Abstract
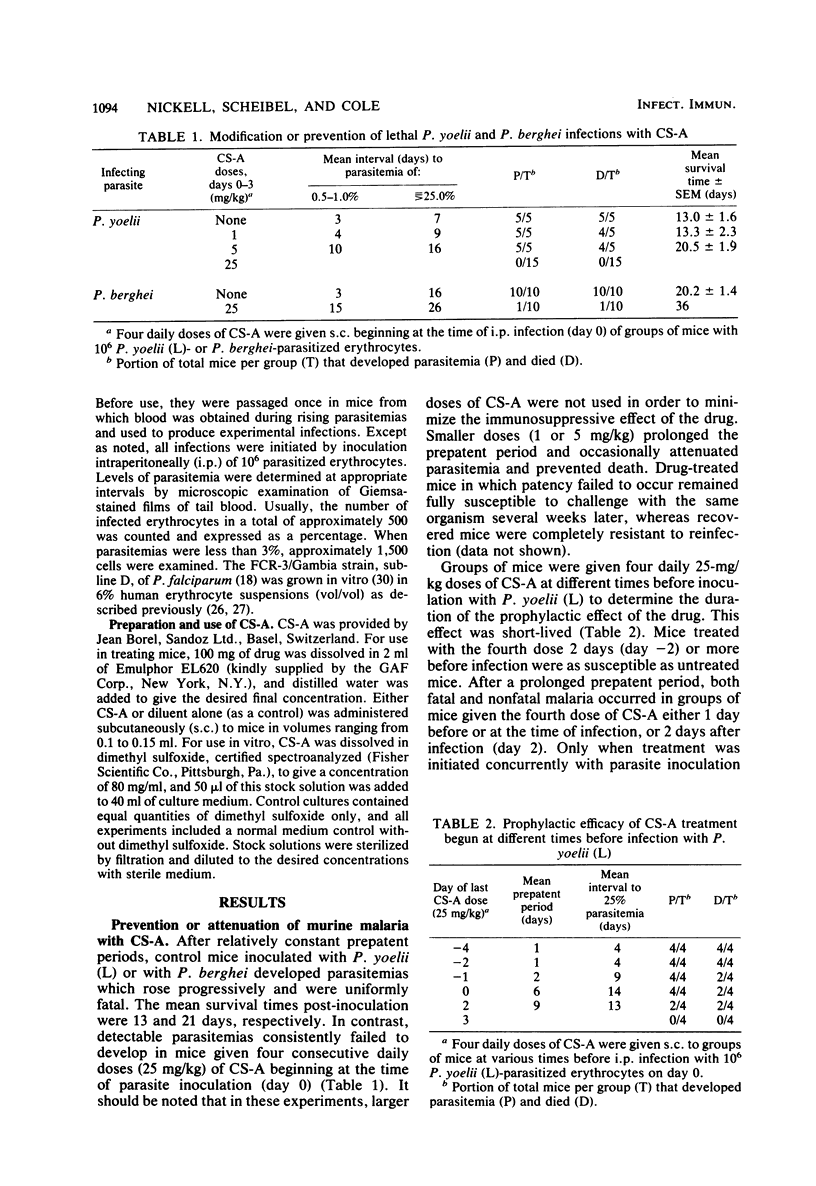
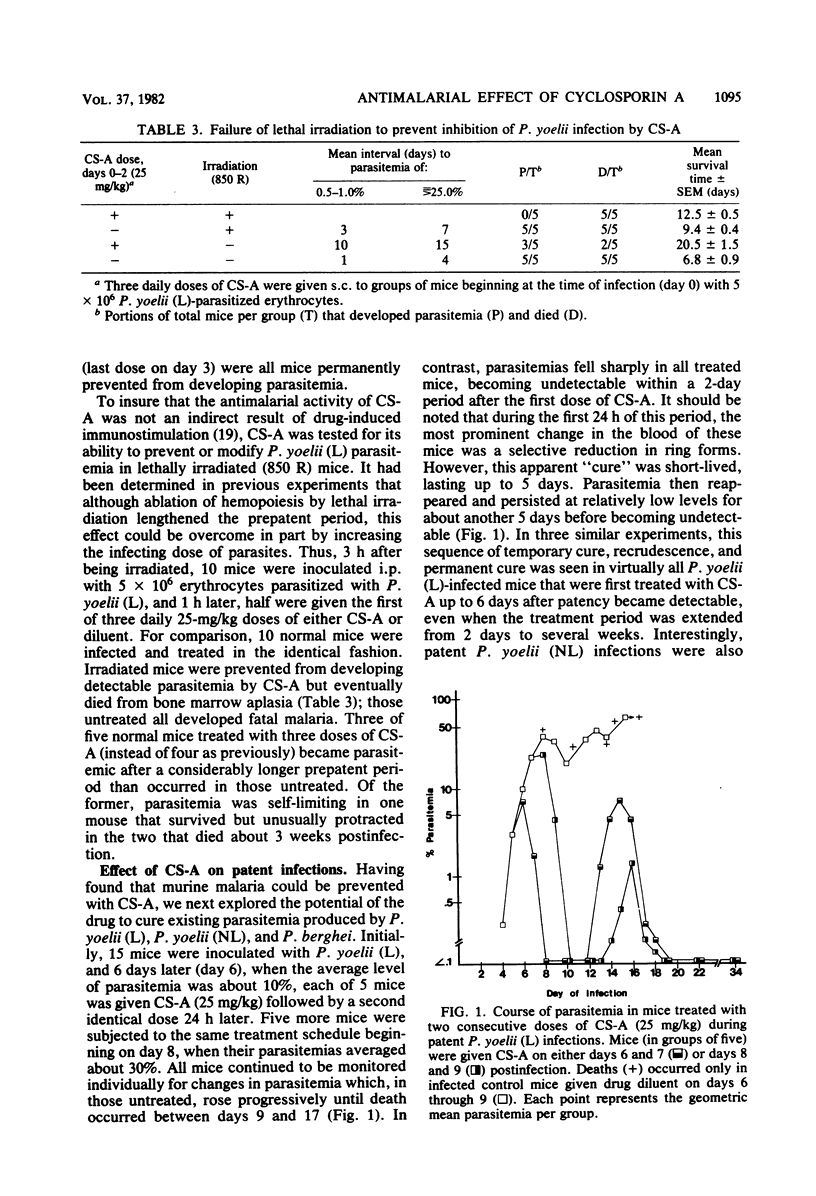
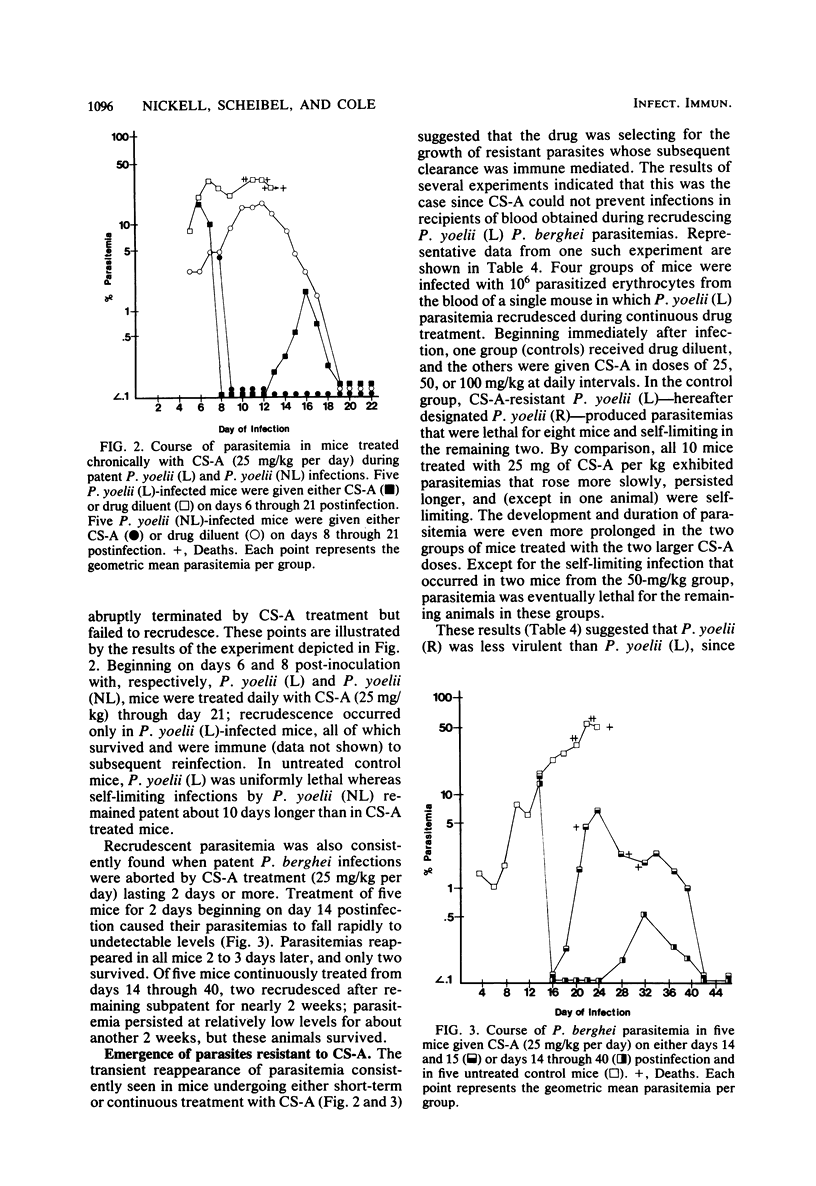
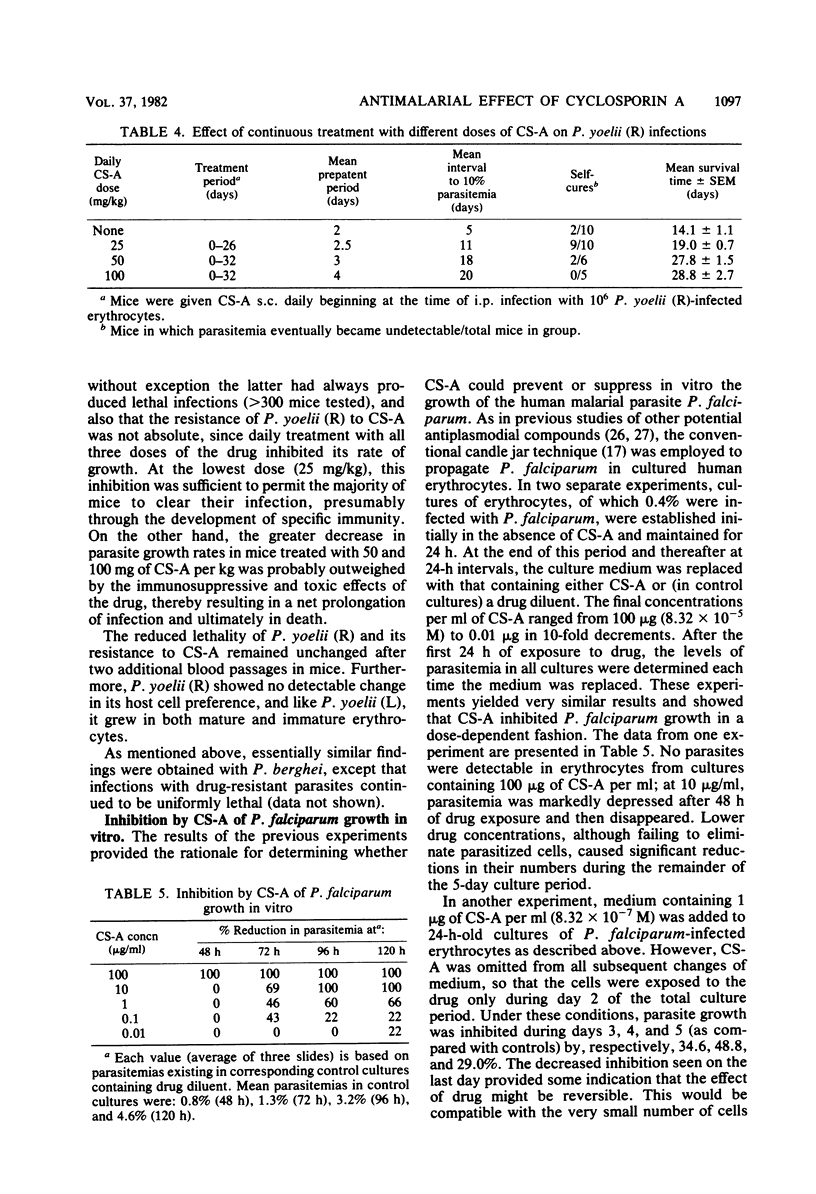
The development and course of normally lethal parasitemias in mice inoculated intraperitoneally with erythrocytic stages of Plasmodium yoelii or Plasmodium berghei were markedly affected by treatment with the antilymphoid drug cyclosporin A (CS-A). When the first of four daily subcutaneous 25-mg/kg doses of CS-A was given at the time of parasite inoculation, patent infections failed to develop. If begun up to 5 days earlier, this same treatment regimen prolonged the prepatent period, attenuated parasitemia, and reduced mortality. In mice with patient infections, two consecutive daily 25-mg/kg doses of CS-A were sufficient to terminate parasitemias which, after several days, reappeared but were self-limiting. This pattern of apparent cure followed by transient recrudescence remained unaltered even when daily treatment with the same drug dose was continued for 3 weeks. Recrudescence was associated with the emergence of parasite populations that were relatively resistant to CS-A and, in the case of P. yoelii, of reduced virulence. In more limited experiments, CS-A was found to be active in vitro against erythrocytic stages of the human malarial parasite palsmodium falciparum. Depending on the concentration of drug in the culture medium, parasite growth was either prevented or inhibited.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale G. H. The genetics of drug resistance in malaria parasites. Bull World Health Organ. 1980;58(5):799–804. [PMC free article] [PubMed] [Google Scholar]
- Borel J. F. Cyclosporin-A--present experimental status. Transplant Proc. 1981 Mar;13(1 Pt 1):344–348. [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Borel J. F. From our laboratories: cyclosporin A. Triangle. 1981;20(3):97–105. [PubMed] [Google Scholar]
- Brown I. N., Allison A. C., Taylor R. B. Plasmodium berghei infections in thymectomized rats. Nature. 1968 Jul 20;219(5151):292–293. doi: 10.1038/219292a0. [DOI] [PubMed] [Google Scholar]
- Bueding E., Hawkins J., Cha Y. N. Antischistosomal effects of cyclosporin A. Agents Actions. 1981 Jul;11(4):380–383. doi: 10.1007/BF01982474. [DOI] [PubMed] [Google Scholar]
- Burckhardt J. J., Guggenheim B. Cyclosporin A: in vivo and in vitro suppression of rat T-lymphocyte function. Immunology. 1979 Apr;36(4):753–757. [PMC free article] [PubMed] [Google Scholar]
- Calne R. Y., White D. J., Rolles K., Smith D. P., Herbertson B. M. Prolonged survival of pig orthotopic heart grafts treated with cyclosporin A. Lancet. 1978 Jun 3;1(8075):1183–1185. doi: 10.1016/s0140-6736(78)90971-6. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C. Babesia microti and Plasmodium berghei yoelii infections in nude mice. Nature. 1974 Nov 22;252(5481):328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- Gordon M. Y., Singer J. W. Selective effects of cyclosporin A on colony-forming lymphoid and myeloid cells in man. Nature. 1979 May 31;279(5712):433–434. doi: 10.1038/279433a0. [DOI] [PubMed] [Google Scholar]
- Green C. J., Allison A. C. Extensive prolongation of rabbit kidney allograft survival after short-term cyclosporin-A treatment. Lancet. 1978 Jun 3;1(8075):1182–1183. doi: 10.1016/s0140-6736(78)90970-4. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., Trigg P. I. Action of pyrimethamine and related drugs against Plasmodium knowlesi in vitr. Parasitology. 1971 Jun;62(3):431–444. doi: 10.1017/s0031182000077581. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Carter R. L., Leuchars E., Davies A. J. The immunological response of CBA mice to P. yoelii. I. General characteristics, the effects of T-cell deprivation and reconstitution with thymus grafts. Immunology. 1977 Jun;32(6):849–859. [PMC free article] [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: establishment of additional strains. Am J Trop Med Hyg. 1978 Jul;27(4):743–746. doi: 10.4269/ajtmh.1978.27.743. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: use of outdated erthrocytes and description of the candle jar method. J Parasitol. 1977 Oct;63(5):883–886. [PubMed] [Google Scholar]
- Kunkl A., Klaus G. G. Selective effects of cyclosporin A on functional B cell subsets in the mouse. J Immunol. 1980 Dec;125(6):2526–2531. [PubMed] [Google Scholar]
- Larsson E. L. Cyclosporin A and dexamethasone suppress T cell responses by selectively acting at distinct sites of the triggering process. J Immunol. 1980 Jun;124(6):2828–2833. [PubMed] [Google Scholar]
- Nguyen-Dinh P., Payne D. Pyrimethamine sensitivity in Plasmodium falciparum: determination in vitro by a modified 48-hour test. Bull World Health Organ. 1980;58(6):909–912. [PMC free article] [PubMed] [Google Scholar]
- Petcher T. J., Weber H., Rüegger A. Crystal and molecular structure of an iodo-derivative of the cyclic undecapeptide cyclosporin A. Helv Chim Acta. 1976 Jun 14;59(5):1480–1489. doi: 10.1002/hlca.19760590509. [DOI] [PubMed] [Google Scholar]
- Powles R. L., Barrett A. J., Clink H., Kay H. E., Sloane J., McElwain T. J. Cyclosporin A for the treatment of graft-versus-host disease in man. Lancet. 1978 Dec 23;2(8104-5):1327–1331. doi: 10.1016/s0140-6736(78)91971-2. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Rank R. G., Weidanz W. P., Finerty J. F. Prevention of recrudescent malaria in nude mice by thymic grafting or by treatment with hyperimmune serum. Infect Immun. 1977 Jun;16(3):821–826. doi: 10.1128/iai.16.3.821-826.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel B., Donatsch P., Götz U., Tschopp M. Cyclosporin receptor on mouse lymphocytes. Immunology. 1980 Dec;41(4):913–919. [PMC free article] [PubMed] [Google Scholar]
- Scheibel L. W., Adler A. Antimalarial activity of selected aromatic chelators. Mol Pharmacol. 1980 Sep;18(2):320–325. [PubMed] [Google Scholar]
- Scheibel L. W., Adler A., Trager W. Tetraethylthiuram disulfide (Antabuse) inhibits the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5303–5307. doi: 10.1073/pnas.76.10.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thommen-Scott K. Antimalarial activity of cyclosporin A. Agents Actions. 1981 Dec;11(6-7):770–773. doi: 10.1007/BF01978803. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Whiting P. H., Cameron I. D., Lessels S. E., Simpson J. G. A toxicological study in rats receiving immunotherapeutic doses of cyclosporin A. Transplantation. 1981 Feb;31(2):121–124. doi: 10.1097/00007890-198102000-00006. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Tutschka P. J., Beschorner W. E., Allison A. C., Burns W. H., Santos G. W. Use of cyclosporin A in allogeneic bone marrow transplantation in the rat. Nature. 1979 Jul 12;280(5718):148–151. doi: 10.1038/280148a0. [DOI] [PubMed] [Google Scholar]
- Walliker D., Sanderson A., Yoeli M., Hargreaves B. J. A genetic investigation of virulence in a rodent malaria parasite. Parasitology. 1976 Apr;72(2):183–194. doi: 10.1017/s0031182000048484. [DOI] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]
- White D. J., Plumb A. M., Pawelec G., Brons G. Cyclosporin A: an immunosuppressive agent preferentially active against proliferating T cells. Transplantation. 1979 Jan;27(1):55–58. [PubMed] [Google Scholar]
- Wiesinger D., Borel J. F. Studies on the mechanism of action of cyclosporin A. Immunobiology. 1980 Jan;156(4-5):454–463. doi: 10.1016/S0171-2985(80)80078-7. [DOI] [PubMed] [Google Scholar]



