Abstract
Patients with chronic obstructive pulmonary disease (COPD) present with a variety of symptoms and pathological consequences. Although primarily viewed as a respiratory disease, COPD has both pulmonary and extrapulmonary effects, which have an impact on many aspects of physical, emotional, and mental well-being. Traditional assessment of COPD relies heavily on measuring lung function, specifically forced expiratory volume in 1 second (FEV1). However, the evidence suggests that FEV1 is a relatively poor correlate of symptoms such as breathlessness and the impact of COPD on daily life. Furthermore, many consequences of the disease, including anxiety and depression and the ability to perform daily activities, can only be described and reported reliably by the patient. Thus, in order to provide a comprehensive view of the effects of interventions in clinical trials, it is essential that spirometry is accompanied by assessments using patient-reported outcome (PRO) instruments. We provide an overview of patient-reported outcome concepts in COPD, such as breathlessness, physical functioning, and health status, and evaluate the tools used for measuring these concepts. Particular attention is given to the newly developed instruments emerging in response to recent regulatory guidelines for the development and use of PROs in clinical trials. We conclude that although data from the development and validation of these new PRO instruments are emerging, to build the body of evidence that supports the use of a new instrument takes many years. Furthermore, new instruments do not necessarily have better discriminative or evaluative properties than older instruments. The development of new PRO tools, however, is crucial, not only to ensure that key COPD concepts are being reliably measured but also that the relevant treatment effects are being captured in clinical trials. In turn, this will help us to understand better the patient’s experience of the disease.
Keywords: patient-reported outcomes, chronic obstructive pulmonary disease, health-related quality of life, questionnaire development, dyspnea, exacerbations
Introduction
Chronic obstructive pulmonary disease (COPD) is a complex disease characterized by multiple symptoms that place a substantial burden on patients’ health and health care systems.1 The effectiveness of treatments in COPD has traditionally been measured by changes in forced expiratory volume in one second (FEV1).2 Spirometry has a central role in diagnosis, but it does not reliably reflect the burden of COPD on a patient’s health status. The change in FEV1 is only modestly associated with change in health status or other patient-reported outcomes (PROs),3–5 which may be a reflection of how individuals experience differing effects on health status, despite the same physiological limitations.5 Furthermore, other symptoms of COPD such as cough and extrapulmonary effects of the disease are not reflected by spirometry,6 the consequences of which may be better captured from the patient’s perspective. Cough and sputum production, for example, can have physical, emotional, and social effects on the patient,7 and skeletal muscle dysfunction, one of the extrapulmonary effects of the disease, can contribute significantly to impaired exercise capacity, in turn affecting a patient’s health status.8 Thus, in order to provide a comprehensive view of the effects of interventions in clinical trials, it is essential that spirometry be accompanied by assessments using PRO instruments.4,9
PROs are outcomes reported directly by patients, usually by self-administered questionnaire or diary. In this way, they capture the individual’s experiences of COPD without any interpretation from third parties, such as health care providers. Occasionally, proxies such as relatives can provide information on patient outcomes when a patient’s cognition or health is severely impaired. However, such responses should be interpreted with care, given that proxy reporting is susceptible to underestimation or overestimation of health status impairment, compared with the responses of patients themselves.10–12
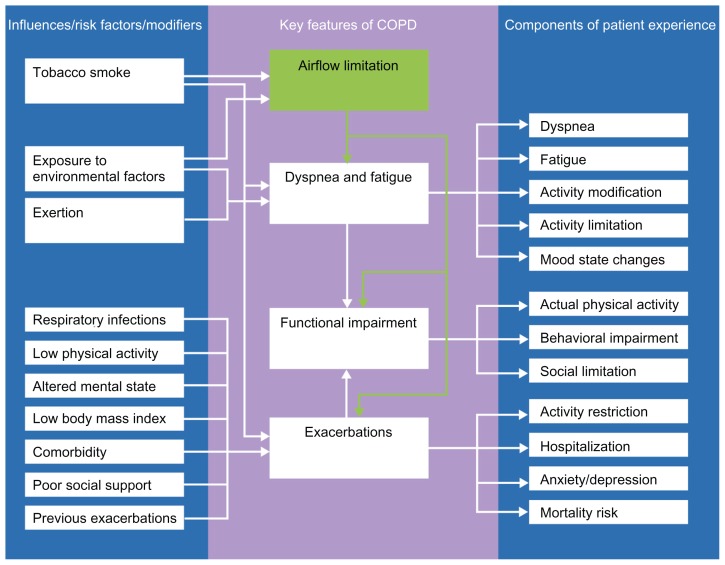
PROs present the patient perspective, quantifying the extent to which the physiological effects of the disease impact on health and functioning. Key concepts in understanding the impact of COPD from the patient’s perspective include breathlessness, fatigue, cough and sputum production, physical functioning, social functioning, and exacerbations (Figure 1), given that these features of the disease often have the greatest impact on patients’ lives.1 This literature review was undertaken to provide an overview of PRO concepts in COPD and the instruments used to evaluate these concepts. The literature was searched to retrieve articles describing tools for measuring outcomes in COPD and the development and use of instruments and PRO measures for assessing symptoms, health status, functioning, and quality of life. Due to the wide scope of the literature and large number of possible search terms, a fully systematic approach was not employed and search terms were not prespecified. Articles were included in the review based on the results of ad hoc searches of the PubMed database conducted during May and June 2011, the authors’ knowledge of the literature, and from the reference lists of retrieved articles.
Figure 1.
Conceptual model of chronic obstructive pulmonary disease and the patient’s experiences of the key features of the condition.103–107
Development and use of PRO instruments
There are a number of tools available for capturing PRO data. Newly developed tools address the relevant concepts and aspects of patients’ lives beyond measuring FEV1 and have been developed to measure treatment benefit in a way that will satisfy regulatory requirements.
In order to capture the effects of therapies that are relevant to patients with COPD, PRO instruments need to be fit for purpose, valid, reliable, and responsive to clinically meaningful treatment effects, understandable to patients and physicians with easily interpreted scoring systems, relevant to health care providers, and acceptable to regulatory authorities. PRO tools that fulfil these criteria may help to advance drug development by increasing our understanding of the efficacy and safety of new therapies. Recent guidance on the development and use of PRO instruments has been prepared by the US Food and Drug Administration (FDA),13,14 which represents the FDA’s current thinking on the use of PRO instruments to support labeling claims for medical products. The guidelines describe the characteristics of a PRO instrument that the FDA will look for to determine the adequacy of the instrument to support the claims made in product labeling where PRO data are being used. According to these new guidelines, for a PRO instrument to be acceptable to regulatory authorities, there must be evidence that there was patient input during item generation and testing, and that the instrument reliably measures the concept of interest in the target population. Consideration will be given to the conceptual framework underlying the design of the instrument and its measurement properties (reliability, validity, and ability to detect change), which should be well established prior to enrolling patients into confirmatory clinical trials of new drugs.
Many of the existing PRO tools available for use in COPD patients were developed before this regulatory guidance was introduced. PRO tools that predate the FDA PRO guidance13 sometimes lack a clear, well defined conceptual framework, or may lack the qualitative foundation of items generated from patient interviews. However, this should be set against the existence of a well documented body of literature detailing the validity, reliability, and responsiveness of such instruments that may have been built up over many years of use. The lack of standardized and accepted PRO tools for assessing the different aspects of COPD can hinder accurate evaluation of the efficacy of new therapies. New PRO instruments are therefore being designed to tackle concepts in COPD that have not previously been evaluable. Several of these are being developed in large-scale collaborations, such as the European Innovative Medicines Initiative PROactive tools and EXAcerbations of COPD Tool (EXACT).15–17 These initiatives aim to improve PRO development and evaluation through cooperation between experts from the pharmaceutical industry, academia, and regulatory authorities.
Other PRO instruments are also in development for the evaluation of specific concepts and to support claims about benefits with a particular treatment. These include the Shortness of Breath with Daily Activity questionnaire (SOBDA), and the Capacity of Daily Living during the Morning (CDLM) questionnaire.18,19
Attributes of effective PRO instruments that are fit for purpose
A PRO can be regarded as a latent construct in the sense that it is not directly measurable but relies upon indicators that can be quantified, such as a patient’s self-reported symptoms.20 How well items in a PRO tool contribute to the concept being measured can be assessed using Rasch modeling,21 which is recognized as an effective application of modern psychometric testing for the development of new PRO instruments. This mathematical modeling tests how well an instrument conforms to a unidimensional model, and ensures that these instruments perform equally for all respondents, a concept termed “invariance”.22 An important function of Rasch analysis is the transformation of an ordinal raw score (ie, patient responses) into a linear, interval-level variable, ultimately producing a linear scale (eg, 0–100).20 The Rasch model can also be used to compare the quality of fit of items measured individually and when tested together.23 The quality of fit to a Rasch unidimensional model will determine whether the instrument has true interval scaling properties; in other words, whether the instrument behaves like a ruler, against which all patients can be measured (by the same standard). When a disease-specific instrument has true interval scaling properties, it may be sensitive to small treatment effects across a broad range of disease severity.2
COPD is a multifactorial disease, with symptoms and structural changes both in the lungs and elsewhere in the body. Even within the lungs, there are several pathological processes, which may be present in different degrees in different patients and, therefore, there is no single or composite summary measure of impaired lung function.3 A PRO instrument, such as a health status questionnaire, provides a means of aggregating into a single score the cumulative effect of the various pathophysiological processes occurring in different organs and systems. It provides an estimate of all of the effects of the disease on the patient. These global outcomes have advantages over specific outcomes that measure just one aspect of the disease, such as FEV1 or depression, in that they give an overall picture of the impact of the disease or response to therapy.24 This feature may be particularly useful when a treatment has multiple beneficial effects, which individually may be too small to register as a change on an assessment of an individual parameter, but collectively may produce noticeable improvement.
PRO instruments for key COPD concepts
Breathlessness
Breathlessness is the symptom most frequently reported by patients with COPD.25 Persistent breathlessness impairs patients’ health-related quality of life, leads to disability and causes patients to make considerable lifestyle adjustments.26,27 Alleviating breathlessness is therefore a primary goal of COPD therapy.1 The most commonly used PRO instruments for assessing breathlessness include the Medical Research Council dyspnea scale,28 the Modified Borg Scale,29 and the Transition Dyspnea Index.30 However, these instruments were developed prior to the FDA guidelines and are unlikely to satisfy the new FDA requirements as stand-alone outcome measures to substantiate claims made in product labeling. Briefly, the Medical Research Council scale was introduced over 50 years ago for patients with chronic bronchitis and comprises a set of five statements about levels of breathlessness during daily activities. Patients select the statement that most closely corresponds to their level of impairment. It is simple to perform, has predictive validity, and correlates well with clinical and pulmonary parameters, but shows poor responsiveness to intervention.31 Originally developed in the 1980s, the Modified Borg scale is a ten-point scale in which patients simply select a point on the scale that matches their perception of their dyspnea. The Modified Borg instrument is easy to perform, can be administered during exercise, and is responsive to intervention, but correlates less well than the Medical Research Council scale with other outcomes.32 The Transition Dyspnea Index was developed in 1984 using data from patients with COPD, asthma, and interstitial fibrosis.30 It is a validated tool that measures changes in the severity of dyspnea in three categories, ie, functional impairment, magnitude of task, and magnitude of effort. It is sensitive to intervention and was originally designed as a physician interview with the patient, but a self-administered computerized (SAC) version of the Transition Dyspnea Index became available in 2004. The SAC Transition Dyspnea Index provides a responsive measure of the severity of breathlessness and avoids any interviewer interpretation; results are collected and analyzed electronically on a continuous scale.33 Comparison studies in patients with COPD have shown that the SAC Transition Dyspnea Index produces data similar to those obtained with the original interviewer-led Transition Dyspnea Index in terms of intensity of breathlessness and response to therapy, and is more responsive to therapy than the Medical Research Council scale.33,34 Other less frequently used breathlessness scales include the Chronic Respiratory Disease Questionnaire (CRQ)-dyspnea component and the University of California San Diego Shortness of Breath Questionnaire (UCSD-SOBQ).35,36 The CRQ-dyspnea component evaluates shortness of breath on a scale of 1–7 and allows patients to identify activities important to them that are restricted by breathlessness. However, as each individial is selecting their own unique list of activities that make them breathless, it is not standardized and direct comparisons cannot be made between patients.37 The current UCSD-SOBQ rates self-reported breathlessness during activities of daily living for 24 activities, and is the result of several modifications of a questionnaire originally described in 1987. The instrument has been shown to be a reliable and valid tool when used to assess dyspnea associated with activities of daily living;36 however, while it has found extensive use in pulmonary rehabilitation, it is largely a research instrument.38
Newly developed PRO tools for assessing breathlessness include the SOBDA questionnaire, the Global Chest Symptoms Questionnaire (GCSQ), the Dyspnea-12, and the Dyspnea Management Questionnaire Computer Adaptive Test (DMQ-CAT).39
Shortness of Breath with Daily Activities questionnaire
The SOBDA is a unidimensional 13-item instrument currently being developed with input from COPD patients and clinical experts for use as a primary or secondary efficacy endpoint in clinical trials.19 It is self-administered via an electronic daily diary, with a weekly average score providing the most representative measure of dyspnea. Preliminary studies indicate that it is reliable and valid for measuring breathlessness with daily activity in COPD patients. Responsiveness to intervention and responder threshold is yet to be confirmed, and relationships between SOBDA scores and other concepts and measures have yet to be elucidated.
Global Chest Symptoms Questionnaire
The GCSQ is a new, validated, and responsive self-administered PRO tool developed to evaluate morning symptoms in patients with COPD.18 The GCSQ shows good-to-high reliability and significant correlation with symptoms, use of rescue medication, and health-related quality of life. The instrument is able to discriminate differences in health-related quality of life between patients, but is insensitive to differences in disease severity. The GCSQ score is significantly responsive to changes with treatment, and the minimally important difference is estimated to be a change of 0.15 points. Unfortunately, the GCSQ was developed using interviews from only a small number of patients with severe COPD and may not fully meet regulatory requirements.
Dyspnea-12
This short instrument for use in COPD, interstitial lung disease, and heart failure was developed in 2009 using patient consultation and a systematic literature review, with subsequent use of Rasch modeling to refine the selection of items. It uses a novel approach based upon descriptions of breathlessness and has good measurement properties when used in its target diseases and asthma.40,41 The minimally important difference is yet to be established.
Dyspnea Management Questionnaire Computer Adaptive Test
The DMQ-CAT, is a multidimensional instrument using a computer adaptive test approach to dyspnea assessment. As a modified version of the Dyspnea Management Questionnaire (DMQ), with an expanded bank of 100 items, it has been shown to capture reliably and validly the four distinct dyspnea domains measured in the DMQ, ie, dyspnea intensity, dyspnea-related anxiety, activity avoidance, and activity self-efficacy.39 However, further studies testing the responsiveness of the DMQ-CAT in detecting dyspnea change after COPD treatment are required.
Physical functioning
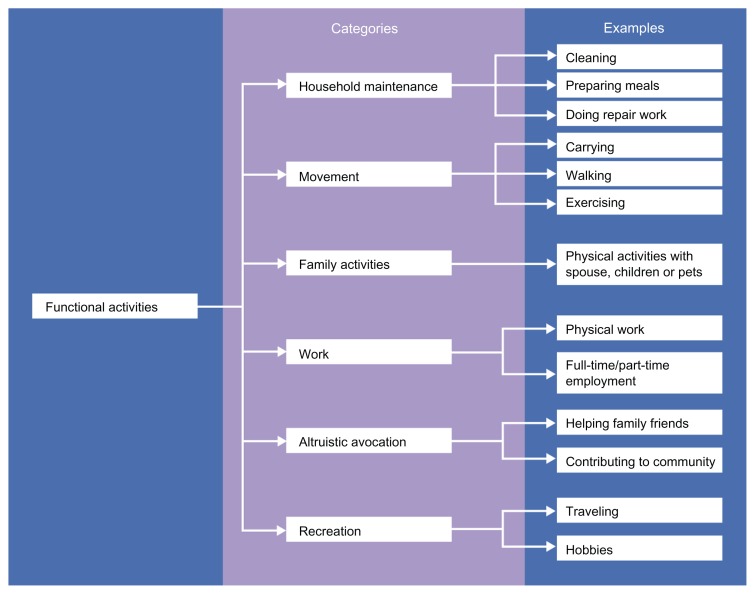
COPD causes considerable impairment of patients’ physical functioning, leading to limitations in their ability to perform daily activities, such as personal care, physical exercise, and attendance at social events.42,43 The most common complaint of patients with COPD is that their condition prevents them from completing their favorite activities.25 Because physical functioning is a broad concept, it must be assessed using a multidomain complex PRO tool in order for the effects of therapies to be interpreted in a clinically meaningful manner.13 The multidimensional nature of physical function in patients with COPD is illustrated in Figure 2.
Figure 2.
Categories and examples of functional activities that are valued by patients.48
A number of subjective instruments that aim to quantify the amount and intensity of physical activity in daily life have been used or adapted for use in patients with COPD, such as Follick’s Diary44 and the Minnesota Leisure Time Physical Activity Questionnaire,45 and have been reviewed by Pitta et al.46 These instruments have not been thoroughly studied in COPD patients and generally lack evidence of validity, reliability, and responsiveness in this population.46,47 In addition, the suitability of many PRO tools measuring physical functioning in the primary care setting has been found to be suboptimal.47
Available PRO tools for measuring physical functioning in COPD do not always provide sufficient coverage of the concepts under investigation.48,49 New PRO tools are being developed to address the unmet need for evaluating physical functioning in COPD patients.
PROactive tools
The European Innovative Medicines Initiative PROactive project is being undertaken by a consortium of 19 partners, comprising academic institutions, a small-to-medium sized enterprise, patient organizations, and eight major pharmaceutical companies, and is partly funded by the European Commission.16 With formal input from the European Medicines Agency and the FDA, European Innovative Medicines Initiative PROactive aims to provide a new PRO instrument for measuring physical activity in COPD that is patient-driven, valid, reliable, and sensitive to change with interventions. Developed from patient interviews, the draft questionnaire includes items evaluating the amount of physical activity, symptoms experienced during physical activity, and physical adaptations that the patient needs to make to cope with the activity. The project is due to be completed in 2014.
The Capacity of Daily Living during the Morning questionnaire
The CDLM questionnaire is a validated PRO tool for patients with COPD, and was developed in 2010 to assess their ability to carry out morning activities, which are particularly bothersome for these patients.18 With this instrument, patients rate their ability to perform different morning activities on a five-point Likert-type scale. Clinical data indicate that the CDLM questionnaire is reliable and responsive to therapy, but is unable to discriminate disease severity. Furthermore, methodological issues, such as the fact that it was developed using interviews from only a small group of patients with severe COPD, may prevent this tool from meeting regulatory requirements.
London Chest Activity of Daily Living questionnaire
The London Chest Activity of Daily Living questionnaire is a short questionnaire designed to assess 15 core activities of daily living in patients with COPD.50 During the development of this questionnaire over 10 years ago, which involved interviews with patients with severe COPD and a literature review, items normally present in other instruments were specifically excluded because they showed poor retest reliability, and no association with perception of global health or activities that were not limited in the majority of patients. The London Chest Activity of Daily Living questionnaire shows moderate-to-good correlations with other PRO instruments, such as the St George’s Respiratory Questionnaire (SGRQ) and the Hospital Anxiety and Depression scale, and has demonstrated high internal consistency.50
Exacerbations
Exacerbations are a hallmark of COPD and have a substantial and often sustained detrimental impact on patients’ health status and quality of life.51,52 Reducing the frequency and severity of exacerbations is an important goal of COPD management and in reducing resource utilization.1,53 The unpredictability of exacerbations contributes to the burden of disease for COPD patients and increases fear. Moreover, fear, anxiety, and depression are all associated with poor adherence or noncompliance with medical treatment, an increased rate of exacerbations, more frequent readmissions to hospital, and higher COPD mortality.54–56 Until recently, there was no standardized PRO instrument for assessing COPD exacerbations.
EXAcerbations of Chronic pulmonary disease Tool
EXACT is a 14-item daily electronic diary designed to measure the severity, frequency, and duration of acute exacerbations in clinical trials of patients with COPD and/or chronic bronchitis. It has been developed by the EXACT-PRO initiative, which is a collaboration between research and clinical specialists in COPD, instrument development experts, and FDA representatives.57–59 Initial testing of EXACT in an observational study of patients with COPD has indicated that it is a reliable, valid, and sensitive tool, with good internal consistency.15 Results of FDA and European Medicines Agency qualification reviews of PRO instruments are expected in 2012.
Health status and quality of life
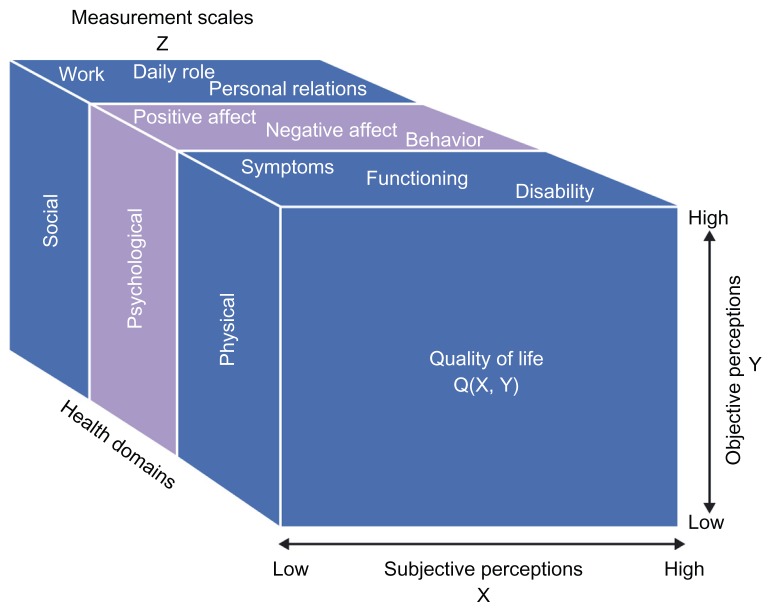
Improving health status is an important goal of COPD management.1,60 Health status and health-related quality of life are terms that are used rather interchangeably, which is not unreasonable given that they both tap into the same patient-reported symptoms and impacts. However, there is an argument for using the term “health status” for standardized measurement of the impact of the disease, since such instruments should be valid for every patient to whom they may be administered. The term health-related quality of life may be better reserved for describing clinical outcome as experienced by the patient, and health status as the marker used to measure that outcome.2 Disease-specific health status questionnaires have been shown to discriminate between different levels of COPD severity.12,61,62 Because health status is a multidimensional concept (Figure 3),63 it requires evaluation with a multidomain PRO instrument to provide useful information about the effects of a treatment.
Figure 3.
Conceptual scheme of domains and variables involved in the assessment of quality of life. © 1996, Massachusetts Medical Society. Reproduced with permission from Testa MA, Simonson DC. Current concepts: assessment of quality-of-life outcomes. N Engl J Med. 1996;334(13):835–840.108
There are numerous PRO instruments designed for evaluating health status in patients with respiratory conditions, the most commonly used being the SGRQ and the CRQ. Furthermore, several PRO tools aimed specifically at assessing health status in patients with COPD have been introduced. These tools take into account aspects of the disease that are most clinically relevant to patients with COPD and which may not be measurable by other methods. They aim to optimize the sensitivity to changes in health status in response to clinical intervention.
St George’s Respiratory Questionnaire for patients with COPD
The SGRQ is a self-administered questionnaire that measures health status in patients with chronic airflow limitation. It contains 50 items, a total score ranging from 0 (perfect health) to 100 (most severe status), and three component scores encompassing symptoms, activity, and impacts. There was significant patient input into its development over 20 years ago because each item response is weighted using explicit patient-derived weights.64,65 The SGRQ has been shown to be reproducible and valid in its ability to detect important changes over time.3,61 It has been used in many trials and has demonstrated responsiveness to pharmacological therapy within 6–8 weeks,66 and was able to identify treatment effects maintained over 3–4 years.67,68 Introduced in 2007, the COPD version of the SGRQ (SGRQ-C) is a revised and slightly shorter version specifically for COPD that produces scores directly analogous to those from the original.69 It was revised using Rasch analysis of responses from COPD patients and validated using data from the original validation study. The SGRQ has been used in ECLIPSE, a large 3-year biomarker study,70 and has been shown to be responsive to therapy.71 A very wide range of translations is available for both versions at http://www.healthstatus.sgul.ac.uk.
Chronic Respiratory Questionnaire
The CRQ is a validated and reliable tool, available as both an interviewer-led and a self-administered questionnaire. Originally developed in 1987 based on the responses of 100 patients with COPD, the questionnaire includes 20 items across four domains, namely dyspnea, fatigue, emotional function, and mastery.72,73 Patients rate their experiences with COPD on a seven-point Likert-type scale, ranging from 1 (maximum impairment) to 7 (no impairment). It is responsive to changes within individuals, but is not suitable for comparisons across populations. It may also lack sensitivity in patients with minor symptoms.74 This widely used questionnaire is available in several languages.75–78
COPD Assessment Test
The COPD Assessment Test (CAT) was developed in 2009 to FDA standards using Rasch modeling of data from over 1500 COPD patients to measure the impact of COPD on health status and aid patient-physician communication, whilst overcoming the obstacle of having to perform a lengthy or complex questionnaire in limited clinic time.79 It is short and simple, comprising eight items that cover a broad range of impacts on COPD patients. Despite its brevity, it provides a reliable measure of COPD severity and can be used routinely. The CAT demonstrates good internal consistency and true interval scaling properties, ensuring that it is relevant for global use, and correlates well with the SGRQ.79–81 It has been shown to be reliable across six European countries.81 Responsiveness to pulmonary rehabilitation has been demonstrated,80 and it has been shown to be responsive to recovery from an exacerbation.82 A very wide range of language versions is available at http://www.CATestonline.org.
Clinical COPD Questionnaire
The Clinical COPD Questionnaire (CCQ) is a short, easy-to-use tool developed in 2003 with both patient and clinical input primarily for assessing health status in the primary care setting, but is also useful for measuring response to intervention in clinical trials and for assessing clinical improvement following smoking cessation.83 It is a self-administered instrument that measures the clinical status of the airways, physical impairment, and emotional dysfunction. The CCQ has shown good reliability, validity, and responsiveness at the group and individual levels.47 The CCQ has also been shown to identify patients at risk of COPD (Global Initiative for Obstructive Lung Disease stage 0).83 This questionnaire is available in 64 different languages at http://www.ccq.nl.
Living with COPD questionnaire
The Living with COPD (LCOPD) questionnaire is a PRO tool designed to assess the patient’s perspective of the overall impact of COPD on their daily life.84 Drafted using the findings from qualitative interviews and focus groups, the questionnaire was refined using Rasch and traditional psychometric analyses to a 22-item instrument. The LCOPD has demonstrated good scaling properties and warrants further investigation to determine its value in evaluating treatment response.
Health authority requirements
Some existing health status tools may not be deemed adequate for determining treatment effects in clinical trials by regulatory authorities, because they were developed before guidance on PRO instrument development and use became available, and they vary widely in the number and type of concepts and items measured.85 However, the FDA has recently allowed presentation of SGRQ clinical trial data in the package insert for indacaterol.
Other concepts of interest
There are several other concepts that are important for COPD outcomes, such as cough, sleep disturbance and fatigue. Hence, the availability of PRO tools designed to measure these concepts may help to elucidate further specific treatment effects.
Cough
Cough is reported to be the exacerbation symptom with the greatest impact on patients’ well-being.25 The Cough Severity Diary is a simple, seven-item PRO instrument in development for use in clinical trials to capture the effects of treatment on cough severity from the patient’s perspective.86 Preliminary testing in 39 patients with chronic or subacute cough shows that the Cough Severity Diary has good correlation with validation instruments and warrants further investigation.86 The Leicester Cough Questionnaire is a self-administered instrument for evaluating health-related quality of life in patients with chronic cough.87 Introduced in 2003, it comprises 19 items with a seven-point Likert-type response scale and takes less than 5 minutes to complete. Patients with chronic cough were involved with item generation and reduction, it has been well validated, and has been shown to be repeatable and responsive to change.87,88 Recent data have indicated that the minimally important difference for the Leicester Cough Questionnaire is a score of 2.5.88
Sleep problems
Patients with COPD frequently experience sleep disturbance, which is often associated with breathlessness.89 It has been shown that “sleep difficulties” is the third most frequently reported symptom of COPD (after dyspnea and fatigue), occurring “almost always” or “always” in 43% of patients.90 An estimated 50% of COPD patients experience sleep problems, which can have significant adverse effects on physical and emotional functioning.91 COPD has been shown to result in problems with initiating and maintaining sleep, excessive daytime sleepiness, altered sleep architecture (especially increased arousals), reduced total sleep time and decreased sleep efficiency.92 In addition, many patients with COPD also have obstructive sleep apnea/hypopnea syndrome.93 However, despite the high frequency of sleep problems and the impact on patients, there is currently a lack of COPD-specific tools for the assessment of sleep in patients with COPD.
Fatigue
Fatigue is a common symptom of COPD and has a detrimental impact on many aspects of patients’ health status.94,95 The Manchester COPD Fatigue Scale is a 27-item questionnaire created in 2009 to address this symptom, which is not well represented in other symptom or health status PRO tools.96 Developed and refined according to patients’ responses to a 57-item pilot scale, it has shown good correlation with validation instruments and correlates well with health status and dyspnea, but its responsiveness to intervention is not yet known.
Symptom severity
Respiratory symptoms are a defining characteristic of COPD but there are no PRO instruments available for assessing changes in their daily severity in response to treatment in clinical trials. The EXACT-Respiratory Symptom (EXACT-RS) is a scoring algorithm for a subset of 11 items within the EXACT to quantify the severity of respiratory symptoms of COPD with three subscale scores, assessing breathlessness, cough and sputum, and chest symptoms. Developed in 2011 using qualitative data from COPD patients, this instrument shows high reliability and validity, allowing evaluation of variation in symptom severity over time.97 Treatment responsiveness and scoring interpretation for the EXACT-RS are yet to be determined.
Work productivity
The Work Productivity and Activity Impairment questionnaire (WPAI) is a well validated instrument for measuring impairments in work and activities and has been adapted for several diseases, including ankylosing spondylitis, gastroesophageal reflux disease, and irritable bowel syndrome.98–100 The COPD version of the instrument (WPAI-COPD) is a seven-item questionnaire that examines the limitations COPD places on patients’ ability to undertake work and daily activities. In a study comparing five different self-administered health-related quality of life questionnaires, the WPAI-COPD was rated “acceptable”, “easy”, or “very easy” to use by 84% of patients with COPD.101 Although the WPAI is not widely used in patients with COPD and appears to be underutilized, a recent international survey incorporated items from this questionnaire to help investigate the impact of COPD on a working-age cohort.102
Conclusion
COPD is a multifactorial disease, characterized by a variety of pulmonary and extrapulmonary changes, which impact upon several aspects of a patient’s life. Although lung function is an essential component of the diagnostic work-up for COPD and an appropriate marker for some aspects of improvement in response to intervention, a more comprehensive approach to evaluating the disease is called for. PROs are now recognized as a crucial element in the assessment of COPD, both for determining the impact of the disease itself and also for evaluating the success or failure of therapeutic interventions. The importance of evaluating the impact of COPD in all aspects of patient’s perceived physical, emotional and mental health, and the responsiveness of these elements to clinical intervention is underlined by new regulatory guidelines that specify strict criteria in the use of PROs to support labeling claims.
Health status questionnaires, such as the SGRQ and the CRQ, provide a comprehensive assessment of the overall effect of the disease and have been well tested in a variety of clinical settings and populations. They are known to be responsive to a wide range of therapeutic interventions, and can provide an overall measure of the response to treatment. Total scores, such as those obtained with the SGRQ, are “black box” measurements and provide little or no information of the specific nature of the benefit or any insight into mechanisms of benefit. A number of more specific tools have been developed to evaluate various aspects of the disease, although many of these were developed prior to the new regulatory guidelines and may only be valid as secondary or supportive outcome measures in clinical trials. Data from the development and validation of new and promising COPD-specific instruments that are acceptable to regulatory authorities are emerging, and new standards in the evaluation of novel therapies for COPD are being set. However, it should be appreciated that it takes several studies and many years for that body of evidence to accumulate. New does not necessarily mean better discriminative or evaluative properties than old. The recent development of the simple eight-item CAT using Rasch methodology is a case in point; it correlates very well with the much longer SGRQ-C, the scoring of which is complex and uses patient-derived item weights.79–81 This suggests that the two instruments address the same underlying construct, but, despite being developed using an approach that followed current FDA guidance, this observation does not mean that CAT is “better”, merely that it is shorter. Indeed this is another piece of evidence for the validity of the SGRQ, even though it is 20 years old.
In many clinical trials a measure of overall treatment efficacy is needed and this can be provided by health status measures. There is a very large body of published evidence concerning the SGRQ in research studies of all kinds. It has been accepted by the European Medicines Agency as a symptomatic outcome measure in COPD trials and it is becoming accepted as an outcome measure for COPD studies by the FDA. A white paper to support that purpose is being put together by a consortium working with the COPD Foundation in the US. For a shorter measure, both the CCQ and CAT have demonstrated validity and responsiveness.
Ideally, there should be a set of standardized comprehensive PRO instruments that are approved by regulatory authorities and used consistently during drug development and research. The ongoing collaborations with regulatory bodies, academia, and the pharmaceutical industry promise to address the need for measures of the COPD concepts, particular for the tools being developed, for example, physical activity in the European Innovative Medicines Initiative PROactive project and exacerbation symptoms in the EXACT initiative, but significant gaps remain. Several individual pharmaceutical companies are committed to the continued development of additional PRO tools to ensure that relevant treatment effects are captured in clinical trials (eg, CDLM, SOBDA). These new instruments may provide insights into COPD, particularly in terms of its impact on patients, and may reveal new treatment outcomes that have been hitherto obscured.
Footnotes
Disclosure
A professional medical writer contracted to CircleScience and funded by Novartis assisted in the preparation of this manuscript. Development of the manuscript was sponsored by Novartis Pharma AG. PWJ, MM, and TvdM have all received lecture and consultancy fees from a range of pharmaceutical companies, including Novartis, but received no fees for the writing of this paper. KK is an employee of Novartis.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2010. [Accessed July 16, 2012]. Available from: http://www.goldcopd.com.
- 2.Jones PW, Agusti AG. Outcomes and markers in the assessment of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(4):822–832. doi: 10.1183/09031936.06.00145104. [DOI] [PubMed] [Google Scholar]
- 3.Jones PW. Health status measurement in chronic obstructive pulmonary disease. Thorax. 2001;56(11):880–887. doi: 10.1136/thorax.56.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Mishima M. Longitudinal deteriorations in patient reported outcomes in patients with COPD. Respir Med. 2007;101(1):146–153. doi: 10.1016/j.rmed.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Westwood M, Bourbeau J, Jones PW, Cerulli A, Capkun-Niggli G, Worthy G. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res. 2011;12:40. doi: 10.1186/1465-9921-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agusti AG, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(2):347–360. doi: 10.1183/09031936.03.00405703. [DOI] [PubMed] [Google Scholar]
- 7.Jones PW. Issues concerning health-related quality of life in COPD. Chest. 1995;107(Suppl 5):187S–193S. doi: 10.1378/chest.107.5_supplement.187s. [DOI] [PubMed] [Google Scholar]
- 8.Gosker HR, Wouters EF, van der Vusse GJ, Schols AM. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr. 2000;71(5):1033–1047. doi: 10.1093/ajcn/71.5.1033. [DOI] [PubMed] [Google Scholar]
- 9.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–468. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 10.Dorman PJ, Waddell F, Slattery J, Dennis M, Sandercock P. Are proxy assessments of health status after stroke with the EuroQol questionnaire feasible, accurate, and unbiased? Stroke. 1997;28(10):1883–1887. doi: 10.1161/01.str.28.10.1883. [DOI] [PubMed] [Google Scholar]
- 11.Low G, Gutman G. Couples’ ratings of chronic obstructive pulmonary disease patients’ quality of life. Clin Nurs Res. 2003;12(1):28–48. doi: 10.1177/1054773803238739. [DOI] [PubMed] [Google Scholar]
- 12.Santiveri C, Espinalt M, Diaz Carrasco FX, Marín A, Miguel E, Jones PW. Evaluation of male COPD patients’ health status by proxies. Respir Med. 2007;101(3):439–445. doi: 10.1016/j.rmed.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Patient-reported Outcome Measures: Use in Medical Product Delvelopment to Support Labelling Claims. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH); 2009. [Accessed July 16, 2012]. Guidance for Industry. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf. [Google Scholar]
- 14.Qualification Process for Drug Development Tools. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER); 2010. [Accessed July 16, 2012]. Draft Guidance for Industry. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM230597.pdf. [Google Scholar]
- 15.Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S EXACT-PRO Study Group. Standardizing measurement of chronic obstructive pulmonary disease exacerbations: reliability and validity of a patient-reported diary. Am J Respir Crit Care Med. 2011;183(3):323–329. doi: 10.1164/rccm.201005-0762OC. [DOI] [PubMed] [Google Scholar]
- 16.proactivecopd.com [homepage on the Internet]PROactive website 2011Available from: http://www.proactivecopd.comAccessed July 16, 2012
- 17.Troosters T, Brindicci C. PROactive COPD, towards a patient reported outcome capturing physical activity in COPD. 2009. [Accessed July 16, 2012]. Available from: http://www.ers-education.org/pages/default.aspx?id=1652&idBrowse=57382&det=1.
- 18.Partridge MR, Miravitlles M, Ståhl E, Karlsson N, Svensson K, Welte T. Development and validation of the Capacity of Daily Living during the Morning questionnaire and the Global Chest Symptoms Questionnaire in COPD. Eur Respir J. 2010;36(1):96–104. doi: 10.1183/09031936.00123709. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox TK, Chen WH, Howard KA, et al. Reliability and validity of the Shortness of Breath with Daily Activities (SOBDA) questionnaire: a new outcome measure for evaluating dyspnea in COPD. Am J Respir Crit Care Med. 2010;181:A5429. doi: 10.1186/1477-7525-11-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yorke J, Russell AM, Swigris J, et al. Assessment of dyspnea in asthma: validation of the Dyspnea-12. J Asthma. 2011;48(6):602–608. doi: 10.3109/02770903.2011.585412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasch G. Probabilistic Models for Some Intelligence and Attainment Tests. Copenhagen, Denmark: Danish Institute for Educational Research; 1960. [Google Scholar]
- 22.Hagquist C, Bruce M, Gustavsson JP. Using the Rasch model in nursing research: an introduction and illustrative example. Int J Nurs Stud. 2009;46(3):380–393. doi: 10.1016/j.ijnurstu.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Vidotto G, Bertolotti G, Carone M, et al. A new questionnaire specifically designed for patients affected by chronic obstructive pulmonary disease; the Italian Health Status Questionnaire. Respir Med. 2006;100(5):862–870. doi: 10.1016/j.rmed.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Jones PW, Kaplan RM. Methodological issues in evaluating measures of health as outcomes for COPD. Eur Respir J Suppl. 2003;41:13s–18s. [PubMed] [Google Scholar]
- 25.Miravitlles M, Anzueto A, Legnani D, Forstmeier L, Forgel M. Patient’s perception of exacerbations of COPD – the PERCEIVE study. Respir Med. 2007;101(3):453–460. doi: 10.1016/j.rmed.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Mazur W, Kupiainen H, Pitkäniemi J, et al. Comparison between the disease-specific Airways Questionnaire 20 and the generic 15D instruments in COPD. Health Qual Life Outcomes. 2011;9:4. doi: 10.1186/1477-7525-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braido F, Baiardini I, Menoni S, et al. Disability in COPD and its relationship to clinical and patient-reported outcomes. Curr Med Res Opin. 2011;27(5):981–986. doi: 10.1185/03007995.2011.563285. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher CM. Standardized questionnaire on respiratory symptoms: a statement prepared and approved by the MRC committee on the aetiology of chronic bronchitis. Br Med J. 1960;2:1665. [Google Scholar]
- 29.Burdon JG, Juniper EF, Killian KJ, Hargreaves FE, Campbell EJ. The perception of breathlessness in asthma. Am Rev Respir Dis. 1982;126(5):825–828. doi: 10.1164/arrd.1982.126.5.825. [DOI] [PubMed] [Google Scholar]
- 30.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest. 1984;85(6):751–758. doi: 10.1378/chest.85.6.751. [DOI] [PubMed] [Google Scholar]
- 31.Gross NJ. Chronic obstructive pulmonary disease outcome measurements: what’s important? What’s useful? Proc Am Thorac Soc. 2005;2(4):267–271. doi: 10.1513/pats.200504-036SR. [DOI] [PubMed] [Google Scholar]
- 32.Ozalevli S, Ucan ES. The comparison of different dyspnoea scales in patients with COPD. J Eval Clin Pract. 2006;12(5):532–538. doi: 10.1111/j.1365-2753.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- 33.Mahler DA, Ward J, Fierro-Carrion G, et al. Development of self-administered versions of modified baseline and transition dyspnea indexes in COPD. COPD. 2004;1(2):165–172. doi: 10.1081/copd-120030829. [DOI] [PubMed] [Google Scholar]
- 34.Mahler DA, Waterman LA, Ward J, McCusker C, ZuWallack R, Baird JC. Validity and responsiveness of the self-administered computerized versions of the baseline and transition dyspnea indexes. Chest. 2007;132(4):1283–1290. doi: 10.1378/chest.07-0703. [DOI] [PubMed] [Google Scholar]
- 35.Aaron SD, Vandemheen KL, Clinch JJ, et al. Measurement of short-term changes in dyspnea and disease-specific quality of life following an acute COPD exacerbation. Chest. 2002;121(3):688–696. doi: 10.1378/chest.121.3.688. [DOI] [PubMed] [Google Scholar]
- 36.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Koplan RM. Chest. 3. Vol. 113. University of California; San Diego: 1998. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire; pp. 619–624. [DOI] [PubMed] [Google Scholar]
- 37.Williams JE, Singh SJ, Sewell L, Guyatt GH, Morgan MD. Development of a self-reported Chronic Respiratory Questionnaire (CRQ-SR) Thorax. 2001;56(12):954–959. doi: 10.1136/thorax.56.12.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones PW. Assessment of disability. In: Barnes PJ, Drazen JM, Rennard SI, Thomson NC, editors. Asthma and COPD: Basic Mechanisms and Clinical Management. San Diego, CA: Elsevier Science Ltd; 2002. [Google Scholar]
- 39.Norweg A, Ni P, Garshick E, O’Connor G, Wilke K, Jette AM. A multidimensional computer adaptive test approach to dyspnea assessment. Arch Phys Med Rehabil. 2011;92(10):1561–1569. doi: 10.1016/j.apmr.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12. Thorax. 2010;65(1):21–26. doi: 10.1136/thx.2009.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yorke J, Swigris J, Russell AM, et al. Dyspnea-12 is a valid and reliable measure of breathlessness in patients with interstitial lung disease. Chest. 2011;139(1):159–164. doi: 10.1378/chest.10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisner MD, Blanc PD, Yelin EH, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med. 2008;121(9):789–796. doi: 10.1016/j.amjmed.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 44.Follick MJ, Ahern DK, Laser-Wolston N. Evaluation of a daily activity diary for chronic pain patients. Pain. 1984;19(4):373–382. doi: 10.1016/0304-3959(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 45.Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 46.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J. 2006;27(5):1040–1055. doi: 10.1183/09031936.06.00064105. [DOI] [PubMed] [Google Scholar]
- 47.Kocks JW, Asijee GM, Tsiligianni IG, Kerstjens HA, van der Molen T. Functional status measurement in COPD: a review of available methods and their feasibility in primary care. Prim Care Respir J. 2011;20(3):269–275. doi: 10.4104/pcrj.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leidy NK. Subjective measurement of activity in chronic obstructive pulmonary disease. COPD. 2007;4(3):243–249. doi: 10.1080/15412550701480414. [DOI] [PubMed] [Google Scholar]
- 49.Stull DE, Leidy NK, Jones PW, Stohl E. Measuring functional performance in patients with COPD: a discussion of patient-reported outcome measures. Curr Med Res Opin. 2007;23(11):2655–2665. doi: 10.1185/030079907x233133. [DOI] [PubMed] [Google Scholar]
- 50.Garrod R, Bestall JC, Paul EA, Wedzicka JA, Jones PW. Development and validation of a standardized measure of activity of daily living in patients with severe COPD: the London Chest Activity of Daily Living scale (LCADL) Respir Med. 2000;94(6):589–596. doi: 10.1053/rmed.2000.0786. [DOI] [PubMed] [Google Scholar]
- 51.Miravitlles M, Ferrer M, Pont A, et al. Exacerbations impair quality of life in patients with chronic obstructive pulmonary disease: a 2-year follow up study. Thorax. 2004;59(5):387–395. doi: 10.1136/thx.2003.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 53.Miravitlles M. Prevention of exacerbations of COPD with pharmacotherapy. Eur Respir Rev. 2010;19(116):119–126. doi: 10.1183/09059180.00002810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones RC, Hyland ME, Hanney K, Erwin J. A qualitative study of compliance with medication and lifestyle modification in chronic obstructive pulmonary disease (COPD) Prim Care Respir J. 2004;13(3):149–154. doi: 10.1016/j.pcrj.2004.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jordan N, Lee TA, Valenstein M, Pirraglia PA, Weiss KB. Effect of depression care on outcomes in COPD patients with depression. Chest. 2009;135(3):626–632. doi: 10.1378/chest.08-0839. [DOI] [PubMed] [Google Scholar]
- 56.de Voogd JN, Wempe JB, Koeter GH, et al. Depressive symptoms as predictors of mortality in patients with COPD. Chest. 2009;135(3):619–625. doi: 10.1378/chest.08-0078. [DOI] [PubMed] [Google Scholar]
- 57.Jones P, Higenbottam T. Quantifying of severity of exacerbations in chronic obstructive pulmonary disease: adaptations to the definition to allow quantification. Proc Am Thorac Soc. 2007;4(8):597–601. doi: 10.1513/pats.200707-115TH. [DOI] [PubMed] [Google Scholar]
- 58.Jones PW, Chen WH, Wilcox TK, Sethi S, Leidy NK EXACT-PRO Study Group. Characterizing and quantifying the symptomatic features of COPD exacerbations. Chest. 2011;139(6):1388–1394. doi: 10.1378/chest.10-1240. [DOI] [PubMed] [Google Scholar]
- 59.Leidy NK, Wilcox TK, Jones PW, et al. Development of the EXAcerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT): a patient-reported outcome (PRO) measure. Value Health. 2010;13(8):965–975. doi: 10.1111/j.1524-4733.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 60.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 61.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 62.Ferrer M, Alonso J, Morera J, et al. Chronic obstructive pulmonary disease stage and health-related quality of life. The Quality of Life of Chronic Obstructive Pulmonary Disease Study Group. Ann Intern Med. 1997;127(12):1072–1079. doi: 10.7326/0003-4819-127-12-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 63.Gupta B, Kant S. Health related quality of life (HRQOL) in COPD. Internet J Pulmon Med. 2009;11:1. [Google Scholar]
- 64.Quirk FH, Jones PW. Patients’ perception of distress due to symptoms and effects of asthma on daily living and an investigation of possible influential factors. Clin Sci. 1990;79(1):17–21. doi: 10.1042/cs0790017. [DOI] [PubMed] [Google Scholar]
- 65.Quirk FH, Baveystock CM, Wilson RC, Jones PW. Influence of demographic and disease related factors on the degree of distress associated with symptoms and restrictions on daily living due to asthma in six countries. Eur Respir J. 1991;4(2):167–171. [PubMed] [Google Scholar]
- 66.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 67.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 68.Tashkin DP, Celli B, Senn S, et al. UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 69.Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St George Respiratory Questionnaire. Chest. 2007;132(2):456–463. doi: 10.1378/chest.06-0702. [DOI] [PubMed] [Google Scholar]
- 70.Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–750. doi: 10.1164/rccm.200904-0492OC. [DOI] [PubMed] [Google Scholar]
- 72.Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax. 1987;42(10):773–778. doi: 10.1136/thx.42.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schünemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic Respiratory Disease Questionnaire (CRQ) COPD. 2005;2(1):81–89. doi: 10.1081/copd-200050651. [DOI] [PubMed] [Google Scholar]
- 74.Morgan MD. Experience of using the CRQ (Chronic Respiratory Questionnaire) Respir Med. 1991;85(Suppl B):23–24. doi: 10.1016/s0954-6111(06)80165-4. [DOI] [PubMed] [Google Scholar]
- 75.Meng NH, Chen FN, Lo SF, Cheng WE. Reliability and validity of the Taiwan (Mandarin Chinese) version of the chronic respiratory questionnaire. Qual Life Res. 2011;20(10):1745–1751. doi: 10.1007/s11136-011-9906-7. [DOI] [PubMed] [Google Scholar]
- 76.Moreira GL, Pitta F, Ramos D, et al. Portuguese-language version of the Chronic Respiratory Questionnaire: a validity and reproducibility study. J Bras Pneumol. 2009;35(8):737–744. doi: 10.1590/s1806-37132009000800004. [DOI] [PubMed] [Google Scholar]
- 77.Puhan MA, Behnke M, Frey M, et al. Self-administration and interviewer-administration of the German Chronic Respiratory Questionnaire: instrument development and assessment of validity and reliability in two randomised studies. Health Qual Life Outcomes. 2004;2(7):1. doi: 10.1186/1477-7525-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vigil L, Guell MR, Morante F, et al. The validity and sensitivity to change of the Spanish self-administered version of the Chronic Respiratory Questionnaire (CRQ-SAS) Arch Bronconeumol. 2011;47(3):343–349. doi: 10.1016/j.arbres.2011.02.016. Spanish. [DOI] [PubMed] [Google Scholar]
- 79.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(5):648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 80.Dodd JW, Hogg L, Nolan J, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax. 2011;66(1):425–429. doi: 10.1136/thx.2010.156372. [DOI] [PubMed] [Google Scholar]
- 81.Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD Assessment Test (CAT) in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35. doi: 10.1183/09031936.00177210. [DOI] [PubMed] [Google Scholar]
- 82.Jones PW, Harding G, Wiklund I, et al. Tests of the responsiveness of the chronic obstructive pulmonary disease (COPD) Assessment Test TM (CAT) following acute exacerbation and pulmonary rehabilitation. Chest. 2012;142:134–140. doi: 10.1378/chest.11-0309. [DOI] [PubMed] [Google Scholar]
- 83.van der Molen T, Willemse BW, Schokker S, ten Hacken NH, Postma DS, Juniper EF. Development, validity and responsiveness of the Clinical COPD Questionnaire. Health Qual Life Outcomes. 2003;1:13. doi: 10.1186/1477-7525-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McKenna S, Meads D, Doward L, et al. Development and validation of the living with chronic obstructive pulmonary disease questionnaire. Qual Life Res. 2011;20(7):1043–1052. doi: 10.1007/s11136-011-9850-6. [DOI] [PubMed] [Google Scholar]
- 85.Stucki A, Stucki G, Cieza A, Schuurmans MM, Kostanjsek N, Ruof J. Content comparison of health-related quality of life instruments for COPD. Respir Med. 2007;101(6):1113–1122. doi: 10.1016/j.rmed.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 86.Vernon M, Kline Leidy N, Nacson A, Nelsen L. Measuring cough severity: development and pilot testing of a new seven-item cough severity patient-reported outcome measure. Ther Adv Respir Dis. 2010;4(4):199–208. doi: 10.1177/1753465810372526. [DOI] [PubMed] [Google Scholar]
- 87.Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MD, Pavord ID. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ) Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yousaf N, Lee KK, Jayaraman B, Pavord ID, Birring SS. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute) Cough. 2011;7(1):4. doi: 10.1186/1745-9974-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reishtein JL. Relationship between symptoms and functional performance in COPD. Res Nurs Health. 2005;28(1):39–47. doi: 10.1002/nur.20054. [DOI] [PubMed] [Google Scholar]
- 90.Kinsman RA, Yaroush RA, Fernandez E, Dirks JF, Schocket M, Fukuhara J. Symptoms and experiences in chronic bronchitis and emphysema. Chest. 1983;83(5):755–761. doi: 10.1378/chest.83.5.755. [DOI] [PubMed] [Google Scholar]
- 91.Shackell BS, Jones RC, Harding G, Pearse S, Campbell J. “Am I going to see the next morning?”A qualitative study of patients’ perspectives of sleep in COPD. Prim Care Respir J. 2007;16(6):378–383. doi: 10.3132/pcrj.2007.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Owens RL, Malhotra A. Sleep-disordered breathing and COPD: the overlap syndrome. Respir Care. 2010;55(10):1333–1344. [PMC free article] [PubMed] [Google Scholar]
- 93.Karachaliou F, Kostikas K, Pastaka C, Bagiatis V, Gourgoulianis KI. Prevalence of sleep-related symptoms in a primary care population – their relation to asthma and COPD. Prim Care Respir J. 2007;16(4):222–228. doi: 10.3132/pcrj.2007.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peters JB, Heijdra YF, Daudey L, et al. Course of normal and abnormal fatigue in patients with chronic obstructive pulmonary disease, and its relationship with domains of health status. Patient Educ Couns. 2011;85(2):281–285. doi: 10.1016/j.pec.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 95.Mollaoglu M, Fertelli TK, Tuncay FO. Fatigue and disability in elderly patients with chronic obstructive pulmonary disease (COPD) Arch Gerontol Geriatr. 2011;53(2):e93–e98. doi: 10.1016/j.archger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 96.Al-shair K, Kolsum U, Berry P, et al. Development, dimensions, reliability and validity of the novel Manchester COPD fatigue scale. Thorax. 2009;64(11):950–955. doi: 10.1136/thx.2009.118109. [DOI] [PubMed] [Google Scholar]
- 97.Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S EXACT-PRO Study Group. Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2011;183(3):323–329. doi: 10.1164/rccm.201005-0762OC. [DOI] [PubMed] [Google Scholar]
- 98.Reilly MC, Bracco A, Ricci JF, Santoro J, Stevens T. The validity and accuracy of the Work Productivity and Activity Impairment questionnaire – irritable bowel syndrome version (WPAI:IBS) Aliment Pharmacol Ther. 2004;20(4):459–467. doi: 10.1111/j.1365-2036.2004.02091.x. [DOI] [PubMed] [Google Scholar]
- 99.Reilly MC, Gooch KL, Wong RL, Kupper H, van der Heijde D. Validity, reliability and responsiveness of the Work Productivity and Activity Impairment Questionnaire in ankylosing spondylitis. Rheumatology (Oxford) 2010;49(4):812–819. doi: 10.1093/rheumatology/kep457. [DOI] [PubMed] [Google Scholar]
- 100.Wahlqvist P, Carlsson J, Stalhammar NO, Wiklund I. Validity of a Work Productivity and Activity Impairment questionnaire for patients with symptoms of gastro-esophageal reflux disease (WPAI-GERD) – results from a cross-sectional study. Value Health. 2002;5(2):106–113. doi: 10.1046/j.1524-4733.2002.52101.x. [DOI] [PubMed] [Google Scholar]
- 101.Ståhl E, Jansson SA, Jonsson AC, Svensson K, Lundbäck B, Andersson F. Health-related quality of life, utility, and productivity outcomes instruments: ease of completion by subjects with COPD. Health Qual Life Outcomes. 2003;1:18. doi: 10.1186/1477-7525-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fletcher MJ, Upton J, Taylor-Fishwick J, et al. COPD uncovered: an international survey on the impact of chronic obstructive pulmonary disease [COPD] on a working age population. BMC Public Health. 2011;11:612. doi: 10.1186/1471-2458-11-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care. 2003;48(12):1204–1213. [PubMed] [Google Scholar]
- 104.Victorson DE, Anton S, Hamilton A, Yount S, Cella D. A conceptual model of the experience of dyspnea and functional limitations in chronic obstructive pulmonary disease. Value Health. 2009;12(6):1018–1025. doi: 10.1111/j.1524-4733.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 105.Evensen AE. Management of COPD exacerbations. Am Fam Physician. 2010;81(5):607–613. [PubMed] [Google Scholar]
- 106.Daudey L, Peters JB, Molema J, et al. Health status in COPD cannot be measured by the St George’s Respiratory Questionnaire alone: an evaluation of the underlying concepts of this questionnaire. Respir Res. 2010;11:98. doi: 10.1186/1465-9921-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andenaes R, Moum T, Kalfoss MH, Wahl AK. Changes in health status, psychological distress, and quality of life in COPD patients after hospitalization. Qual Life Res. 2006;15(2):249–257. doi: 10.1007/s11136-005-0890-7. [DOI] [PubMed] [Google Scholar]
- 108.Testa MA, Simonson DC. Current concepts: assessment of quality-of-life outcomes. N Engl J Med. 1996;334(13):835–840. doi: 10.1056/NEJM199603283341306. [DOI] [PubMed] [Google Scholar]