Abstract
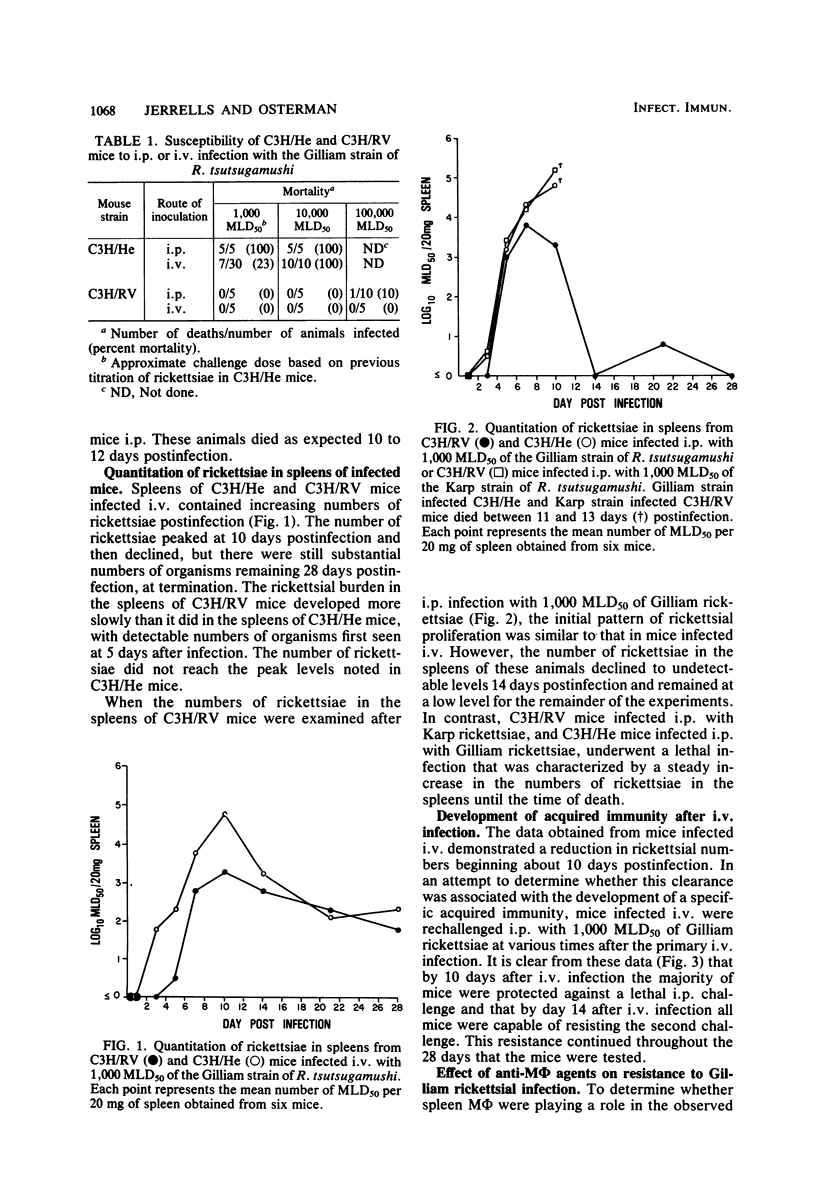
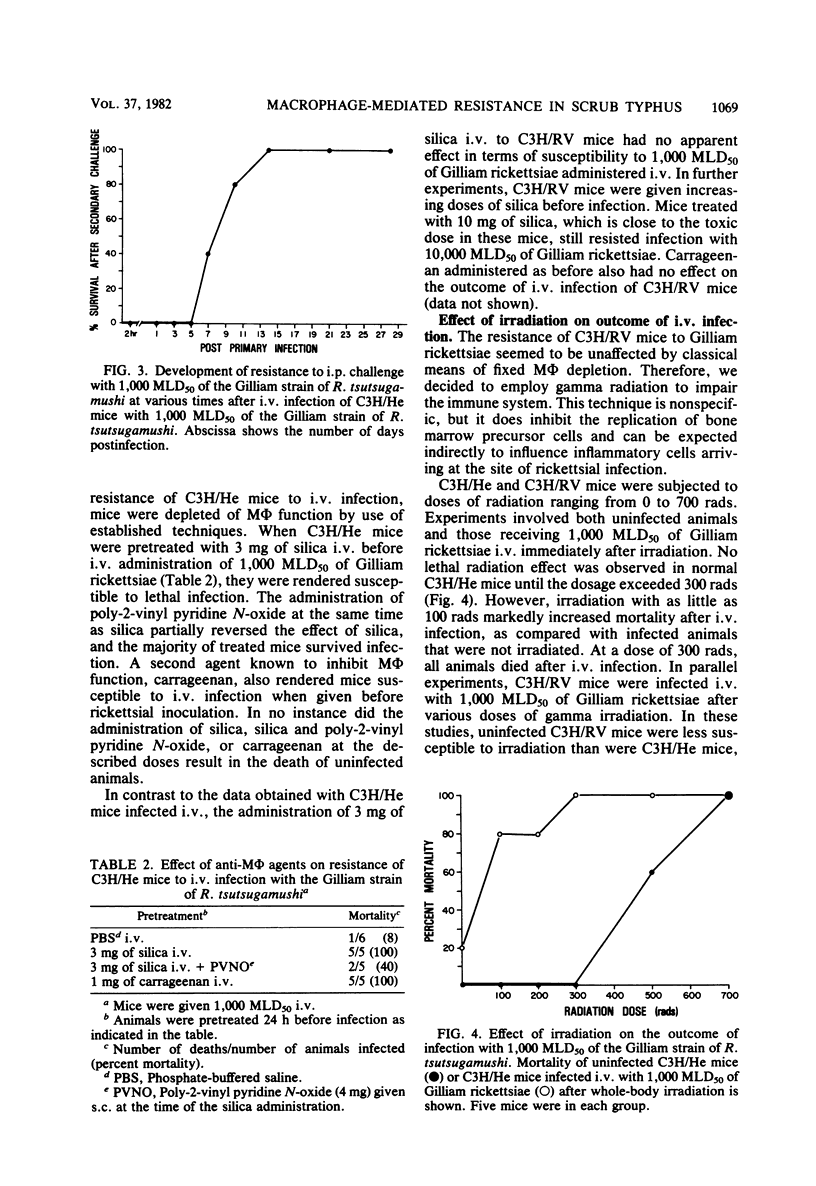
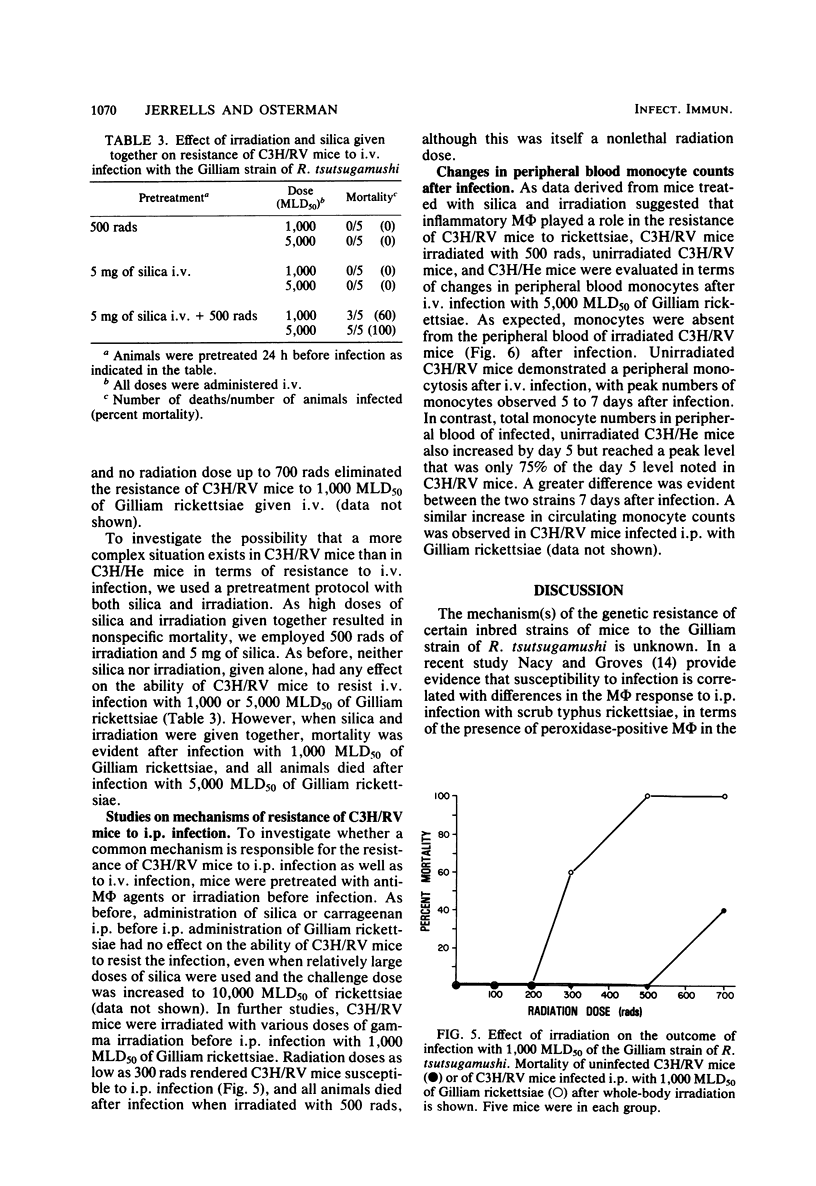
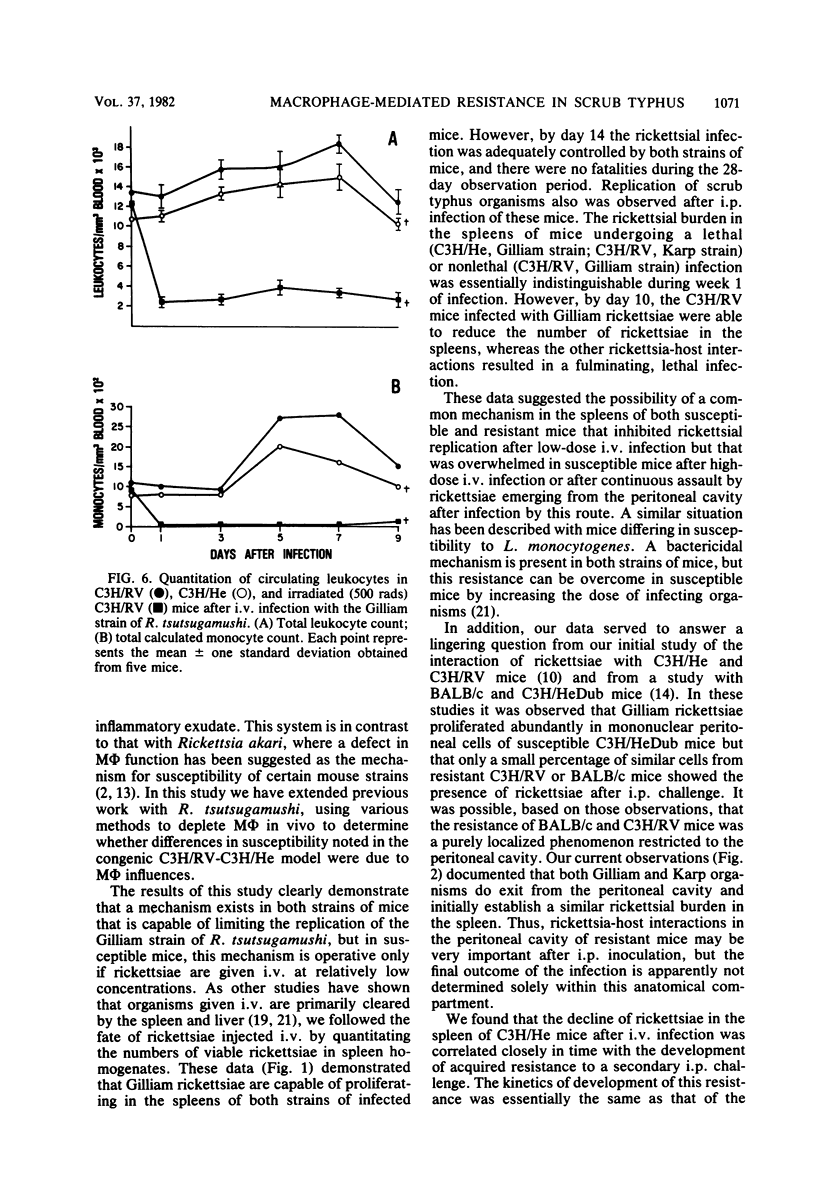
Mechanisms of innate resistance to infection with the Gilliam strain of Rickettsia tsutsugamushi were examined using congenic strains of mice resistant (C3H/RV) or susceptible (C3H/He) to intraperitoneal infection. Both strains of mice were resistant to infection with 1,000 50% mouse lethal doses of rickettsiae if given intravenously. In both systems rickettsial replication occurred after intravenous infection, as evidenced by an increase in rickettsial numbers in the spleens of infected animals, followed by a decrease in rickettsiae to low levels by day 14 postinfection. Administration of the antimacrophage agents silica and carrageenan to C3H/He mice intravenously rendered these animals susceptible to lethal infection. Neither irradiation nor silica given individually rendered C3H/RV mice susceptible to intravenous infection. However, if silica and irradiation were given together, a lethal infection occurred after intravenous infection. C3H/RV mice became susceptible to lethal infection after sublethal doses of irradiation only if they were infected intraperitoneally. Administration of silica or carrageenan had no effect on the outcome of intraperitoneal infection of these mice with Gilliam rickettsiae. These data suggest that both strains of mice share innate resistance mechanisms to intravenous infection that consist of fixed macrophages. Resistance of C3H/RV mice to intraperitoneal infection, in contrast, apparently was dependent only on an irradiation-sensitive process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Moore V. L., Stevens J. O. Strain variation in BCG-induced chronic pulmonary inflammation in mice. I. Basic model and possible genetic control by non-H-2 genes. J Immunol. 1977 Jul;119(1):343–347. [PubMed] [Google Scholar]
- Anderson G. W., Jr, Osterman J. V. Host defenses in experimental rickettsialpox: genetics of natural resistance to infection. Infect Immun. 1980 Apr;28(1):132–136. doi: 10.1128/iai.28.1.132-136.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C., Kongshavn P. A., Skamene E. Enhanced primary resistance to Listeria monocytogenes in T cell-deprived mice. Immunology. 1977 Apr;32(4):529–537. [PMC free article] [PubMed] [Google Scholar]
- Cheers C., McKenzie I. F. Resistance and susceptibility of mice to bacterial infection: genetics of listeriosis. Infect Immun. 1978 Mar;19(3):755–762. doi: 10.1128/iai.19.3.755-762.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Geiger B., Gallily R. Effect of X-irradiation on various functions of murine macrophages. Clin Exp Immunol. 1974 Apr;16(4):643–655. [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Osterman J. V. Host defenses in experimental scrub typhus: genetics of natural resistance to infection. Infect Immun. 1978 Feb;19(2):583–588. doi: 10.1128/iai.19.2.583-588.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves M. G., Rosenstreich D. L., Taylor B. A., Osterman J. V. Host defenses in experimental scrub typhus: mapping the gene that controls natural resistance in mice. J Immunol. 1980 Sep;125(3):1395–1399. [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: delayed-type hypersensitivity responses of inbred mice. Infect Immun. 1982 Jan;35(1):117–123. doi: 10.1128/iai.35.1.117-123.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: inflammatory response of congenic C3H mice differing at the Ric gene. Infect Immun. 1981 Mar;31(3):1014–1022. doi: 10.1128/iai.31.3.1014-1022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M. H., Wheelock E. F. Effects of intravenous silica on immune and non-immune functions of the murine host. J Immunol. 1975 Jul;115(1):41–48. [PubMed] [Google Scholar]
- Meltzer M. S., Nacy C. A. Macrophages in resistance to rickettsial infection: susceptibility to lethal effects of Rickettsia akari infection in mouse strains with defective macrophage function. Cell Immunol. 1980 Sep 1;54(2):487–490. doi: 10.1016/0008-8749(80)90229-4. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Groves M. G. Macrophages in resistance to rickettsial infections: early host defense mechanisms in experimental scrub typhus. Infect Immun. 1981 Mar;31(3):1239–1250. doi: 10.1128/iai.31.3.1239-1250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Osterman J. V. Host defenses in experimental scrub typhus: role of normal and activated macrophages. Infect Immun. 1979 Nov;26(2):744–750. doi: 10.1128/iai.26.2.744-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Formal S. B. Effect of silica on the innate resistance of inbred mice to Salmonella typhimurium infection. Infect Immun. 1979 Aug;25(2):513–520. doi: 10.1128/iai.25.2.513-520.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks S. C., Jr, Osterman J. V., Hetrick F. M. Plaque assay and cloning of scrub typhus rickettsiae in irradiated L-929 cells. J Clin Microbiol. 1977 Jul;6(1):76–80. doi: 10.1128/jcm.6.1.76-80.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Sadarangani C., Skamene E., Kongshavn P. A. Cellular basis for genetically determined enhanced resistance of certain mouse strains to listeriosis. Infect Immun. 1980 May;28(2):381–386. doi: 10.1128/iai.28.2.381-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. J., Fiset P., Wisseman C. L., Jr Simple, differential staining technique for enumerating rickettsiae in yolk sac, tissue culture extracts, or purified suspensions. J Clin Microbiol. 1979 Mar;9(3):437–440. doi: 10.1128/jcm.9.3.437-440.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A. Phenotypic expression of genetically controlled host resistance to Listeria monocytogenes. Infect Immun. 1979 Jul;25(1):345–351. doi: 10.1128/iai.25.1.345-351.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skamene E., Kongshavn P. A., Sachs D. H. Resistance to Listeria monocytogenes in mice: genetic control by genes that are not linked to the H-2 complex. J Infect Dis. 1979 Feb;139(2):228–231. doi: 10.1093/infdis/139.2.228. [DOI] [PubMed] [Google Scholar]
- VOLKMAN A., GOWANS J. L. THE ORIGIN OF MACROPHAGES FROM BONE MARROW IN THE RAT. Br J Exp Pathol. 1965 Feb;46:62–70. [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]



