Abstract
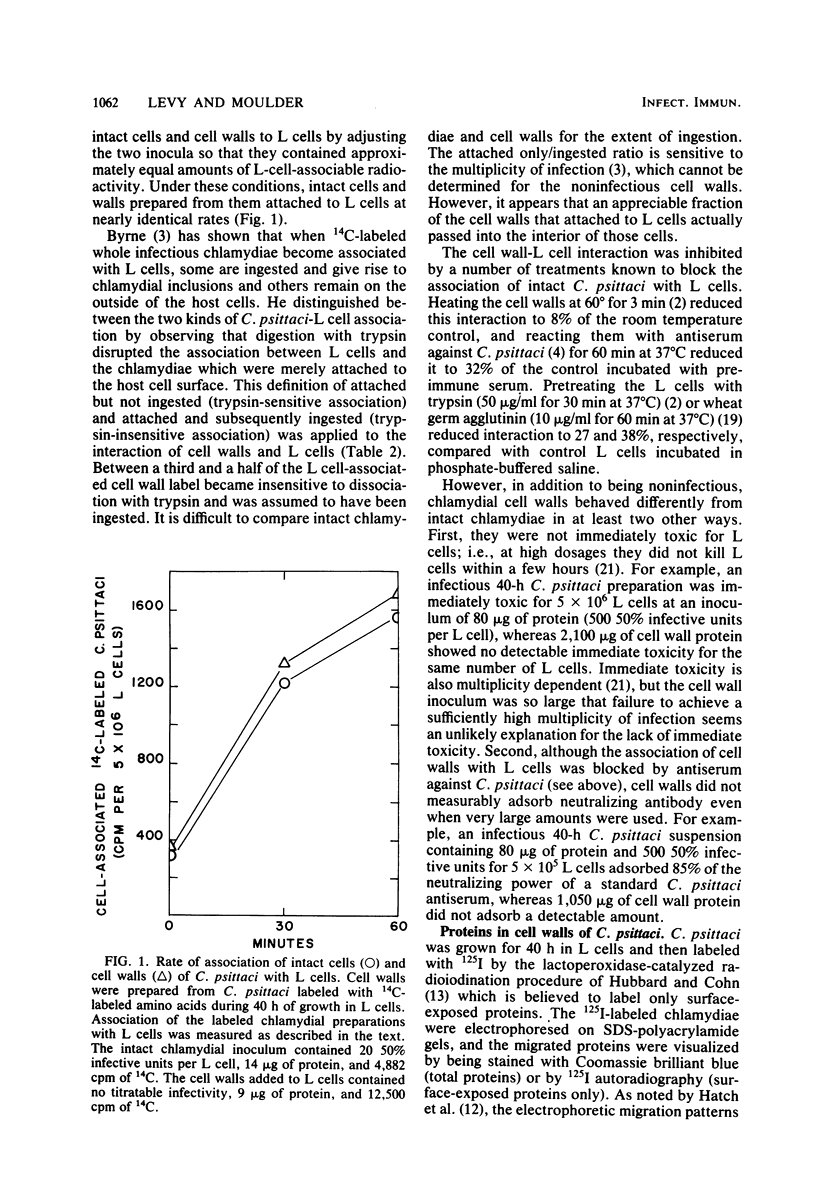
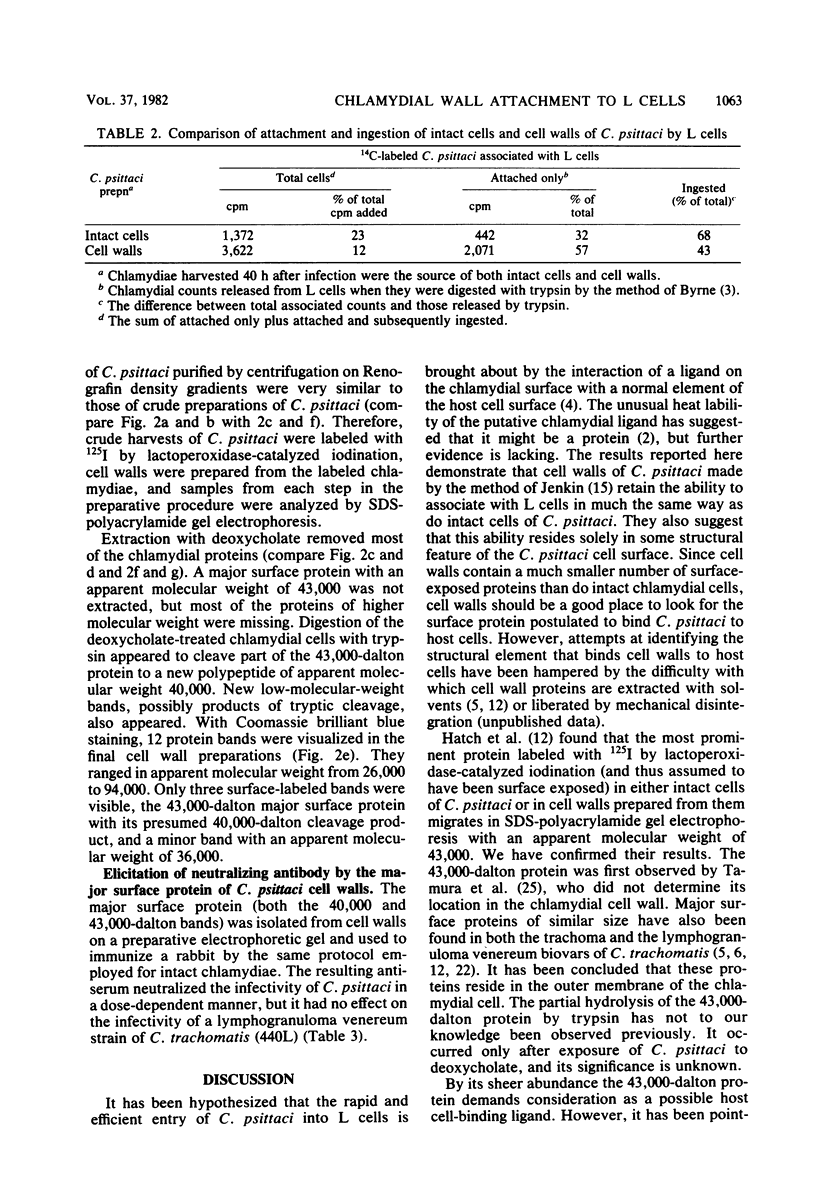
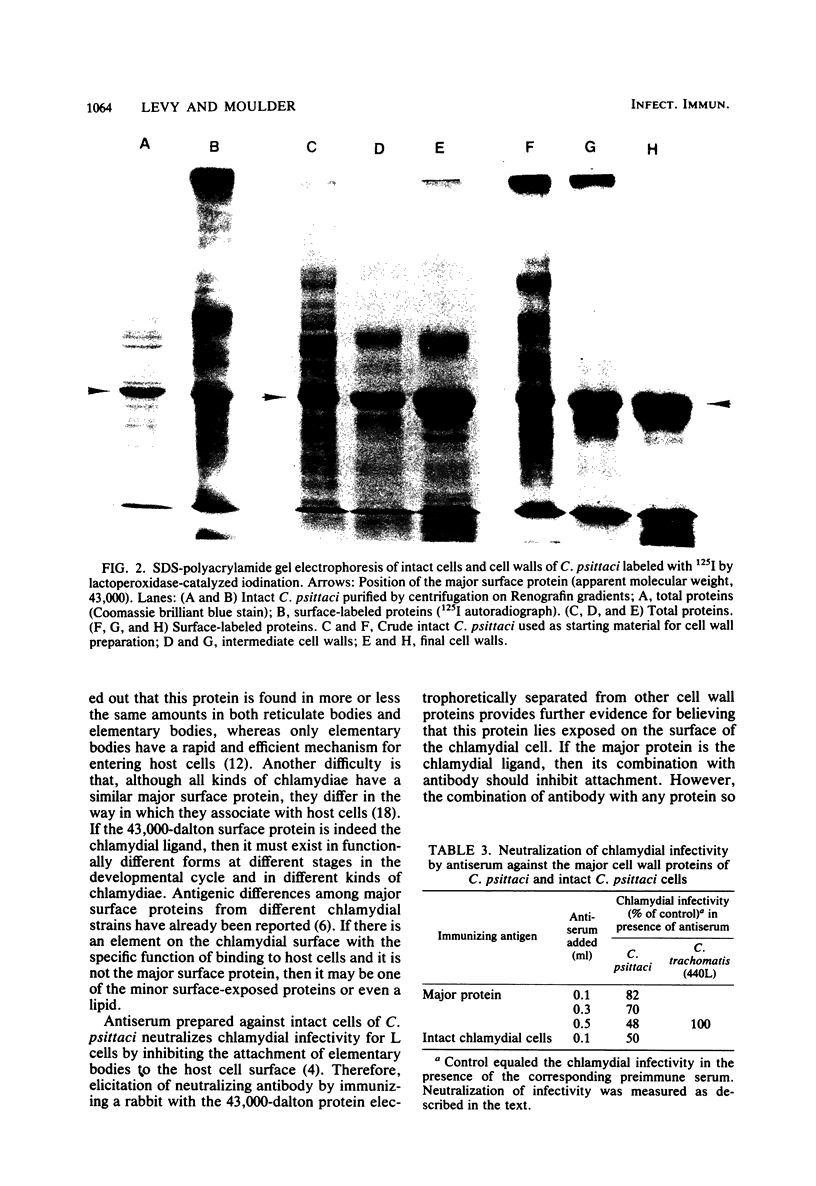
14C-labeled cell walls of the 6BC strain of Chlamydia psittaci, prepared from intrinsically labeled chlamydial cells by digestion with deoxycholate and trypsin, associated with mouse fibroblasts (L cells) in a manner comparable to that of intact C. psittaci. Almost half of the host cell-associated cell walls were not dissociated by trypsin, suggesting that they had been attached and then ingested. The attachment of cell walls to L cells was inhibited by a number of treatments known to block association of intact C. psittaci with L cells: heating the cell walls for 3 min or reacting them with antiserum against intact C. psittaci, or pretreating the L cells with trypsin or wheat germ agglutinin. Unlike intact cells of C. psittaci, cell walls were not immediately toxic for L cells, and they did not measurably adsorb neutralizing antibody. As revealed by making cell walls from intact C. psittaci labeled with 125I by lactoperoxidase-catalyzed iodination, cell walls contained a much smaller number of surface-labeled proteins than did whole chlamydial cells. The most abundant surface-labeled protein was one with an apparent molecular weight of 43,000. In the final step of cell wall preparation, tryptic digestion of deoxycholate-extracted cells, this major surface protein was partially cleaved to a 40,000-dalton product. When the major surface protein (both the 43,000- and 40,000-dalton moieties) was electrophoretically separated from the other cell wall proteins and used to immunize a rabbit, antibodies that neutralized the infectivity of intact C. psittaci were elicited. It was concluded that cell walls retain the ability to associate with L cells in much the same way as do intact cells of C. psittaci, but, despite the simpler structure of cell walls, the element that binds C. psittaci to host cells cannot yet be identified.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Kinetics of phagocytosis of Chlamydia psittaci by mouse fibroblasts (L cells): separation of the attachment and ingestion stages. Infect Immun. 1978 Feb;19(2):607–612. doi: 10.1128/iai.19.2.607-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Requirements for ingestion of Chlamydia psittaci by mouse fibroblasts (L cells). Infect Immun. 1976 Sep;14(3):645–651. doi: 10.1128/iai.14.3.645-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. T., Moulder J. W. Loss of inorganic ions from host cells infected with Chlamydia psittaci. Infect Immun. 1978 Mar;19(3):827–832. doi: 10.1128/iai.19.3.827-832.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory W. W., Byrne G. I., Gardner M., Moulder J. W. Cytochalasin B does not inhibit ingestion of Chlamydia psittaci by mouse fibroblasts (L cells) and mouse peritoneal macrophages. Infect Immun. 1979 Jul;25(1):463–466. doi: 10.1128/iai.25.1.463-466.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale T. L., Bonventre P. F. Shigella infection of Henle intestinal epithelial cells: role of the bacterium. Infect Immun. 1979 Jun;24(3):879–886. doi: 10.1128/iai.24.3.879-886.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P., Vance D. W., Jr, Al-Hossainy E. Identification of a major envelope protein in Chlamydia spp. J Bacteriol. 1981 Apr;146(1):426–429. doi: 10.1128/jb.146.1.426-429.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN H. M. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol. 1960 Nov;80:639–647. doi: 10.1128/jb.80.5.639-647.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg K. R., Horoschak K. D., Moulder J. W. Toxicity of low and moderate multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1977 Nov;18(2):531–541. doi: 10.1128/iai.18.2.531-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. K. Interaction between a trachoma strain of Chlamydia trachomatis and mouse fibroblasts (McCoy cells) in the absence of centrifugation. Infect Immun. 1981 Feb;31(2):584–591. doi: 10.1128/iai.31.2.584-591.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. J. Wheat germ agglutinin blockage of chlamydial attachment sites: antagonism by N-acetyl-D-glucosamine. Infect Immun. 1979 Sep;25(3):946–953. doi: 10.1128/iai.25.3.946-953.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Hatch T. P., Byrne G. I., Kellogg K. R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1976 Jul;14(1):277–289. doi: 10.1128/iai.14.1.277-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari S. H., Ward M. E. Polypeptide composition of Chlamydia trachomatis. J Gen Microbiol. 1981 Apr;123(2):197–207. doi: 10.1099/00221287-123-2-197. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Tamura A., Tanaka A., Manire G. P. Separation of the polypeptides of Chlamydia and its cell walls by polyacrylamide gel electrophoresis. J Bacteriol. 1974 Apr;118(1):139–143. doi: 10.1128/jb.118.1.139-143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]