Abstract
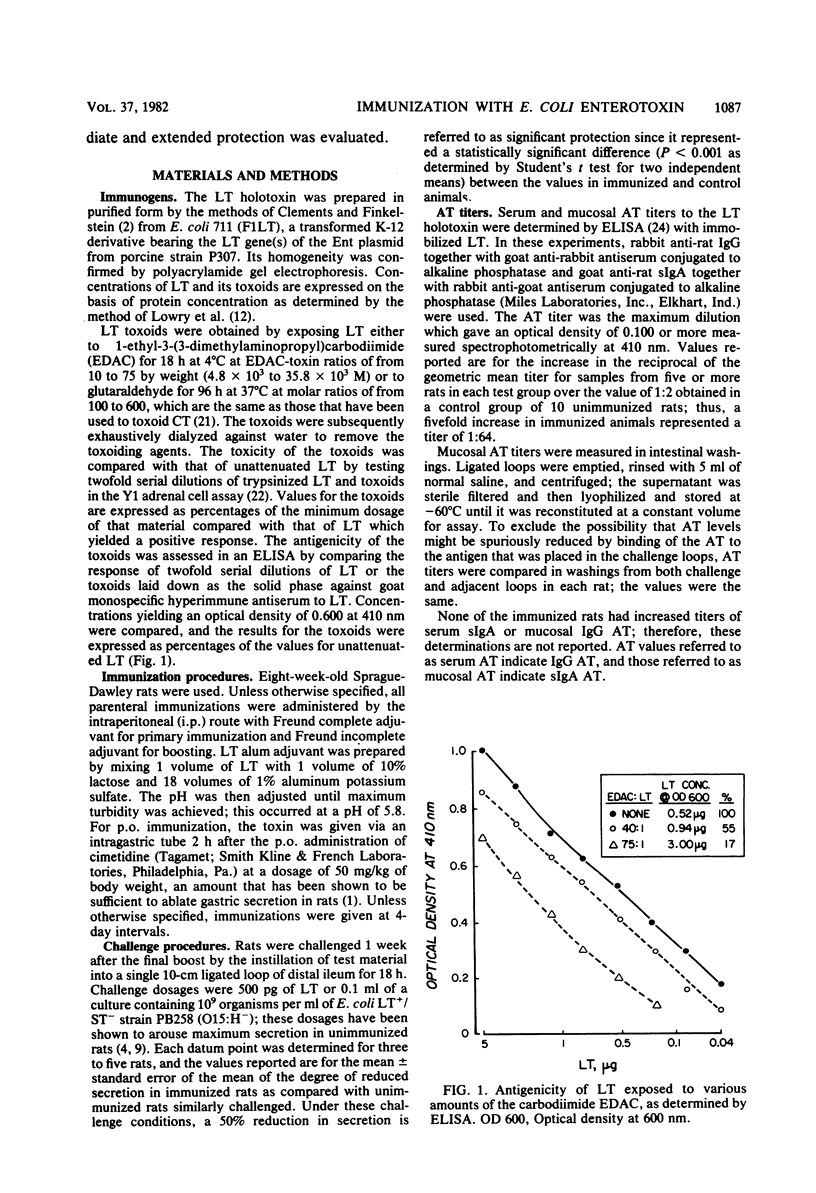
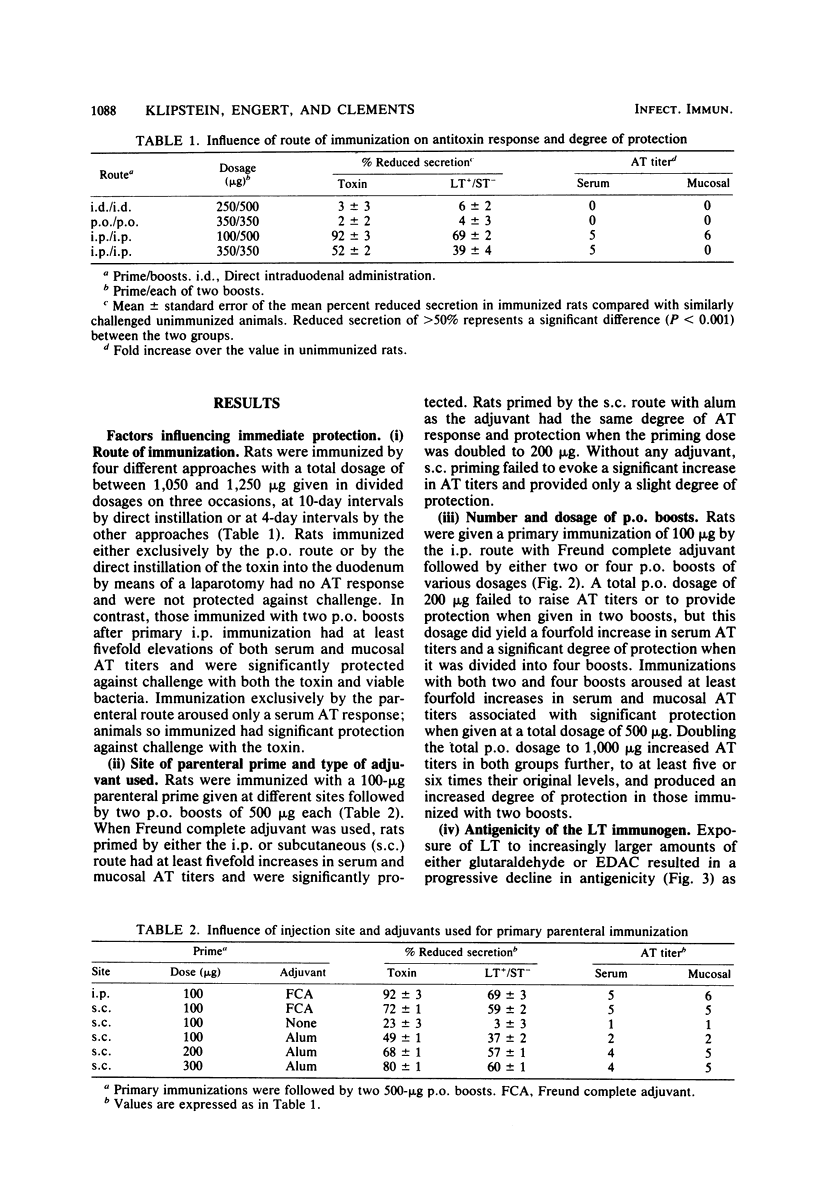
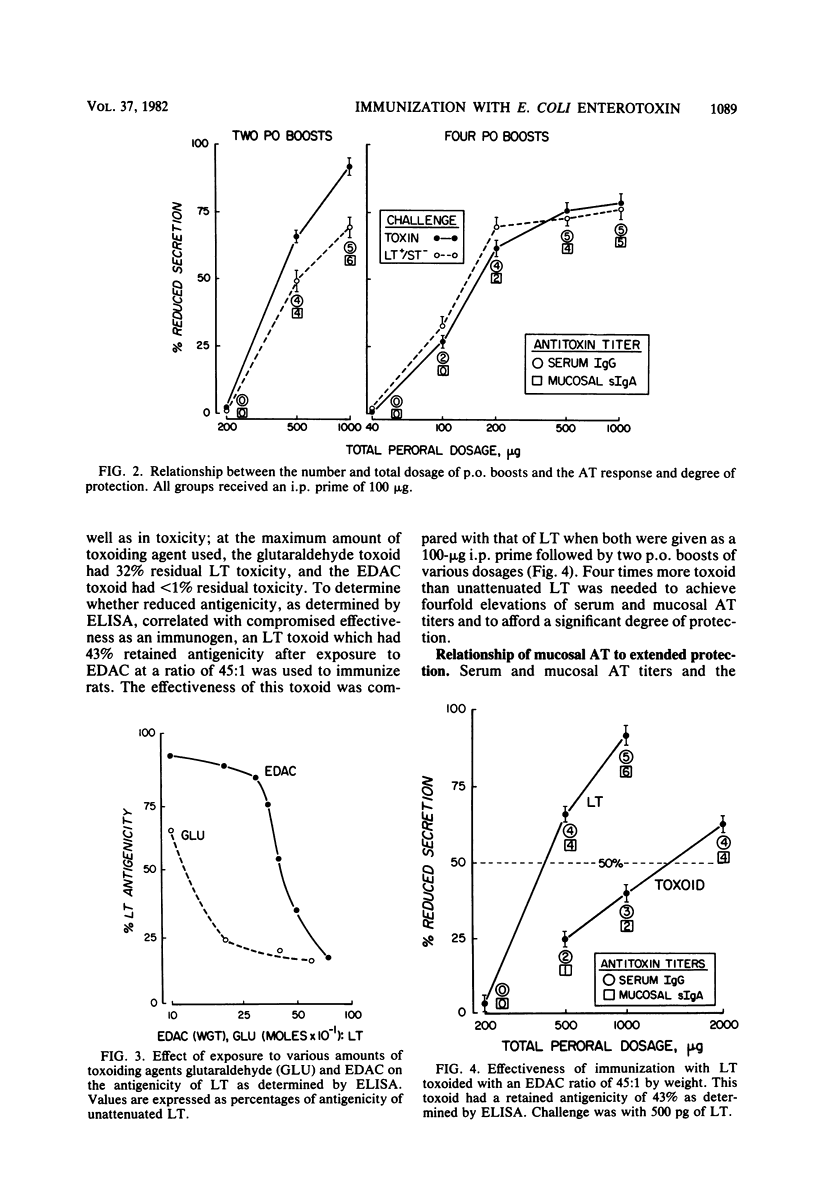
Specific serum and mucosal antitoxin levels were determined by enzyme-linked immunosorbent assays in rats immunized with Escherichia coli heat-labile enterotoxin (LT). Immunization by means of a parenteral prime followed by peroral boosts was the only approach that aroused titers of both serum immunoglobulin G (IgG) antitoxin and mucosal secretory IgA antitoxin that were increased fourfold or more over control values. Primary parenteral immunization was effective when given either intraperitoneally or subcutaneously with either Freund complete adjuvant or alum as the adjuvant. The magnitude of the nucosal secretory IgA antitoxin response and the degree of protection against challenge with either LT or viable LT-producing organisms were related to the number and dosage of peroral boosts. LT antigenicity, as determined by enzyme-linked immunosorbent assay, was progressively reduced by toxoiding it with increasing amounts of glutaraldehyde or a carbodiimide; when LT antigenicity was reduced by greater than 50%, the effectiveness of the toxoid in stimulating mucosal antitoxin and providing protection was compromised. Strong protection extended for more than 6 weeks only in rats immunized with a sufficient peroral dosage of LT to arouse mucosal secretory IgA antitoxin titers at least fourfold greater than those of controls. These observations indicate that the ability of LT to stimulate a mucosal secretory IgA antitoxin response is dependent on the antigenicity, route, and dosage of this immunogen; they suggest that the duration of protection in animals immunized by the peroral route is related to the extent of arousal of mucosal secretory IgA antitoxin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brimblecombe R. W., Duncan W. A., Durant G. J., Emmett J. C., Ganellin C. R., Leslie G. B., Parsons M. E. Characterization and development of cimetidine as a histamine H2-receptor antagonist. Gastroenterology. 1978 Feb;74(2 Pt 2):339–347. [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Immunization of rats with heat-labile enterotoxin provides uniform protection against heterologous serotypes of enterotoxigenic Escherichia coli. Infect Immun. 1981 Jun;32(3):1100–1104. doi: 10.1128/iai.32.3.1100-1104.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Protection in rats immunized with Escherichia coli heat-stable enterotoxin. Infect Immun. 1981 Nov;34(2):637–639. doi: 10.1128/iai.34.2.637-639.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Influence of route of administration on immediate and extended protection in rats immunized with Escherichia coli heart-labile enterotoxin. Infect Immun. 1980 Jan;27(1):81–86. doi: 10.1128/iai.27.1.81-86.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Protective effect of active immunization with purified Escherichia coli heat-labile enterotoxin in rats. Infect Immun. 1979 Mar;23(3):592–599. doi: 10.1128/iai.23.3.592-599.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Protective effect of immunization of rats with holotoxin or B subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Jan;31(1):144–150. doi: 10.1128/iai.31.1.144-150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Respective contributions to protection of primary and booster immunization with Escherichia coli heat-labile enterotoxin in rats. Infect Immun. 1981 Jan;31(1):252–260. doi: 10.1128/iai.31.1.252-260.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Short H. B. Protective effect of immunization with heat-labile enterotoxin in gnotobiotic rats monocontaminated with enterotoxigenic Escherichia coli. Infect Immun. 1980 Apr;28(1):163–170. doi: 10.1128/iai.28.1.163-170.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange S., Hansson H. A., Molin S. O., Nygren H. Local cholera immunity in mice: intestinal antitoxin-containing cells and their correlation with protective immunity. Infect Immun. 1979 Mar;23(3):743–750. doi: 10.1128/iai.23.3.743-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Engel P. F. Antitoxic immunity to cholera in dogs immunized orally with cholera toxin. Infect Immun. 1980 Feb;27(2):632–637. doi: 10.1128/iai.27.2.632-637.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sircar B. K. Induction of a mucosal antitoxin response and its role in immunity to experimental canine cholera. Infect Immun. 1978 Jul;21(1):185–193. doi: 10.1128/iai.21.1.185-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Koster F. T. Priming and suppression of the intestinal immune response to cholera toxoid/toxin by parenteral toxoid in rats. J Immunol. 1980 Jan;124(1):307–311. [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B. Immune response of the intestinal mucosa to cholera toxoid. J Infect Dis. 1977 Aug;136 (Suppl):S113–S117. doi: 10.1093/infdis/136.supplement.s113. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. S., Bonde G., McCann T., Rubin B. A., Tint H. Development of a purified cholera toxoid. II. Preparation of a stable, antigenic toxoid by reaction of purified toxin with glutaraldehyde. Infect Immun. 1974 Feb;9(2):304–317. doi: 10.1128/iai.9.2.304-317.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack D. A., Sack R. B. Test for enterotoxigenic Escherichia coli using Y-1 adrenal cells in miniculture. Infect Immun. 1975 Feb;11(2):334–336. doi: 10.1128/iai.11.2.334-336.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A., Lange S., Holmgren J. Correlation between intestinal synthesis of specific immunoglobulin A and protection against experimental cholera in mice. Infect Immun. 1978 Jul;21(1):1–6. doi: 10.1128/iai.21.1.1-6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley J. H., Keren D. F., Hamilton S. R., Brown G. D. Local (immunoglobulin A) immune response by the intestine to cholera toxin and its partial suppression with combined systemic and intra-intestinal immunization. Infect Immun. 1978 Feb;19(2):589–597. doi: 10.1128/iai.19.2.589-597.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



