choices completed during the session. Repeated-measures one-way ANOVA demonstrated a significant main effect of phenmetrazine treatment on cocaine (F3,9=7.2, p<0.01) and total choices (F3,9=6.3, p<0.05), but not food choices. Post-hoc analysis revealed that treatment with 0.32 mg/kg/h phenmetrazine significantly (p< 0.05) decreased both total and cocaine choices compared to baseline. For cocaine intake (lower right panel), repeated-measures one-way ANOVA demonstrated a significant main effect of phenmetrazine treatment (F3,9=18.1, p<0.001) and post-hoc analysis revealed that treatment with 0.032 mg/kg/h and 0.32 mg/kg/h phenmetrazine significantly decreased cocaine intake compared to baseline.
Figure 2.
Effects of continuous 7-day treatment with (+)-phenmetrazine (0.032–0.32 mg/kg/hr) on choice between cocaine and food. Other details as in Fig. 1.
Effects of methamphetamine treatment on cocaine choice
Fig. 3 (upper left panel) shows that methamphetamine did not significantly alter the cocaine choice dose-effect curve or cocaine ED50 values (Table 1). There were also individual differences in methamphetamine effects on cocaine choice, with two monkeys displaying dose-dependent rightward shifts in the cocaine choice curve and increases in cocaine choice ED50 values and two monkeys displaying dose-dependent leftward shifts in the cocaine choice curve and decreases in cocaine choice ED50 values (Fig. 7). Repeated-measures two-way ANOVA demonstrated a significant effect of cocaine dose (F4,12=29.8, p<0.001), no significant effect of methamphetamine treatment and a significant cocaine dose and methamphetamine treatment interaction (F12,36=3.2, p<0.01). Post-hoc analysis revealed that treatment with 0.1/hr methamphetamine significantly decreased choice of the 0.032 mg/kg/inj cocaine dose. Figure 3 (lower left panel) also shows the number of total, food and cocaine choices completed during the session. Repeated-measures one-way ANOVA demonstrated a significant main effect of methamphetamine treatment on total choices (F3,9=5.7, p<0.05), but not cocaine or food choices. Post-hoc analysis revealed that treatment with 0.1 mg/kg/h methamphetamine significantly (p< 0.05) decreased total choices compared to baseline. For cocaine intake (lower right panel), repeated-measures one-way ANOVA demonstrated a significant main effect of methamphetamine treatment (F3,9=4.8, p<0.05) and post-hoc analysis revealed that treatment with 0.1 mg/kg/h methamphetamine significantly decreased cocaine intake.
Figure 3.
Effects of continuous 7-day treatment with (+)-methamphetamine (0.032–0.1 mg/kg/hr) on choice between cocaine and food. Other details as in Fig. 1.
Effects of napthylisopropylamine treatment on cocaine choice
Fig. 4 (upper left panel) shows that napthylisopropylamine did not significantly alter the cocaine choice dose-effect curve or cocaine ED50 values (Table 1, Fig. 7). Repeated measures two-way ANOVA revealed a significant effect of cocaine dose (F4,4=667.0, p<0.001), but no significant effect of napthylisopropylamine treatment and no significant cocaine dose and napthylisopropylamine treatment interaction. Figure 4 (lower left panel) also shows the number of total, food and cocaine choices completed during the session. Repeated measures one-way ANOVAs demonstrated no significant main effect of napthylisopropylamine treatment on total, food, or cocaine choices. For cocaine intake (lower right panel), repeated measures one-way ANOVA demonstrated no significant main effect of napthylisopropylamine treatment. Higher doses of napthylisopropylamine were not tested because the highest dose of 1.0 mg/kg/h produced robust rate-decreasing effects and overt sedation in two monkeys.
Figure 4.
Effects of continuous 7-day treatment with naphthylisopropylamine (0.1–1.0 mg/kg/hr) on choice between cocaine and food. Other details as in Fig. 1, except that 1.0 mg/kg/hr napthylisopropylamine was tested in only three monkeys.
Effects of fenfluramine treatment on cocaine choice
Fig. 5 (upper left panel) shows that fenfluramine did not significantly alter the cocaine choice dose-effect curve or cocaine ED50 values (Table 1; Fig. 7). Repeated measures two-way ANOVA revealed a significant effect of cocaine dose (F4,12=28.9, p<0.001), but no significant effect of fenfluramine treatment and no significant cocaine dose and fenfluramine treatment interaction. Figure 5 (lower left panel) also shows the number of total, food and cocaine choices completed during the session. Repeated measures one-way ANOVAs demonstrated no significant main effect of fenfluramine treatment on total, food choices or cocaine choices. For cocaine intake (lower right panel), repeated measures one-way ANOVA demonstrated no significant main effect of fenfluramine treatment. Higher doses of fenfluramine were not tested because the highest dose of 0.32 mg/kg/h produced robust rate-decreasing effects in two monkeys.
Figure 5.
Effects of continuous 7-day treatment with (±)-fenfluramine (0.1–0.32 mg/kg/hr) on choice between cocaine and food. Other details as in Fig. 1.
Effects of removing cocaine or food availability
Fig. 6 (upper panels) shows that removing cocaine availability decreased choice of cocaine-associated stimuli, whereas removing food availability increased cocaine choice, producing a leftward shift in the cocaine choice dose-effect curve and a significant decrease in the cocaine ED50 value (Table 1). Removing food availability also decreased the number of choices completed during the first two components of test sessions, when no food and little or no cocaine (0.0032 mg/kg/inj) were available. Repeated measures two-way ANOVA revealed a significant effect of cocaine dose (F4,12=57.1, p<0.001) and environmental manipulation (F2,6=44.5, p<0.001) and a significant cocaine dose and environmental manipulation interaction (F8,23=10.0, p<0.001). Post-hoc analysis revealed that removing cocaine availability significantly decreased choice of the 0.032 and 0.1 mg/kg/inj cocaine doses and removing food availability significantly increased choice of the 0.0032 and 0.1 mg/kg/inj cocaine doses. Figure 6 (bottom left panel) shows the number of total, food and cocaine choices completed during the session. Repeated measures one-way ANOVAs demonstrated a significant main effect of treatment condition on total choices (F2,6=24.0, p<0.01), cocaine choices (F2,6=26.7, p<0.01), and food choices (F2,6=89.6, p<0.001). Post-hoc analysis revealed that removing cocaine availability significantly decreased choice of cocaine-associated stimuli and significantly increased food choices with no significant effect on total choices. In contrast, removing food availability significantly decreased total choices and choice of food-associated stimuli while producing a significant increase in cocaine choices.
Effects of continuous monoamine releaser treatment on total, food and cocaine choices
Because of the individual differences observed during monoamine releaser treatment, we examined the effects of each monoamine releaser on total, food and cocaine choices based on efficacy to produce a rightward shift in the cocaine choice dose-effect curve, rather than drug dose as in figures 1–5. Specifically, drug doses were selected based on individual results as the highest dose that produced a rightward shift in the cocaine choice dose-effect curve without producing more than a 20% decrease in the total number of choices compared to baseline (Fig. 8). This criterion for total choices was to select treatment doses that did not disrupt overall responding and may be representative of a potential side effect. If no dose produced a rightward shift in the cocaine choice dose-effect curve in an individual monkey, the highest dose that did not produce more than a 20% decreasing in total choices was then selected. For m-fluoroamphetamine, the doses represented are 0.032 mg/kg/h (n=1), 0.1 mg/kg/h (n=2) and 0.32 mg/kg/h (n=1). For phenmetrazine, the doses represented are 0.032 mg/kg/h (n=2), 0.1 mg/kg/h (n=1) and 0.32 mg/kg/h (n=1). For methamphetamine, the doses represented are 0.032 mg/kg/h (n=2) and 0.056 mg/kg/h (n=2). For napthylisopropylamine, the doses represented are 0.032 mg/kg/h (n=1) and 0.32 mg/kg/h (n=3). For fenfluramine, the doses represented are 0.1 mg/kg/h (n=2) and 0.32 mg/kg/h (n=2). Paired t-tests were conducted to compare each treatment to baseline conditions within each experimental endpoint. Removing cocaine availability significantly increased the number of food choices. Treatment with m-fluoroamphetamine and phenmetrazine trended (p = 0.09) towards a significant increase in food choices. The number of cocaine choices was significantly reduced by removing cocaine availability and by treatment with m-fluoroamphetamine and phenmetrazine. Methamphetamine, napthylisopropylamine and fenfluramine did not significantly alter either food or cocaine choices.
Figure 8.
Effects of experimental manipulations on total, food and cocaine choices. Doses of each monoamine releaser were selected based on individual data as described in Results. Horizontal axis: Experimental endpoint. Vertical axis: Number of choices per session. Each bar shows mean±SEM from a group of 4 monkeys, except ”baseline,” which shows data from all 8 monkeys. Asterisk indicates significantly (p< 0.05) different from baseline within an experimental endpoint.
DISCUSSION
The present study compared effects of a range of monoamine releasers on choice between cocaine and food by rhesus monkeys. There were three main findings. First, efficacy to decrease cocaine choice correlated with pharmacological selectivity to release DA/NE vs. 5HT. Thus, continuous 7-day treatment with the DA/NE-selective monoamine releasers m-fluoroamphetamine and (+)-phenmetrazine produced significant decreases in cocaine choice and trended towards a reciprocal increase in food choice in rhesus monkeys. Conversely, compounds with lower selectivity to release DA/NE vs. 5HT (methamphetamine, napthylisopropylamine, fenfluramine) produced only non-selective decreases in responding without significantly affecting the allocation of choice between cocaine and food. Second, none of the monoamine releasers produced as robust a reallocation of behavior as substituting saline for cocaine. These findings suggest that even the most effective monoamine releasers did not eliminate the reinforcing effects of cocaine. Finally, there was concordance between effects of most drugs in the present study, which used a concurrent choice schedule of cocaine and food availability, and effects of the same drugs in previous studies that used a multiple, second-order schedule of sequential cocaine and food availability (Negus et al., 2007, 2009). However, the efficacies of phenmetrazine and methamphetamine to reduce cocaine choice were less than was predicted based on their selectivities to reduce cocaine- vs. food-maintained responding under the multiple schedule. These findings suggest that schedule conditions may influence medication effects on cocaine self-administration in preclinical studies and that caution should be exerted in extrapolating from results using any one schedule.
Baseline cocaine choice and effects of reinforcer magnitude
Consistent with numerous studies from our laboratory and others using nonhuman primates, cocaine maintained a dose-dependent increase in choice of cocaine vs. food (Nader and Woolverton, 1992; Negus, 2003; Czoty et al., 2005a; Bergman, 2008; Banks and Negus, 2010). The impact of reinforcer magnitude on drug vs. food choice in the present study was also examined by eliminating food or cocaine delivery while retaining presentation of the food- or cocaine-associated stimuli. As expected, elimination of food delivery produced a leftward shift in the cocaine choice dose-effect curve and a greater than 5-fold increase in the potency of cocaine to maintain responding on the cocaine-associated key. In contrast, eliminating cocaine delivery decreased cocaine-associated choice and increased food choice. These results confirm the sensitivity of the procedure to experimental manipulations that alter the consequences of responding on the cocaine- and food-associated keys and provide a framework for interpreting effects of monoamine releasers.
Effects of monoamine releasers on choice between cocaine and food
The present study evaluated cocaine vs. food choice during maintenance on monoamine releasers selected to represent points along a continuum of DA/NE-selectivity to 5HT-selectivity, and the efficacy of these releasers to decrease cocaine choice correlated with their pharmacological selectivity to release DA/NE vs. 5HT. These results confirm and extend previous findings that maintenance on DA/NE-selective monoamine releasers decreases cocaine self-administration across a broad range of conditions. For example, treatment with the highly DA/NE-selective releaser d-amphetamine for periods ranging from 3–28 consecutive days produced selective and sustained decreases in cocaine self-administration under concurrent choice and other schedules of reinforcement in rodents, nonhuman primates and humans (Mello and Negus, 1996; Negus, 2003; Negus and Mello, 2003a,b; Chiodo et al., 2008; Czoty et al., 2010; Greenwald et al., 2010; Rush et al., 2010). These preclinical results with d-amphetamine agree with clinical evidence for the efficacy of amphetamine treatment to reduce cocaine use in placebo-controlled, double-blind clinical trials (Grabowski et al., 2001). Together, these preclinical and clinical findings have been interpreted to suggest that maintenance on DA/NE-selective monoamine releasers may selectively decrease the reinforcing effects of cocaine and be useful in treating cocaine abuse (Herin et al., 2010). However, in the present study, even the highly selective DA/NE-selective releaser m-fluoroamphetamine failed to reduce cocaine choice and increase food choice to the same degree as eliminating cocaine availability. This suggests that maintenance on monoamine releaser up to doses that produce nonselective behavioral disruption may reduce but not eliminate the reinforcing effects of cocaine.
A particular focus of the present study was the degree to which monoamine releaser effects on cocaine self-administration under the present concurrent schedule of cocaine and food availability might correspond to effects reported previously on cocaine self-administration maintained in rhesus monkeys under a multiple schedule of sequential cocaine and food availability (Negus et al., 2007, 2009). In particular, we hypothesized that medication efficacy to decrease cocaine vs. food choice under the concurrent schedule would be predicted by medication selectivity to decrease cocaine- vs. food-maintained responding under the multiple schedule. This hypothesis was supported only for releasers at the extremes of the continuum from DA/NE-selectivity to 5HT-selectivity. For example, seven-day treatment with the DA/NE-selective releaser m-fluoroamphetamine (a.k.a. PAL-353; 0.032–0.32 mg/kg/h) produced dose-dependent and relatively selective decreases in cocaine- vs. food-maintained responding under the multiple schedule, and these same doses of m-fluoroamphetamine dose-dependently decreased cocaine vs. food choice in the present study. Conversely, the nonselective DA/NE/5HT releaser napthylisopropylamine (a.k.a. PAL-287, 0.1–1.0 mg/kg/h) and the 5HT-selective releaser fenfluramine (0.1–1.0 mg/kg/h) produced nonselective decreases in cocaine- vs. food-maintained responding under the multiple schedule and also decreased overall responding without modifying cocaine choice under the choice schedule. Other manipulations have also produced concordant results across these two schedules of cocaine self-administration. For example, both removal of cocaine availability (Negus et al., 1995) and treatment with amphetamine (Negus, 2003; Negus and Mello, 2003b) selectively decreased cocaine self-administration under the multiple schedule and also decreased cocaine choice under the concurrent schedule. Conversely, treatment with the mu-opioid receptor agonist methadone non-selectively decreased cocaine- vs. food-maintained responding under the multiple schedule and also decreased overall responding without affecting cocaine choice under the concurrent schedule (Negus and Mello, 2004).
In comparison to results with the treatments described above, the effects of phenmetrazine and methamphetamine were less concordant across schedules of cocaine self-administration. Under the multiple schedule, phenmetrazine and methamphetamine produced more selective reductions in cocaine- vs. food-maintained responding than any other releaser tested, including m-fluoroamphetamine and amphetamine (Negus and Mello, 2003b; Negus et al., 2007, 2009). However, in the present study, phenmetrazine was no more effective than m-fluoroamphetamine in decreasing cocaine choice, and methamphetamine dose-dependently decreased cocaine choice in two monkeys but increased cocaine choice in two other monkeys. Thus, the efficacy of these releasers to reduce cocaine vs. food choice under the concurrent schedule was less then might have been expected from their selectivity to reduce cocaine- vs. food-maintained responding under the multiple schedule. Discrepant effects across the concurrent and multiple schedules have also been reported for other treatments. For example, the kappa opioid receptor agonist U50,488 and the dopamine receptor antagonist flupenthixol produced marginally selective decreases in cocaine self-administration maintained under the multiple schedule of cocaine- and food-maintained responding, but both compounds increased cocaine- vs. food-choice under the concurrent schedule (Negus et al., 1996, 1997; Negus, 2003, 2004). Taken together, these results argue against the hypothesis that medication effects on cocaine vs. food choice under this concurrent schedule can be reliably predicted from medication effects on sequential cocaine- vs. food-maintained responding under the multiple schedule. More generally, these results support the view that medication effects on responding for drug and nondrug reinforcers may be determined in part by the schedules of reinforcement.
Implications for medications development
Preclinical assays of drug self-administration provide one experimental tool used to assess candidate medications for the treatment of drug abuse (Mello and Negus, 1996), and concurrent schedules such as the one used in this study may be advantageous for reasons discussed in the Introduction. However, the ultimate utility of these procedures in medications development will depend in part on their utility for predicting medication effects on cocaine self-administration by humans. Translational predictions of this sort are complicated by a host of factors that include the potential for differences in schedules of cocaine delivery between preclinical studies, human laboratory studies and human clinical trials. Nonetheless, translational data do exist for some classes of compounds that suggest some predictive validity of medication effects in preclinical choice procedures. For example, as noted above, amphetamine maintenance decreased cocaine vs. food choice by rhesus monkeys (Negus, 2003), cocaine vs. money choice in human laboratory studies (Greenwald et al., 2010; Rush et al., 2010), and cocaine use by cocaine-dependent humans in clinical trials (Grabowski et al., 2001). Conversely, maintenance on kappa opioid agonists or dopamine receptor antagonists increased cocaine vs. food choice by rhesus monkeys (Woolverton and Balster, 1981; Negus, 2003, 2004), trended toward an increase in cocaine vs. money choice in human laboratory studies (Evans et al., 2001; Haney et al., 2001; Walsh et al., 2001) and either increased cocaine use or worsened treatment retention in clinical trials (Grabowski et al., 2000; Haney and Spealman, 2008). Of the drugs examined in the present study, only methamphetamine has been evaluated for its effects on cocaine use by humans (Mooney et al., 2009). In this controlled clinical trial, sustained-release methamphetamine decreased cocaine use and craving in eight of 25 patients who completed the trial; however, methamphetamine treatment did not improve treatment compliance relative to placebo, and most patients either violated the treatment protocol (8/25) or were lost to follow up (9/25). This clinical variability in response to methamphetamine may be related to the individual variability in responses to methamphetamine reported in this study, wherein methamphetamine reduced cocaine choice by two subjects, but increased cocaine choice in two other subjects. Further research will be required to assess the degree of predictive validity afforded by preclinical choice procedures, and determinants of individual variability in the valence and magnitude of medication effects also warrant further study.
ACKNOWLEDGEMENTS
The authors would like to thank Jennifer Gough for technical support of this study.
Footnotes
Conflicts of Interest and Source of Funding: The authors declare that this work was funded by National Institutes of Heath grants R01-DA26946, R01-DA012790 and T32-DA07027 from the National Institute on Drug Abuse, National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arnold JM, Roberts DCS. A Critique of Fixed and Progressive Ratio Schedules Used to Examine the Neural Substrates of Drug Reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Effects of Extended Cocaine Access and Cocaine Withdrawal on Choice Between Cocaine and Food in Rhesus Monkeys. Neuropsychopharmacology. 2010;35:493–504. doi: 10.1038/npp.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J. Medications for stimulant abuse: agonist-based strategies and preclinical evaluation of the mixed-action D-sub-2 partial agonist aripiprazole (Abilify) Exp Clin Psychopharmacol. 2008;16:475–483. doi: 10.1037/a0014398. [DOI] [PubMed] [Google Scholar]
- Chiodo K, Läck C, Roberts D. Cocaine self-administration reinforced on a progressive ratio schedule decreases with continuous D-amphetamine treatment in rats. Psychopharmacology. 2008;200:465–473. doi: 10.1007/s00213-008-1222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL. The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend. 2008;96:1–15. doi: 10.1016/j.drugalcdep.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther. 2005a;312:96–102. doi: 10.1124/jpet.104.073411. [DOI] [PubMed] [Google Scholar]
- Czoty PW, McCabe C, Nader MA. Effects of the 5-HT(1A) agonist (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behav Pharmacol. 2005b;16:187–191. doi: 10.1097/00008877-200505000-00008. [DOI] [PubMed] [Google Scholar]
- Czoty P, Martelle J, Nader M. Effects of chronic D-amphetamine administration on the reinforcing strength of cocaine in rhesus monkeys. Psychopharmacology. 2010;209:375–382. doi: 10.1007/s00213-010-1807-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Walsh SL, Levin FR, Foltin RW, Fischman MW, Bigelow GE. Effect of flupenthixol on subjective and cardiovascular responses to intravenous cocaine in humans. Drug Alcohol Depend. 2001;64:271–283. doi: 10.1016/s0376-8716(01)00129-6. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Silverman P, Schmitz JM, Stotts A, Creson D, Bailey R. Risperidone for the treatment of cocaine dependence: randomized, double-blind trial. J Clin Psychopharmacol. 2000;20:305–310. doi: 10.1097/00004714-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Sustained Release d-Amphetamine Reduces Cocaine but not /`Speedball/'-Seeking in Buprenorphine-Maintained Volunteers: A Test of Dual-Agonist Pharmacotherapy for Cocaine/Heroin Polydrug Abusers. Neuropsychopharmacology. 2010;35:2624–2637. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Wurster RM, Brady JV. Discrete-trial choice procedure: effects of naloxone and methadone on choice between food and heroin. Pharmacol Rev. 1975;27:357–365. [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008;199:403–419. doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology. 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann NY Acad Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J. Effects of oral methamphetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2009;101:34–41. doi: 10.1016/j.drugalcdep.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Woolverton WL. Choice between cocaine and food by rhesus monkeys: effects of conditions of food availability. Behav Pharmacol. 1992;3:635–638. [PubMed] [Google Scholar]
- Negus SS. Rapid Assessment of Choice between Cocaine and Food in Rhesus Monkeys: Effects of Environmental Manipulations and Treatment with d-Amphetamine and Flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- Negus SS, Banks ML. Making the right choice: lessons from drug discrimination for research on drug reinforcement and drug self-administration. In: Glennon RA, Young R, editors. Drug Discrimination: Applications to medicinal chemistry and drug studies. John Wiley and Sons; Holboken, NJ: 2011. pp. 361–388. [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d -amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic methadone treatment on cocaine- and food-maintained responding under second-order, progressive-ratio and concurrent-choice schedules in rhesus monkeys. Drug Alcohol Depend. 2004;74:297–309. doi: 10.1016/j.drugalcdep.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lukas SE, Mendelson JH. Diurnal patterns of cocaine and heroin self-administration in rhesus monkeys responding under a schedule of multiple daily sessions. Behav Pharmacol. 1995;6:763–775. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lamas X, Mendelson JH. Acute and chronic effects of flupenthixol on the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1996;278:879–890. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin C-E. Effects of Kappa Opioids on Cocaine Self-Administration by Rhesus Monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine Releasers with Varying Selectivity for Dopamine/Norepinephrine versus Serotonin Release as Candidate “Agonist” Medications for Cocaine Dependence: Studies in Assays of Cocaine Discrimination and Cocaine Self-Administration in Rhesus Monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective Suppression of Cocaine- versus Food-Maintained Responding by Monoamine Releasers in Rhesus Monkeys: Benzylpiperazine, (+)Phenmetrazine, and 4-Benzylpiperidine. J Pharmacol Exp Ther. 2009;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–159. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Fink-Jensen A, Woldbye D, Wörtwein G, Sager T, Holm R, Pepe L, Barak Caine S. Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology. 2008;201:43–53. doi: 10.1007/s00213-008-1245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci FJ. Can replacement therapy work in the treatment of cocaine dependence? And what are we replacing anyway? Addiction. 2007;102:1888–1889. doi: 10.1111/j.1360-0443.2007.02014.x. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- Woolverton WL, Balster RL. Effects of antipsychotic compounds in rhesus monkeys given a choice between cocaine and food. Drug Alcohol Depend. 1981;8:69–78. doi: 10.1016/0376-8716(81)90088-0. [DOI] [PubMed] [Google Scholar]