SUMMARY
There are many detailed articles regarding accidents and local complications in dental implantation. Comparison of the data they report is not always easy because different criteria have been followed in the various classifications and there is confusion between the terms accident and complication. The aim of this paper is to propose a classification that considers the timing of the events and makes a distinction between the two terms. Accidents are events that occur during surgery and complications are all the pathological conditions that appear postoperatively. The proper diagnostic procedures and surgical techniques for complications prevention and treatment are also described.
Keywords: complications, failures, prevention, treatment, osseointegrated implants
RIASSUNTO
Le pubblicazioni relative agli incidenti e alle complicanze locali in chirurgia implantare sono numerose e dettagliate, anche se i dati non sempre sono di facile confronto sia per la diversità dei criteri adottati nell’elaborare le classificazioni, sia per la confusione esistente tra i termini di incidente e complicanza. Scopo del presente lavoro è quello di proporre una classificazione che tenga in considerazione la cronologia degli eventi e si basi sulla differenza esistente tra i due termini, per cui si devono considerare incidenti gli eventi che si verificano durante l’intervento e complicanze tutte le condizioni patologiche che si manifestano nel periodo successivo alla fase chirurgica. Vengono descritti inoltre le procedure diagnostiche e gli accorgimenti di tecnica operatoria necessari alla loro prevenzione e al loro trattamento.
The diverse methods and terminology used in the literature regarding accidents and complications call for descriptive criteria that may be universally accepted irrespective of the different focus of single studies. The proposed classification (Tab. 1). considers the timing of events and is based on a distinction between the terms accident and complication. Accidents are events that occur during surgery and complications are all the conditions that appear postoperatively. Early-stage complications appear in the immediate postoperative period and interfere with healing, and late-stage complications arise during the process of osseointegration.
Table 1.
Local complications in dental implant surgery.
Early-stage complications
|
Late complications
|
Early-stage complications
Early-stage complications may involve the maxillary sinus or the mandibular bone, soft tissues and nerve trunks adjacent to the implant site. Not all the mechanisms responsible for these complications are known but the most common causes are an excessively traumatic surgical approach, bone overheating during osteotomy, and bacterial contamination of the host site.
Infection
Infections arising during the first few postoperative days present with edema, exudate and pain. They are caused by bacterial contamination during surgery either directly via accidental contact with the implants or indirectly from gloves or instruments. The risks of such a complication may be reduced by following the surgical principles of asepsis (1, 2). This measure is advisable even though a retrospective analysis by Scharf and Tarnow (3) comparing 273 implants inserted under “sterile” conditions and 113 implants placed under “clean” conditions showed no statistically significant differences in the success rates of the two groups.
Besides a sterile working area and a clean environment, an aseptic protocol includes disinfection of the perioral skin with solutions containing povidone-iodine and alcohol, disinfection of the oral mucosa with 0.2% chlorhexidine (which significantly reduces the bacterial count in the saliva for over 4 hours), and cleansing of surgical gloves in sterile saline to remove dust or contaminants. Further preventive measures are the administration of antibiotic therapy before and after the procedure and the prescription of proper oral hygiene at home with mouthwashes containing 0.12% chlorhexidine during the first two weeks after the procedure. A recent study reported that the use of chlorhexidine was associated with significant reductions in infections showing a 4.1% decrease in the test group and 8.7 % reduction in the control group (4).
Edema
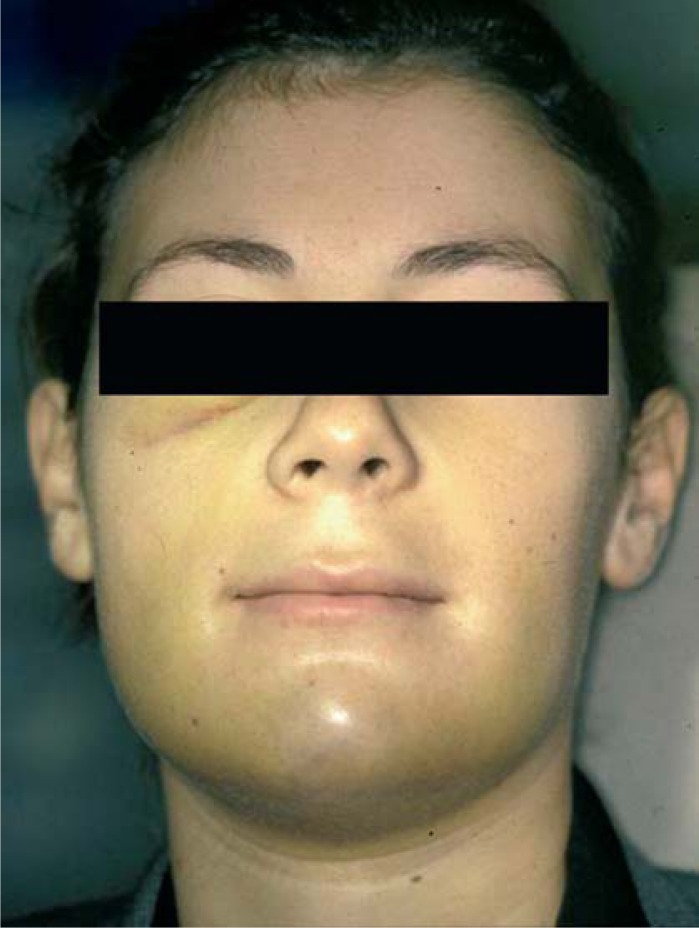
Edema is the accumulation of excess plasma fluid (transudate) in the interstitial spaces (at least a 10% increase). It is correlated to the extent of surgical trauma and to the duration of surgery. Edema is a complication when there is a considerable accumulation of fluid because this may negatively affect healing and create discomfort to the patient during food intake and oral hygiene maintenance (Fig. 1). Atraumatic surgical techniques minimizing tissue damage, the application of ice packs and the administration of corticosteroids will prevent or limit edema after implant surgery.
Figure 1.
Postoperative edema.
Ecchymoses and haematomas
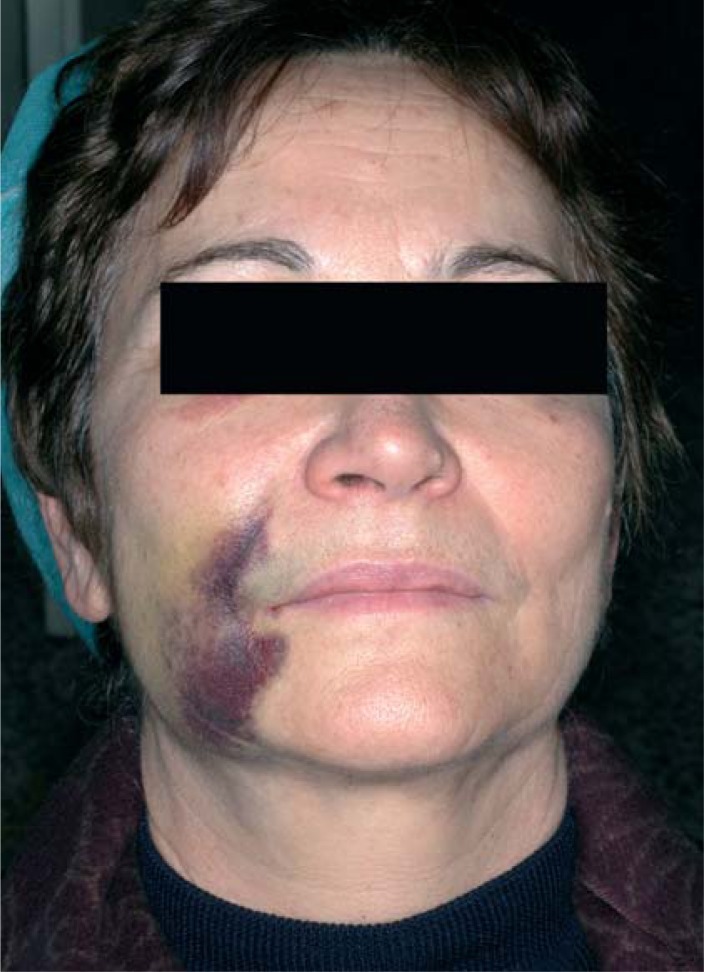
Blood effusions infiltrating surface tissues (ecchymoses) and circumscribed blood collections (hematomas) are not common after implant surgery. Particularly long and complex procedures, lack of patient compliance with the instructions received for the immediate postoperative period (application of ice packs, compression and tamponade, and cold liquid diet), vessel fragility, especially typical in elderly patients, and failure to discontinue antiplatelet therapy before surgery (5) may favor the appearance of ecchymoses and hematomas (Fig. 2).
Figure 2.
Ecchymose and haematoma.
Although they are associated with a greater risk of infection, ecchymoses and hematomas do not generally require any particular treatment. Topical skin applications of heparin-containing medications will help them resorb. If there is a recent hematoma between the bone and the mucoperiosteal flap, it should be drained and external compression will be applied on the soft tissues to avoid relapses (6).
Emphysema
Emphysema is a very rare complication resulting from a sudden rise of the intraoral pressure. This may occur when a patient sneezes and air is forced through the mucoperiosteal tissue of a not perfectly approximated flap and into the muscular interstices at the interface between the muscular fascia and soft tissues (6). Clinically it presents with swelling of half of the face, extending at times to the neck and thorax. A characteristic crackling sound is evinced upon palpation.
Massages and compression with ice packs will help resorb the air entrapped in the tissues thus leading to fast and spontaneous regression of the emphysema.
Measures for preventing this complication include avoiding the use of high-velocity instruments to prepare the bone bed or irrigation of the wound with hydrogen peroxide and ensuring a perfect approximation of incised edges when suturing. Patients will be instructed blow their nose gently and avoid sneezing during the first few days after surgery.
Bleeding
Failure to stabilize the flap, tearing of soft tissues caused by tight or sharp suture material, masticatory trauma and traumas resulting from early temporization or an inappropriately modified temporary prosthesis are all causes of postoperative bleeding. Treatment will consist of eliminating the causes of bleeding and implementing the normal procedures to promote hemostasis (compression and tamponade with surgical gauzes soaked in tranexamic acid). If the bleeding does not stop, the flap will be re-elevated, the clotted blood removed and new sutures applied to fully immobilize the soft tissues and promote clot formation and stabilization.
Flap dehiscence
Dehiscence is opening of the surgical wound edges exposing part or all of the implant head and/or surrounding bony tissues. Etiologically, flap dehiscence may result from a number of causative factors: a very thin mucosa; failure to ensure passive reapproximation and closure of the flap margins, that will thus be unable to counter the intramural mechanical stress due to muscle/bone interaction; presence of large edema or hematomas; insufficient or excessive tension on the suture, causing soft tissue necrosis due to impaired blood supply; functional movements, such as mastication, phonation or deglutition; previous prosthodontic surgery or radiation therapy affecting the vascularity of the flap; sudden trauma of edentulous segments by the opposing dentition; premature use of a removable denture; incomplete tightening of the cover screw, often as a result of the presence of blood residues inside the implant; bone débris produced during osteotomy or implant insertion and trapped under the periosteum; cigarette smoking and the local effects of nicotine (presence of cytotoxic and vasoactive substances) as well as its systemic effects (altered granulocytes and T cells, impaired production of antibodies and vasomotor substances) (Fig. 3) (5, 7, 8).
Figure 3.
Flap dehiscence one week after surgery.
Treatment will vary based on the extent of exposure. If it is small, no surgical correction is required because the granulation tissue that forms will promote healing by secondary intention. A granulation tissue formation process lasting over two weeks may require refreshing the epithelial wound margins with a diamond bur. A large dehiscence will be treated by removing the sutures and resuturing.
Dehiscences may be prevented by 1) careful preoperative assessment of the soft tissues to measure the amount of keratinized mucosa present and planning of augmentation procedures as appropriate; 2) minimally invasive flap elevation and reflection with careful removal of any bone débris beneath; 3) proper suturing; 4) sensible temporization with appropriate modifications, rebasing and relining; and 5) delaying the use of removable dentures until two weeks after surgery.
Sensory disorders
Temporary or permanent sensory impairment may result from injuries to nerve trunks during implant surgery. Quantitatively, sensory disorders may entail enhanced perception of a stimulus (hyperesthesia), reduced sensitivity (hypoesthesia) or no sensation (anesthesia). Qualitatively, disorders are distinguished based on the perception of a different stimulus from the one applied (paresthesia or dysesthesia).
Patients may express the changes they subjectively perceive with a variety of words and ways (9). After a self-administered questionnaire was completed by 266 patients treated with osseointegrated mandibular implants, Ellies reported that symptoms included numbness, tingling, hot and cold, pain, swelling, hardening, burning, loss of saliva, prickling, tickle, electrical shock sensation (54–64%), itch and effects on phonation, on the intake of solid or liquid foods, on deglutition, and on taste. The lower jaw was more affected and the most common sites were the lower lip (54–64%), chin (46–58%), gum tissues (32–45%), and the tongue (11–16%) (10–12).
Sensory alterations prevail in the mandible (there is a lesser likelihood of sensory impairment in the maxilla) with values ranging from 1.7 to 43.5% for temporary alterations and from 5% to 15% for permanent alterations over one year after surgery (11).
Establishing the prognosis is not simple. The main factor affecting duration and reversibility is the nature of the damage. Reversible conditions comprise compression by edema or hematomas and excessive stretching of the mental nerve during flap reflection, as long as stretching is not too sudden and the 8% elastic limit is not exceeded (9). The outcome of disorders caused by implant placement close to the inferior alveolar nerve is variable since immediate implant removal often leads to sensory recovery. By contrast, injuries to the inferior alveolar nerve or to the mental nerve during osteotomy produce permanent sensory alteration with the occurrence of hyperalgesia (13). Diagnosis of a nerve injury is performed in two stages. The early-stage diagnosis will take place immediately after the injury occurs while late-stage diagnosis will include more thorough investigations. In the early stage, the symptoms reported by the patient will be assessed and panorex x-rays performed to identify any pathological changes that may be associated with the injury (e.g. penetration of an implant into the mandibular canal). If such changes are not radiographically visible, a wait and see attitude is advisable since the paresthesia and/or hypoesthesia reported by the patient may result from a “stunned nerve syndrome” (neuropraxia). By contrast, if the injury is observed intraoperatively and/or symptoms persist or worsen, clinical and laboratory tests will be required. Clinical investigations include mechanoceptive, thermal, electric, nociceptive, and chemical tests that will be repeated monthly starting from the first month after the accident to assess changes in the function of the affected nerve. Investigations of the lingual nerve will be supplemented with gustatory sensitivity tests.
Laboratory tests include electrophysiological measurements (somato-sensory evoked potentials - TSEP) the Blink Reflex test, and advanced imaging techniques (computerized tomography and nuclear magnetic resonance).
Nerve trunk injuries may be treated medically or surgically depending on the extent of the pathological alterations and the neurological symptoms reported by the patient. In the immediate postoperative period combination drug therapies with NSAIDs, cortisones, proteoliyic enzymes, antibiotics, and vitamins (C and E) are administered to reduce compression of the nerve trunk by edema or hematomas, to prevent the development of infections and to block fibrous tissue scarring. In the first month after implant surgery, the aim of treatment is to promote nerve regeneration. Hence, vitamins C and D, vasodilators (naftidrofuryl) and ozone therapy will be administered to prevent ischemia due to an increased blood supply demand during the regeneration process. Physical therapy, with magnetotherapy, low level laser therapy, and transcutaneous electric nerve stimulation (TENS) will be ensured. Should these therapeutic regimens fail, drug therapy will be administered with anticonvulsants (carbamazepine, diphenylhydantoin, valproic acid) or associations of tricyclic antidepressants and psychotropic drugs (phenotiazine) to control central pain induced by cortical hyperactivity. Surgical therapy (microneurosurgery) may be required to restore integrity of the injured nerve trunk and of nerve function. Three techniques have been described in the literature for nerve reconstruction: 1) neurorrhaphy or direct end-to-end anastomosis with an epineural or interfascicular suture; 2) grafting of autologous nerve tissue taken from various sites; and 3) tubulization of the nerve stumps.
Late complications
Late-stage complications are the result of noxious events occurring during surgery or healing. They mainly concern osseointegrating implants and the surrounding bone tissue and have causative mechanisms that often remain obscure.
Perforation of the mucoperiosteum
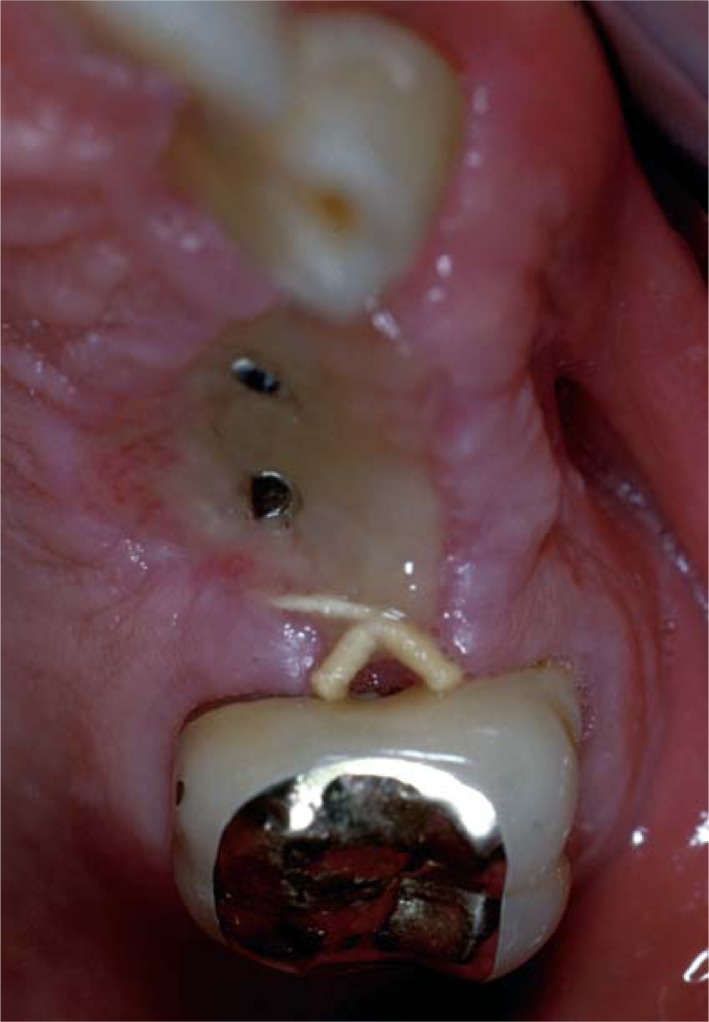
Spontaneous exposure of the cover screw is a frequent complication. It could be due to extremely thin tissues surrounding the implant, acute mechanical trauma, pressure from an inadequate rebase of a removable denture, causing atrophy or necrosis of the mucosa, or to loosening of the cover screw resulting in tension, irritation and displacement of the overlying soft tissues (Fig. 4) (5, 14, 15).
Figure 4.
Spontaneous exposure of the cover screw.
The frequency of perforations of the mucoperiosteum was described to range from 2% to 11% in a review of five articles by Goodacre and coworkers (16).
Perforations of the mucoperiosteum may concur in bacterial plaque build-up eventuating in inflammation, damage to the peri-implant mucosa and bone loss. The existence of a statistically significant correlation between implant exposure during healing and crestal bone resorption was confirmed by Toljanic et al. in a 5-year prospective study on 275 implants in 50 patients (17).
There is broad consensus that perforations of the mucoperiostium do not require treatment since tight closure of the flap is not indispensable for healing and osseointegration (18). In a few cases, however, in order to facilitate oral hygiene, the area of exposure may be extended and the cover screw replaced with a healing screw. Conversely, when there is early exposure of the crestal bone, a flap should be elevated to cover the mucoperiosteal perforation with or without grafts or membranes (17).
An accurate preoperative assessment of the soft tissues can avert this complication. The quantity of keratinized mucosa present will be measured and if it is inadequate, grafting of palatal connective tissue can be aptly scheduled concomitant with implant insertion.
Maxillary sinusitis
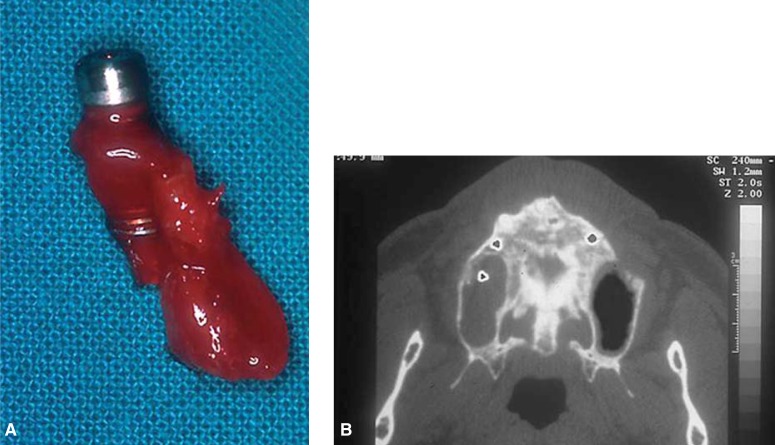
Sinusitis is a complication resulting from bacterial contamination of the maxillary sinus during surgery performed under non-aseptic conditions. Bacterial contamination may also occur during healing for wound dehiscence or because of implant displacement into the sinus causing a foreign body reaction and chronic infection (19–21). Sinusitis may present as acute and then become chronic, if the cause is not detected and removed, or it may present as chronic from the start. Acute cases present with pain, edema, swelling, reddened soft tissues overlying the involved sinus and purulent drainage through the homolateral nasal cavity (19–20). In chronic cases, there is less exudates but more massive proliferation of mucosa, thickening of the membrane and metaplasia of the epithelium; polypoid masses may partially fill the sinus leading to exudate retention. Air in the sinus will decrease considerably and the antral content will become progressively radiopaque until it is completely and permanently opacified.
Treatment of sinusitis includes systemic therapy with antibiotics, chlorhexidine mouthwashes, irrigations with saline through the nasal orifice, and the use of nasal decongestants. If the infection worsens or a dislodged implant is in the sinus, radical revision surgery of the maxillary sinus will be required and the antral mucosa completely removed (Fig. 5a–5b) (19–21).
Figure 5a–b.
Maxillary sinusitis for implant displacement into the sinus.
Maxillary sinusitis may be prevented by carefully screening patients before implant surgery to identify individuals with sinusitis or predisposing factors, administering prophylactic antibiotic therapy and strictly observing the surgical principles of asepsis.
Mandibular fractures
Mandibular fractures are rare complications which may occur during osseointegration (before the implants are uncovered and loaded), after restoration (for the removal of non-osseointegrated implants) or as the result of a trauma (16, 22–24). The cases reported in the literature regard only endosseous implants placed in severely resorbed crests.
The exact mechanism through which mandibular fractures occur is unknown, but the consistent finding of fracture lines passing through implant sites strongly suggests that this is the weakest and most susceptible area during osseointegration where stresses converge and the greatest loss of bone density occurs. This is why it has often been hypothesized that there may be a correlation between the occurrence of mandibular fractures and metabolic or bone remodeling disorders, such as osteoporosis and osteomalacia (22).
The clinical signs of mandibular fractures are pain, swelling, impaired function and fistulae in the fracture area (23, 24).
Diagnosis consists of clinical and radiographic examination of the patient. During the clinical evaluation, small movements of bony fragments, crackling sounds, and signs of infection will be identified. Radiographs will characteristically show a radiolucent area through the implant site (25). This is not always easy to identify and often requires more enhanced investigations, such as high resolution CT and technetium 99m-methylene diphosphonate bone scans, as suggested by Rothman and co-workers (23).
Aligned fractures with only numbness of the fractured area will be treated with antibiotic therapy and a soft diet. The patient will be kept under observation since healing is usually uneventful. Malaligned fractures require reduction and immobilization of the fractured segment to restore mandibular shape and function, especially in view of future prosthodontic rehabilitation. There is no need to remove the implant to ensure effective healing and osseointegration if there is no inflammation or implant loosening and the fractured fragments can be stabilized and fixed adequately (25, 26). It is not easy to predict the outcome of highly resorbed malaligned mandibular fractures because of the reduced blood supply and the poor bone regeneration potential.
Adequate precautions need to be taken before and during implantation surgery and during healing to prevent mandibular fractures. The bone should be at least 7 mm in height and 6 mm in width (24) and if this is not the case, procedures for ridge expansion or augmentation must be performed. During surgery, preparation of multiple bone beds is to be avoided. At least 5 mm of hard tissue should be left between one site and the other to permit an adequate distribution of forces (24). After preparing the implant sites, the surgeon should ensure that a few millimeters of buccal and oral cortical bone remain. Finally, over-screwing of the implant into the bone is to be avoided. During healing the patient will be instructed to keep the mandible at rest to protect it from stress.
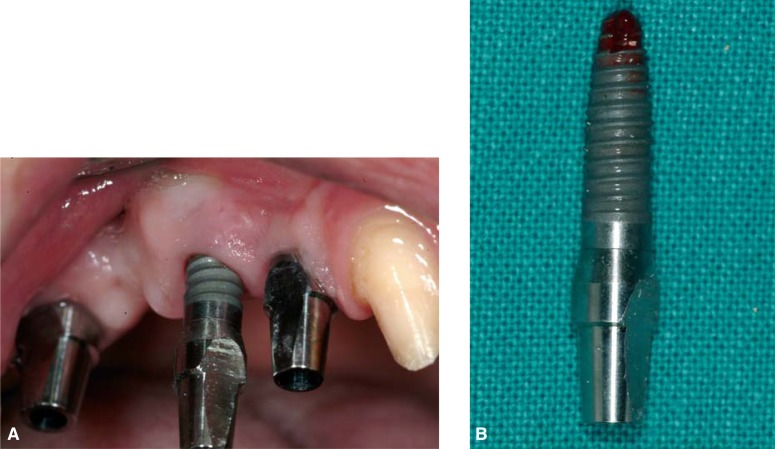
Failed osseointegration
Lack of osseointegration is diagnosed at phase II surgery or restoration when the implant is loaded. It is one of the worst complications since it inevitably results in loss of the implant (Fig. 6a–6b) (27).
Figure 6a–b.
Loss of the implant for lack of osseointegration.
The main causes for lack of osseointegration include reduced healing capacity, occlusal loading during osseointegration, failure to follow the planned protocol, technical errors during surgery (such as accidental contamination of the implant surface) (5–7), and especially bone overheating during implant site preparation. When a temperature of over 47°C is reached for 1 minute intraosseous blood vessels coagulate and extended necrotic areas are formed which become radiographically visible after 2–4 weeks (6).
Clinically, lack of osseointegration is diagnosed when the implant has loosened and a muffled sound is heard upon percussion. Radiographic evidence consists of a small radiolucent margin around the implant indicating that there is no direct contact between the bone and the implant (27–29).
Treatment will require removal of the loose implant and accurate debridement of the area involved so that a new implant may be inserted after healing has taken place.
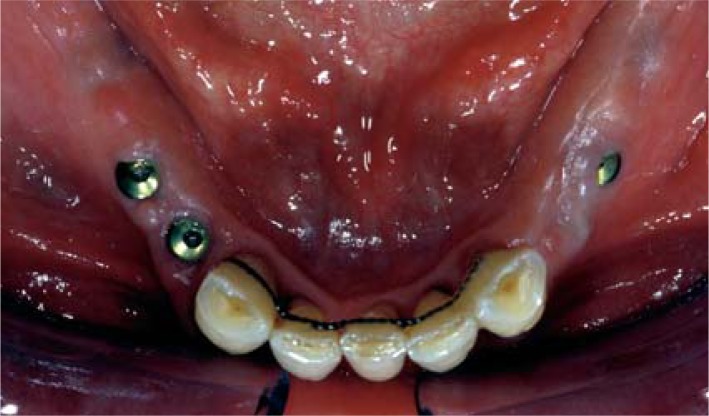
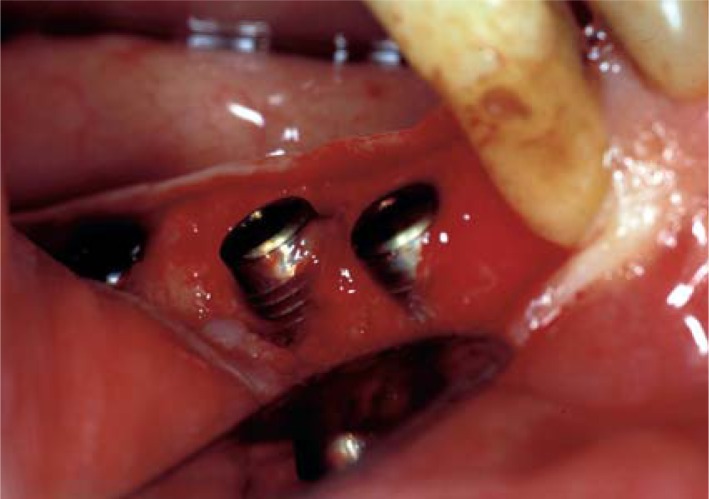
Bony defects
A horizontal or vertical bony defect around an implant is a complication that may be observed on assessing the bone-implant interface at the time of phase II surgery (Fig. 7).
Figure 7.
A vertical bony defect around two implants at the time of phase II surgery.
The causes that may lead to bone defects are:
1) direct trauma to the bone or an insult to the periosteum reducing vascularity, 2) decreased bone density, 3) implant placement into fresh extraction sockets, 4) wrong inclination of the implant, 5) excessive torque during insertion, 6) the presence of a bone dehiscence not treated at phase I surgery, 7) an extremely thin alveolar crest, 8) wound dehiscence during healing, 9) perforation of the mucoperiosteum, 10) postoperative infection, 11) excessive loading by the temporary prosthesis, or 12) the patient’s bad habits (17, 30).
Bony defects are not easy to identify since the patients are asymptomatic. Hence the crestal bone-implant interface should always be examined radiographically before connecting the abutment. If a bone defect is suspected, it is recommended to incise and elevate a flap to directly evaluate the size of the defect after curetting any epithelial tissue present.
Treatment will differ based on the type and extent of bone loss. Hence, in the presence of:
– a vertical defect of less than 2 mm, horizontal osteoplasty can be performed to reduce the defect without compromising the restorations or the cosmetic result;
– a vertical defect of more than 2 mm involving less than half of the implant, autologous bone taken from an intraoral site may be grafted. When the bone loss is greater than 25% of the circumference of the implant, grafting may be combined with a membrane. Uncovering of the implants will be postponed by 2–4 months;
– a small horizontal defect, apical repositioning of the soft tissues will be performed with cleaning of the exposed threads to avoid plaque buildup;
– a larger horizontal defect, a graft of autologous bone will be performed and a membrane positioned after accurate curettage of the area to help restore blood supply and promote new bone formation. Uncovering of the implants will be postponed by 3–4 months;
– a vertical or horizontal defect deeper than half the length of the implant, the implant will have to be removed because of inadequate stability and cosmetics.
Bone defects may be prevented by identifying and avoiding all the conditions that may engender the risk of such an event. Hence, treatment planning will take into account the quantity and quality of bone present. Proper surgical techniques will be performed and careful postoperative management ensured to protect the surgical wound from excessive stress.
Periapical implant lesion
“Periapical implant lesion” designates a pathological area of osteolysis at the apex of an osseointegrated implant (27, 31–33). The incidence of periapical implant lesions has been reported to increase when implant therapy was extended to cases of partial edentulism (31–35).
The most frequent cause is contamination of the apical portion of an implant by microbial flora from adjacent endodontically or periodontally involved teeth. Other causes include: 1) accidental sectioning of the neurovascular bundle of neighboring teeth, 2) pre-existing bone infections, 3) the presence of foreign bodies (endodontic material, fractured instruments in the canals, etc.) or root fragments, 4) sinus infections, 5) contamination of the implant surface during manufacturing or insertion and 6) compression of bone débris on the bottom of the implant site by excessively forceful implant insertion causing ischemia, necrosis and bone sequestration (31–35).
Periapical implant lesions are “inactive” if no symptoms are present and “infected” when signs of acute inflammation or a fistula are present. Inactive lesions are similar to bone scars around the apex of a tooth. They require no therapy, but only careful clinical and radiographic follow-up. By contrast, infected lesions must be treated surgically and the procedure will vary based on the extension of the lesion. Conservative surgery will be performed for small lesions. The infected site will be accessed to entirely remove the inflammatory tissue around the apex of the implant. Some cases may also require removal of part of the implant (36). A more radical surgical approach will be necessary when osseointegration is compromised or localized osteomyelitis is present. In this case the implant will be removed together with the infected tissue (31–35). Adjacent teeth with affected pulp or periodontal disease will also be treated and the apical seal of devitalized teeth checked for leakage.
Periapical implant lesions may be prevented through a careful preoperative examination of the periodontal and endodontic conditions of the patient and eradication of any microbial foci. This approach might not suffice since bacteria have been shown to exist around the apex of pulpectomized teeth without any signs or symptoms. Finally, surgery should be as minimally invasive as possible and care will be taken to remove any bone débris from the bottom of the implant site before implantation.
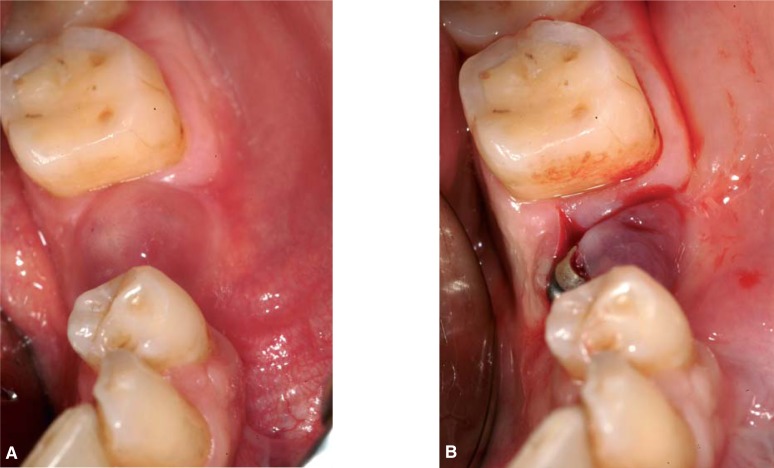
Infection
The main cause of late-stage infection is contamination of recently inserted implants by the pathogenic microflora of natural teeth. Contamination of the implants may be favored by the presence of necrotic and traumatized bony tissue and/or an impaired host defense mechanisms (7, 37). Characteristic clinical features are edema, swelling, purulent exudate, pain on palpation or fistulae (Fig. 8a–8b). Relatively marked bone resorption may be found on radiographs (38).
Figure 8.
a. Infection at the time of phase II surgery, characterized from edema and purulent exudates.
b. A flap has been elevated to drain the abscess.
If there is no bone involvement, a flap will be elevated and reflected to drain the abscess or remove the granulation tissue. Sterile saline will be used to irrigate the area and local antibiotic treatment with a tetracycline solution will be administered. If there is bone resorption a guided bone regeneration protocol will be followed. Postoperative antibiotic therapy in both cases will consist of amoxillicin + clavulanic acid 2 g daily and metronidazole 750 mg daily. The patient will be instructed to maintain accurate oral hygiene with 0.12% chlorhexidine rinses.
Conclusions
Local complications arising during implant surgery may be the main determinants of the outcome of the entire rehabilitation program. Hence, the prevention of complications is a priority objective for the surgeon. Careful clinical and radiographic examination of each case, accurate planning of procedures, the use of proper surgical techniques and appropriate instruments and the correct management of healing and osseointegration all concur in preventing such events.
References
- 1.Friberg B. Sterile operating conditions for the placement of intraoral implants. J Oral Maxillofac Surg. 1996;54:1334–1336. doi: 10.1016/s0278-2391(96)90493-0. [DOI] [PubMed] [Google Scholar]
- 2.Kraut RA. Clean operating conditions for the placement of intraoral implants. J Oral Maxillofac Surg. 1996;54:1337–1338. doi: 10.1016/s0278-2391(96)90494-2. [DOI] [PubMed] [Google Scholar]
- 3.Sharf DR, Tarnow DP. Success rates of osseointegration for implants placet under sterile versus clean conditions. J Periodontol. 1993;64(10):954–956. doi: 10.1902/jop.1993.64.10.954. [DOI] [PubMed] [Google Scholar]
- 4.Lambert PM, Morris HF, Ochi S. The influence of 0.12% chlorexidine digluconate rinses on the incidence of infectious complications and implant success. J Oral Maxillofac Surg. 1997;55(suppl 5):25–30. doi: 10.1016/s0278-2391(16)31194-6. [DOI] [PubMed] [Google Scholar]
- 5.Worthington P, Bolender CL, Taylor TD. The swedish system of osseointegrated implants: problems and complications encountered during a 4- year trial period. Int J Oral Maxillof Implants. 1987;2:77–84. [PubMed] [Google Scholar]
- 6.Lauc T, Kobler P. Early post-operative complications in oral implantology. Coll Antropol. 1998;22:251–257. [PubMed] [Google Scholar]
- 7.Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants: (II) Etiophatogenesis. Eur J Oral Sci. 1998;106:721–764. doi: 10.1046/j.0909-8836..t01-6-.x. [DOI] [PubMed] [Google Scholar]
- 8.Haas R, Haimbock W, Mailath G, Watzek G. The relationship of smoking on peri-implant tissue: a retrospective study. J Prosthet Dent. 1996;76:592–596. doi: 10.1016/s0022-3913(96)90435-7. [DOI] [PubMed] [Google Scholar]
- 9.Dao T, Mellor A. Sensory disturbances associated with implant surgery. Int J Prosthodont. 1998;11:462–469. [PubMed] [Google Scholar]
- 10.Ellies L. Altered sensation following mandibular implant surgery: a retrospective study. J Prosthet Dent. 1992;68:664–671. doi: 10.1016/0022-3913(92)90384-m. [DOI] [PubMed] [Google Scholar]
- 11.Ellies LG, Hawker PB. The prevalence of altered sensation associated with implant surgery. Int J Oral Maxillofac Implants. 1993;8:674–679. [PubMed] [Google Scholar]
- 12.Ellies LG. The incidence of altered sensation of the mental nerve after mandibular implant placement. J Oral Maxillofac Surg. 1999;57:1410–1412. doi: 10.1016/s0278-2391(99)90720-6. [DOI] [PubMed] [Google Scholar]
- 13.Wismeijer D, van Waas MA, Vermeeren JI, Kalk W. Patients’ perception of sensory disturbances of the mental nerve before and after implant surgery: a prospective study of 110 patients. Br J Oral Maxillofac Surg. 1997;35(4):254–9. doi: 10.1016/s0266-4356(97)90043-7. [DOI] [PubMed] [Google Scholar]
- 14.Jemt T, Laney WR, Herris D, Henry PJ, Krogh PH, Polizzi G. Osteointegrated implants for single tooth replacement: a 1-year report from a multicenter prospective study. Int J Oral Maxillof Impl. 1991;6:29–36. [PubMed] [Google Scholar]
- 15.Tal H. Spontaneus early exposure of submerged implants: classification and clinical observations. J Periodontal. 1999;70:213–219. doi: 10.1902/jop.1999.70.2.213. [DOI] [PubMed] [Google Scholar]
- 16.Goodacre C, Kan J, Rungcharassaeng K. Clinical complications of osseointegrated implants. J Prosthet Dent. 1999;81:537–552. doi: 10.1016/s0022-3913(99)70208-8. [DOI] [PubMed] [Google Scholar]
- 17.Toljanic J, Banakis M, Willes L, Graham L. Soft tissue exposure of endosseous implants between stage I and stage II surgery as a potential indicator of early crestal bone loss. Int J Oral Maxillof Implants. 1999;14:436–441. [PubMed] [Google Scholar]
- 18.Buser D, Bragger U, Lang NP, Nyman S. Regeneration and anlargement of jaw bone guided tissue regeneration. Clin Oral Impl Res. 1990;1:22–32. doi: 10.1034/j.1600-0501.1990.010104.x. [DOI] [PubMed] [Google Scholar]
- 19.Ueda M, Kaneda T. Maxillary sinusitis caused by dental implants: report of two cases. J Oral Maxillof Surg. 1992;50:285–287. doi: 10.1016/0278-2391(92)90328-w. [DOI] [PubMed] [Google Scholar]
- 20.Quiney RE, Brimble E, Hodge M. Maxillary sinusitis from dental osseointegrated implants. J Laryng Otol. 1990;104:333–334. doi: 10.1017/s0022215100112630. [DOI] [PubMed] [Google Scholar]
- 21.Regev E, Smith RA, Perrott DH, Pogrel MA. Maxillary sinus complications related to endosseous implants. J Oral Maxillofac Surg. 1995;10:451–461. [PubMed] [Google Scholar]
- 22.Mason M, Triplett G, Van Sickels J, Parel S. Mandibular fractures through endosseous cylinder implants: report of cases and review. J Oral Maxillofac Surg. 1990;48:311–317. doi: 10.1016/0278-2391(90)90401-m. [DOI] [PubMed] [Google Scholar]
- 23.Rothman SLG, Schwarz MS, Chafetz NI. High-resolution computerized tomography and nuclear bone scanning in the diagnosis of postoperative stress fractures of the mandible: a clinical report. Int J Oral Maxillofac Implants. 1995;10:765–768. [PubMed] [Google Scholar]
- 24.Raghoebar GM, Stellingsma K, Batenburg RH, Vissink A. Etiology and management of mandibular fractures associated with endosteal implants in the atrophic mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:553–9. doi: 10.1067/moe.2000.105237. [DOI] [PubMed] [Google Scholar]
- 25.Shonberg DC, Stith HD, Jameson LM, Chai JY. Mandibular Fracture Through an Endosseous Implant. Int J Oral Maxillofac Implants. 1992;7:401–404. [Google Scholar]
- 26.Eyrich G, Gratz K, Sailer H. Surgical treatment of fractures of the edentulous mandible. J Oral Maxillofac Surg. 1997;55:1081–1087. doi: 10.1016/s0278-2391(97)90284-6. [DOI] [PubMed] [Google Scholar]
- 27.Esposito M, Thomsen P, Ericson L, Lekholm U. Histopathologic observations on early oral implant failures. Int J Oral Maxillof Implants. 1999;14:798–810. [PubMed] [Google Scholar]
- 28.Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants: (I) Success criteria and epidemiology. Eur J Oral Sci. 1998;106:527–551. doi: 10.1046/j.0909-8836..t01-2-.x. [DOI] [PubMed] [Google Scholar]
- 29.Schmid J. Pathogenesis of implant failures. Periodontology 2000. 1994;4:127–138. doi: 10.1111/j.1600-0757.1994.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 30.Block MS, Kent JN. Factors associated with soft-and hard-tissue compromise of endosseous implants. J Oral Maxillofac Surg. 1990 Nov;48(11):1153–60. doi: 10.1016/0278-2391(90)90531-6. [DOI] [PubMed] [Google Scholar]
- 31.Piattelli A, Scarano A, Balleri P, Favero G. Clinical and histologic evaluation of an active implant periapical lesion: a case report. Int J Oral Maxillofac Impl. 1998;13:713–716. [PubMed] [Google Scholar]
- 32.Piattelli A, Scarano A, Piattelli M, Podda G. Implant periapical lesions: clinical, histologic and histochemical aspects. A case report. Int J Periodont Rest Dent. 1998;18:181–187. [PubMed] [Google Scholar]
- 33.Piattelli A, Scarano A, Piattelli M. Abscess formation around the apex of a maxillary root form implant: clinical and microscopical aspects. A case report. J Periodontol. 1995;66(10):899–903. doi: 10.1902/jop.1995.66.10.899. [DOI] [PubMed] [Google Scholar]
- 34.Shaffer MD, Juruaz DA, Haggerty PC. The effect of periradicular endodontic pathosis on the apical region of adjacent implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(5):578–81. doi: 10.1016/s1079-2104(98)90349-3. [DOI] [PubMed] [Google Scholar]
- 35.Chaffee NR, Lowden K, Tiffee JC, Cooper LF. Periapical abscess formation and resolution adjacent to dental implants: a clinical report. J Prosthet Dent. 2001;85(2):109–12. doi: 10.1067/mpr.2001.113353. [DOI] [PubMed] [Google Scholar]
- 36.Reiser GM, Nevins M. The implant periapical lesion: etiology, prevention, and treatment. Compend Contin Educ Dent. 1995;16(8):768, 770, 772. passim. [PubMed] [Google Scholar]
- 37.Rosenberg ES, Torosian JP, Slots J. Microbial differences in 2 clinically distinct types of failures of osseointegrated implants. Clin Oral Impl Res. 1991;2:135–144. doi: 10.1034/j.1600-0501.1991.020306.x. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann B, Bragger U, Hammerle CHF, Fourmousis I, Lang NP. Treatment of an early implant failure according to the principles of guided tissue regeneration (GTR) Clin Oral Impl Res. 1992;3:42–48. doi: 10.1034/j.1600-0501.1992.030107.x. [DOI] [PubMed] [Google Scholar]