Abstract
PURPOSE
To assess the impact of geographic health services factors on the timely diagnosis of autism.
METHODS
Children residing in central North Carolina were identified by records-based surveillance as meeting a standardized case definition for autism. Individual-level geographic access to health services was measured by the density of providers likely to diagnose autism, distance to early intervention service agencies and medical schools, and residence within a Health Professional Shortage Area. We compared the presence of an autism diagnosis by age 8 and timing of first diagnosis across level of accessibility, using Poisson regression and Cox proportional hazards regression and adjusting for family and neighborhood characteristics.
RESULTS
Of 206 identified cases, 23% had no previous documented diagnosis of autism. Most adjusted estimates had confidence limits including the null. Point estimates across analyses suggested that younger age at diagnosis was found for areas with many neurologists and psychiatrists and proximal to a medical school but not areas with many primary care physicians or proximal to early intervention services agencies.
CONCLUSIONS
Further study of the distribution of medical specialists diagnosing autism may suggest interventions to promote the early diagnosis, and initiation of targeted services, for children with autism spectrum disorders.
Keywords: Autism, Diagnosis, Health Services Accessibility
INTRODUCTION
One in a hundred children in the United States has an autism spectrum disorder (ASD) (1). Intensive early intervention can improve outcomes for these children (2, 3). Although many of these children will receive intervention support services before an official diagnosis is made, autism-specific interventions may be delayed. Increasingly, evidence supports that the timing of an autism diagnosis is important. Younger age at autism diagnosis is one of the best-known predictors of functional outcome (4, 5). Unfortunately, timely diagnosis of autism is not the norm. A 2- to 4-year delay between a parent’s first expression of concern and an eventual autism diagnosis is a well-documented problem (2, 6, 7). Nearly 25% of children with autism may not be diagnosed until entering school (8) and some are missed entirely (9, 10).
Identifying factors to support the timely diagnosis of autism is of great public health importance. Earlier autism diagnosis has been consistently associated with more severe autism (8), male gender (10), greater family socioeconomic status (11), and white race (12), whereas factors that could be addressed with societal interventions are understudied. Urban residence, which may serve as a proxy for greater health care resources, has been found to be associated with earlier autism diagnoses (11) and with increased autism prevalence (13). A greater number of physicians and school-based health centers in a state have also been associated with higher state-wide administrative autism prevalence (14). However, the association between individual-level geographic access to health services and the timeliness of autism diagnoses has not been studied.
To address this important knowledge gap, we explored the impact of geographic access to health care services at birth on subsequent autism diagnoses, hypothesizing that closer proximity to autism-specific agencies and greater density of health providers would be associated with earlier autism diagnoses.
METHODS
Study Population and Autism Diagnosis
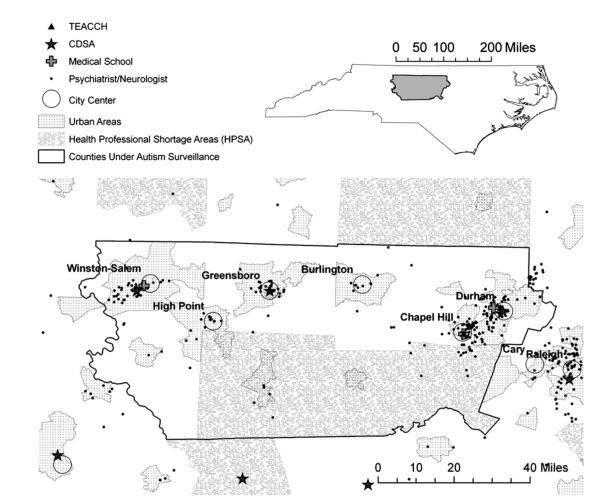
We constructed a population-based cohortof 206 children who resided in a defined area at birth and were determined to meet surveillance criteria for ASD at the age of 8 years by the Autism and Developmental Disabilities Monitoring Network in North Carolina (NC-ADDM). In brief, this active surveillance methodology relied on a review of all available developmental evaluation records from educational and health agencies for children with any developmental disability (9, 15). Trained clinician review staff evaluated the records of children meeting a minimal criteria suggesting that autism was possible and compared a child’s documented behavioral symptoms to standardized criteria for ASD based on the Diagnostic and Statistical Manual IV. Surveillance designation of ASD does not rely on the presence of a previous autism diagnosis. NC-ADDM surveillance required a child’s residence in the central North Carolina counties of Alamance, Chatham, Davidson, Durham, Forsyth, Guilford, Orange, and Randolph, surrounding the cities of Durham, Chapel Hill, Winston-Salem, High Point, and Greensboro at 8 years of age during 2002 and 2004 (Fig. 1). Participants in our study were also required to reside in the same eight-county region at the time of birth to obtain a corresponding in-state birth certificate and to assure inclusion of developmental records throughout the child’s life.
FIGURE 1.
Map showing locations of selected health services exposures in central North Carolina. Children with birth residence within counties under surveillance for autism, outlined in black, were included. Shown are urban areas, Health Professional Shortage Areas, North Carolina medical schools, and autism-specific agencies: Division for the Treatment and Education of Autistic and related Communication-handicapped Children (TEACCH) and North Carolina’s early intervention service agency, the Children’s Developmental Service Agencies (CDSA). Locations of specialist physicians (neurologists and psychiatrists) are shown. Psychologists and primary care physician locations had similar distributions.
Our outcome was the presence of a previous autism diagnosis by 8 years of age and the age in days at first autism diagnosis. An autism diagnosis was defined as documentation of (i) a diagnosis of ASD Not Otherwise Specified, Asperger’s disorder, Autistic Disorder, or Pervasive Developmental Disorder Not Otherwise Specified; (ii) an ICD-9 code of 299.0 (Autistic Disorder) or 299.8 (Other pervasive developmental disorder); or (iii) North Carolina public school designation of autism for the purposes of special education.
Variables pertaining to the child’s autism, such as the presence of comorbid intellectual disabilities and the degree of impairment, were obtained from the autism surveillance record. Other covariates were obtained from the child’s linked birth certificate record (e.g., maternal education and prenatal care) or by obtaining census block-group data corresponding to the child’s birth residence: median household income and urban residence.
Characterizing Health Services Accessibility
To explore the relative impact of different types of providers, services, and global indicators of health care accessibility, we constructed seven measures of geographic access that may be relevant to autism diagnosis:(i) densities of physician specialists (psychiatrists, pediatric psychiatrists, neurologists, and pediatric neurologists); (ii) doctoral-level psychologists (reporting a primary specialty in clinical, counseling, developmental, educational, or school psychology and not practicing in business/industry), who often make autism diagnoses (8); and (iii) density of primary care physicians (pediatricians and family or general practitioners) who often see children before referring them to specialists (11). These providers’ specialties and primary practice addresses from 1994 and 1996 were obtained from the North Carolina Health Professions Data System’s annual provider-report data from licensing boards (16). We measured proximity to publicly funded facilities known to diagnose autism in North Carolina: (iv) the Division of Treatment and Education of Autistic and related Communication handicapped Children (TEACCH) and (v) the Children’s Developmental Service Agencies (CDSA), North Carolina’s early intervention service agencies, and (vi) the nearest North Carolina medical school. We also determined whether the child’s residence was (vii) within a census tract that was federally designated as a geographic-level Health Professional Shortage Area (HPSA) for primary care physicians in the mid-1990s (17).
Ninety-nine percent of health services providers from the entire state of North Carolina were geocoded. Geocoding methods, in order of priority, were using the commercial services of Mapping Analytics (Rochester, NY; 55%), geocoding after finding more complete address information by the use of internet searches of the practice name (5%), using an adjacent year’s Health Professions Data System record (8%), assigning hospital and university addresses to the center of the university complex (6%), or assigning to a random point location within the zip code to prevent assigning providers on top of each other (25%).
Children’s residential addresses at birth from their North Carolina birth certificates were geocoded with ArcGIS 9.2 (Environmental Systems Research Institute, Redlands, CA) automatic criteria (78%) or after interactive review including verification with other data (16%). The remaining addresses were assigned to the zip+4 centroid (3%) or a postal delivery–weighted zip code centroid (3%). We also geocoded each child’s age 8 address obtained from surveillance records and compared this with the birth certificate address to determine whether a child had moved within the region.
We calculated individual-level accessibility measures using a geographic information system, including providers throughout the state of North Carolina. Density was measured as the count of providers within road network polygons constructed around each child’s residential address from the birth certificate, using a radii of 20 miles because this distance corresponds to the recommended catchment for pediatric medical care recommended by the Graduate Medical Education National Advisory Committee (18). Proximity involved identifying the closest facility and measuring the distance along the road network.
Statistical Analyses
We dichotomized health services accessibility exposures by using round numbers (e.g., 100 providers, 20 miles) that did not yield small cell sizes. Results from dichotomized exposures were similar to those in which we used several categories. We used Kaplan-Meier product limit estimators to generate survival curves and calculate median survival times and 95% confidence limits by category of exposure. We used Cox proportional hazards regression to estimate hazard ratios with the Efron method to handle ties in the age at diagnosis (19), including follow-up time until December 31 of their surveillance year for children without a previous diagnosis of ASD. We also estimated risk ratios of not being diagnosed across level of health services accessibility. To do this we dichotomized the outcome into having or not having a diagnosis at age 8 and using poisson regression with a robust variance estimator (20). We adjusted for a priori factors thought to be diagnostic determinants and to vary by residential location: child’s intellectual disabilities, race, maternal education, age, marital status, census block-group median household income, and urban residence.
To assess the impact of residential mobility and consequent exposure misclassification on our estimates, we performed a sub analysis restricted to the 83 children who lived in the same residence at birth and age 8. For this subgroup accessibility measures at birth would characterize accessibility at the time of autism recognition.
RESULTS
Of 206 surveillance-identified autism cases born in the region, 48(23%) had nodiagnosis of autism on record. Three children had a diagnosis for which the date could not be determined. The mean age at first diagnosis among those with a diagnosis was 57 months (median, 51; range, 21–100 months). Characteristics of the children in relation to the presence and timing of an autism diagnosis are shown in Table 1.
TABLE 1.
Child and family characteristics and age at and presence of an autism diagnosis among confirmed autism cases from NC-ADDM 2002 and 2004 born in the surveillance region
| Autism diagnosis |
||||
|---|---|---|---|---|
| n | %None | %Late (>52 months) | %Early (≤52 months) | |
| Child characteristics | ||||
| Sex | ||||
| Female | 27 | 22% | 44% | 33% |
| Male | 179 | 23% | 36% | 40% |
| Race | ||||
| Non-Hispanic white | 118 | 22% | 37% | 41% |
| Non-Hispanic black | 75 | 24% | 39% | 37% |
| Other | 12 | 33% | 25% | 42% |
| Developmental loss or plateau | ||||
| No | 160 | 28% | 43% | 29% |
| Yes | 46 | 9% | 17% | 74% |
| Intellectual Disabilities (Intelligence Quotient) | ||||
| None (71–) | 104 | 28% | 41% | 31% |
| Mild/moderate impairment (35–70) | 62 | 21% | 44% | 35% |
| Severe impairment (<35) | 34 | 12% | 15% | 74% |
| Degree of autism impairment | ||||
| Mild | 137 | 28% | 39% | 34% |
| Moderate/severe | 69 | 14% | 35% | 51% |
| Family Characteristics | ||||
| Maternal education | ||||
| <High school | 30 | 40% | 37% | 23% |
| High school degree | 104 | 25% | 38% | 38% |
| College degree | 71 | 14% | 38% | 48% |
| Married | ||||
| No | 60 | 30% | 43% | 27% |
| Yes | 146 | 21% | 3% | 45% |
| Maternal age, years | ||||
| 22 or younger | 37 | 41% | 30% | 30% |
| 23–34 | 141 | 22% | 38% | 40% |
| 35 or older | 28 | 7% | 43% | 50% |
| Prenatal care begun in first trimester | ||||
| No | 24 | 21% | 50% | 29% |
| Yes | 182 | 24% | 36% | 41% |
| Moved between birth and age 8 years | ||||
| No | 85 | 28% | 34% | 38% |
| Yes | 121 | 20% | 40% | 41% |
| Median household income, $/year | ||||
| ≤$30,000 | 50 | 34% | 36% | 30% |
| $30,000≤$40,000 | 45 | 18% | 38% | 44% |
| $40,000≤$50,000 | 52 | 25% | 37% | 38% |
| $50,000≤$60,000 | 34 | 21% | 26% | 53% |
| >$60,000 | 25 | 12% | 56% | 32% |
NC-ADDM = Autism and Developmental Disabilities Monitoring Network in North Carolina.
Health services were more concentrated near city centers and consequently many of the accessibility exposure measures were correlated (Fig. 1). Densities of individual primary care providers, specialists, and psychologists were especially similar, reflected by Spearman rank correlations (r) > 0.9. Density of specialist physicians and distance to the nearest medical school were highly concordant: both measures classified 112 children with better accessibility (e.g., more providers or closer proximity) and 91 with worse accessibility (e.g., fewer providers or further distance). Most children living in urban areas had better geographic access to providers, although a few urban areas had low provider densities (results not shown).
The median ages at diagnosis were 3–16 months earlier for children living in areas dense in specialist physicians and psychologists, proximal to a medical school, and not HPSA, and were 4–5 months later for children living in areas dense in primary care physicians or proximal to a TEACCH or CDSA facility (Table 2). However, in adjusted analyses hazard ratios and risk ratios were attenuated toward the null with confidence limits including the null.
TABLE 2.
Associations between health services accessibility measures at birth and age at and presence of an autism diagnosis among confirmed autism cases from NC-ADDM 2002 and 2004 born in the surveillance region
| Autism diagnosis |
Median age at diagnosis in months (95% CL) |
Hazard ratio of time to diagnosis (95% CL) |
Risk ratio of previous diagnosis (95% CL) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| %None |
%Late (>52 m) |
%Early (≤52m) |
Among all surveillance- recognized ASD |
Among Prior Diagnosis of ASD |
Unadjusted |
Adjusted* |
Adjusted*,† Nonmovers |
Adjusted* |
||
| n | n = 48 | n = 77 | n = 81 | n = 203 | n = 155 | n = 203 | n = 196 | n = 83 | n = 199 | |
| Number within 20 miles | ||||||||||
| Primary care physicians | ||||||||||
| 13 < 100 | 34 | 26% | 32% | 41% | 59 (50–89) | 50 (47–59) | Ref | Ref | Ref | Ref |
| 100–600 | 172 | 23% | 38% | 39% | 63 (55–74) | 52 (46–58) | 1.1 (0.7–1.7) | 1.0 (0.6–1.7) | 0.6 (0.2–1.9) | 1.0 (0.7–2.4) |
| Specialist physicians | ||||||||||
| 0 < 100 | 93 | 31% | 37% | 32% | 70 (55–86) | 53 (48– 59) | Ref | Ref | Ref | Ref |
| 100–424 | 113 | 17% | 38% | 45% | 59 (49–77) | 49 (43– 59) | 1.4 (1.0–1.9) | 1.2 (0.8–1.7) | 2.2 (1.1–4.4) | 1.1 (0.9–1.3) |
| Psychologists | ||||||||||
| 1 < 100 | 131 | 26% | 35% | 39% | 65 (53–77) | 50 (46–55) | Ref | Ref | Ref | Ref |
| 100–484 | 75 | 19% | 41% | 40% | 62 (52–81) | 53 (46–62) | 1.1 (0.8–1.6) | 0.9 (0.6– 1.3) | 1.2 (0.6– 2.4) | 1.0 (0.8–1.3) |
| Distance to the nearest | ||||||||||
| TEACCH | ||||||||||
| 20–101 miles | 73 | 23% | 32% | 45% | 59 (48–81) | 50 (44–62) | Ref | Ref | Ref | Ref |
| <20 miles | 133 | 23% | 41% | 36% | 64 (55–74) | 52 (46–56) | 1.0 (0.7–1.3) | 0.8 (0.6–1.2) | 0.4 (0.2–0.8) | 1.0 (0.8–1.1) |
| CDSA | ||||||||||
| 20–41 miles | 38 | 32% | 29% | 39% | 60 (49–94) | 49 (45–54) | Ref | Ref | Ref | Ref |
| <20 miles | 168 | 21% | 39% | 39% | 64 (55–74) | 52 (47–58) | 1.2 (0.8–1.9) | 1.4 (0.8–2.3) | 0.9 (0.3–2.4) | 1.2 (0.9–1.5) |
| Medical school | ||||||||||
| 20–90 miles | 92 | 33% | 37% | 30% | 73 (58, 89) | 53 (48–59) | Ref | Ref | Ref | Ref |
| <20 miles | 114 | 16% | 38% | 47% | 57 (49, 74) | 49 (43–58) | 1.5 (1.1–2.1) | 1.3 (0.9–1.9) | 2.5 (1.3–4.9) | 1.1 (0.9–1.4) |
| Residence within a | ||||||||||
| HPSA | ||||||||||
| Yes | 27 | 41% | 30% | 30% | 73 (53–NE‡) | 52 (47–54) | Ref | Ref | Ref | Ref |
| No | 179 | 21% | 39% | 41% | 62 (54–74) | 50 (47–58) | 1.6 (1.0–2.8) | 1.4 (0.8–2.5) | 0.9 (0.3–2.3) | 1.3 (1.0–1.8) |
ASD = autism spectrum disorder; CDSA = Children’s Developmental Service Agencies; CL = confidence limit; HPSA = Health Professional Shortage Areas; NC-ADDM = Autism and Developmental Disabilities Monitoring network in North Carolina; TEACCH = Division for the Treatment and Education of Autistic and related Communication-handicapped Children.
Adjusted models include race (non-Hispanic white, non-Hispanic black, other), maternal education (quadratic splines), maternal age (quadratic splines), marital status (married and not married), 100% urban census block group (yes, no), median block group household income (quadratic splines), and child’s intellectual disabilities (none, mild/moderate, severe). Cox regression models used to estimate hazard ratios also include a continuous time-interaction for child’s intellectual disabilities, as indicated by evaluations of the proportional hazards assumption.
These estimates were restricted to 83 children who did not change residential address between birth and age 8 years, for whom health services accessibility exposures calculated at birth would be the same throughout childhood.
95 percentile of the age at diagnosis estimated from Kaplan Meier curves was not estimable (NE).
Children from families that did not relocate compared with those that did had on average an earlier diagnosis with an ASD (Table 1), similar accessibility exposures, mothers that were slightly older, more educated, and more likely to be married, in less urban block groups with higher incomes (results not shown). Hazard ratios from models restricted to children who did not relocate between birth and age 8 years, for which exposure measures assigned at birth also pertained to the time of diagnosis, were imprecise and in some cases, exaggerated compared with estimates including all children (Table 2). For example, neurologist and psychiatrist density and proximity to a medical school, hazard ratios were larger, suggesting earlier diagnosis with better accessibility to these services.
DISCUSSION
We evaluated the associations between geographic access to health services and the timing of autism diagnoses among children with surveillance-identified autism by using a population-based design and including children meeting standardized autism criteria but with variability in the presence and timing of a prior diagnosis. We characterized geographic access at the individual level by using a geographic information system, which yields improved accuracy compared with aggregate measures (21). We focused on density measures, which have been shown to be related to health care utilization (22) and outcomes (23) and that may better capture the component of access termed availability that refers to the relationship between the volume of services and client need (24).
Estimates of association were likely attenuated by limitations of our exposure measures. Health services utilization data were not available and so our exposure measures were limited to geographic access, which can be thought of as potential access to services. We knew provider specialties but not whether they actually performed autism evaluations. The geographic accessibility measures themselves were subject to several sources of misclassification, especially residential mobility of families, which is high (25). Estimates limited to families that did not relocate, for which geographic exposure measures may be more relevant to the time of diagnosis, suggested that associations with neurologists, psychiatrists, and medical schools may hold. Alternately, estimates for these families may reflect selection factors: that residentially-stable families are unique in some way.
Residual confounding also likely influenced our observed associations. Although we controlled for maternal education, median household income, and urbanicity, these variables are imperfect proxies for the complex determinants of diagnosis. We lacked information on other ways in which families differ in their ability to recognize a child’s developmental problem or obtain care, such as having adequate health insurance.
Several of our measures of geographic access to health services were not associated with earlier autism diagnoses. It is possible that our study area was saturated with appropriate health services, limiting the range of variation of our exposures. Additional explanations include that parents of children with autism are highly motivated to obtain a diagnosis regardless of geographic barriers, or that autism diagnosis is influenced more by interpersonal networks than geographic location (26).
We lacked the sample size to generate statistical precision, but the pattern of results was consistent, suggesting that aspects of the geographic distribution of health services like residing in an area dense in neurologists and psychiatrists may support the timely diagnosis of ASD. Studies of the reasons why these measures of accessibility were slightly associated with earlier diagnoses may help in forming interventions to improve the timeliness of diagnosis. Our replication of the finding that almost one quarter of children meeting surveillance criteria for ASD have not received a diagnosis, and presumably autism-targeted services, by age 8 years, deserves highlighting. Studies to search for modifiable factors promoting earlier diagnoses for these children are urgently needed.
Acknowledgments
We thank the North Carolina schools and agencies that provided help and cooperation in the review of developmental records, Molly Wen for assistance using Network Analyst, Kristen Rappazzo for geocoding assistance, and Dr. Michelle Mayer for her contributions to this project prior to her death.
This research was supported in part by grants from the Centers for Disease Control and Prevention and the National Institute of Environmental Health Sciences (P30ES10126) and the National Institute of Environmental Health Sciences (T32 ES007018). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Selected Abbreviations and Acronyms
- ASD
autism spectrum disorder
- NC-ADDM
Autism and Developmental Disabilities Monitoring Network in North Carolina
- TEACCH
Division of Treatment and Education of Autistic and related Communication handicapped Children
- CDSA
Children’s Developmental Service Agencies
- HPSA
Health Professional Shortage Area
REFERENCES
- 1.Kogan MD, Blumberg SJ, Shieve LA, Boyle CA, Perrim JM, Ghandour RM, et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics. 2009;124:1395–1403. doi: 10.1542/peds.2009-1522. [DOI] [PubMed] [Google Scholar]
- 2.Bryson SE, Rogers SJ, Fombonne E. Autism spectrum disorders: Early detection, intervention, education, and psychopharmacological management. Can J Psychiatry. 2003;48:506–516. doi: 10.1177/070674370304800802. [DOI] [PubMed] [Google Scholar]
- 3.Volkmar F, Cook EH, Jr, Pomeroy J, Realmuto G, Tanguay P. Practice parameters for the assessment and treatment of children, adolescents, and adults with autism and other pervasive developmental disorders. American Academy of Child and Adolescent Psychiatry Working Group on Quality Issues. J Am Acad Child Adolesc Psychiatry. 1999;38(12 Suppl):32S–54S. doi: 10.1016/s0890-8567(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 4.Harris SL, Handleman JS. Age and IQ at intake as predictors of placement for young children with autism: A four- to six-year follow-up. J Autism Dev Disord. 2000;30:137–142. doi: 10.1023/a:1005459606120. [DOI] [PubMed] [Google Scholar]
- 5.Turner LM, Stone WL, Pozdol SL, Coonrod EE. Follow-up of children with autism spectrum disorders from age 2 to age 9. Autism. 2006;10:243–265. doi: 10.1177/1362361306063296. [DOI] [PubMed] [Google Scholar]
- 6.Siegel B, Pliner C, Eschler J, Elliott GR. How children with autism are diagnosed: Difficulties in identification of children with multiple developmental delays. J Dev Behav Pediatr. 1988;9:199–204. [PubMed] [Google Scholar]
- 7.Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH, Jr, Dawson G, et al. Practice parameter: Screening and diagnosis of autism: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- 8.Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. J Dev Behav Pediatr. 2006;27(2 Suppl):S79–S87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56:12–28. [PubMed] [Google Scholar]
- 10.Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, et al. Timing of identification among children with an autism spectrum disorder: Findings from a population-based surveillance study. J Am Acad Child Adolesc Psychiatry. 2009;48:474–483. doi: 10.1097/CHI.0b013e31819b3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;116:1480–1486. doi: 10.1542/peds.2005-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandell DS, Wiggins LD, Carpenter LA, Daniels J, DiGuiseppi C, Durkin MS, et al. Racial/ethnic disparities in the identification of children with autism spectrum disorders. Am J Public Health. 2009;99:493–498. doi: 10.2105/AJPH.2007.131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Arch Dis Child. 2006;91:8–15. doi: 10.1136/adc.2004.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandell DS, Palmer R. Differences among states in the identification of autistic spectrum disorders. Arch Pediatr Adolesc Med. 2005;159:266–269. doi: 10.1001/archpedi.159.3.266. [DOI] [PubMed] [Google Scholar]
- 15.Van Naarden Braun K, Pettygrove S, Daniels J, Miller L, Nicholas J, Baio J, et al. Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56:29–40. [PubMed] [Google Scholar]
- 16.North Carolina Health Professions Data System, maintained by the Cecil G. Sheps Center Available at: http://www.shepscenter.unc.edu/hp/ index.html.
- 17.Department of Health and Human Services Designation of medically underserved populations and health professional shortage areas: Proposed rule. 1998. pp. 46538–46555. [PubMed]
- 18.Ricketts TC, Savitz LA, Gesler WM, Osborne DN, editors. Geographic Methods for Health Services Research. University Press of America, Inc.; Lanham, MD: 1994. [Google Scholar]
- 19.Hertz-Picciotto I, Rockhill B. Validityand efficiency ofapproximationmethods for tied survival times in Cox regression. Biometrics. 1997;53:1151–1156. [PubMed] [Google Scholar]
- 20.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 21.Fortney J, Rost K, Warren J. Comparing alternative methods of measuring geographic access to health services. Health Services Outcomes Res Methodol. 2000;1:173–184. [Google Scholar]
- 22.Leonard C, Stordeur S, Roberfroid D. Association between physician density and health care consumption: A systematic review of the evidence. Health Policy. 2009;91:121–134. doi: 10.1016/j.healthpol.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Sarma S, Peddigrew C. The relationship between family physician density and health related outcomes: The Canadian evidence. Cah Sociol Demogr Med. 2008;48:61–105. [PubMed] [Google Scholar]
- 24.Penchansky R, Thomas JW. The concept of access: Definition and relationship to consumer satisfaction. Med Care. 1981;19:127–140. doi: 10.1097/00005650-198102000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Urayama KY, Von Behren J, Reynolds P, Hertz A, Does M, Buffler PA. Factors associated with residential mobility in children with leukemia: Implications for assigning exposures. Ann Epidemiol. 2009;19:834–840. doi: 10.1016/j.annepidem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu KY, King M, Bearman PS. Social influence and the autism epidemic. AJS. 2010;115:1387–1434. doi: 10.1086/651448. [DOI] [PMC free article] [PubMed] [Google Scholar]