Abstract
Increased tumor-associated macrophages (TAMs) are reported to be associated with poor prognosis in classic Hodgkin lymphoma (CHL). We investigated the prognostic significance of TAMs in the E2496 Intergroup trial, a multicenter phase 3 randomized controlled trial comparing ABVD and Stanford V chemotherapy in locally extensive and advanced stage CHL. Tissue microarrays were constructed from formalin-fixed, paraffin-embedded tumor tissue and included 287 patients. Patients were randomly assigned into training (n = 143) and validation (n = 144) cohorts. Immunohistochemistry for CD68 and CD163, and in situ hybridization for EBV-encoded RNA were performed. CD68 and CD163 IHC were analyzed by computer image analysis; optimum thresholds for overall survival (OS) were determined in the training cohort and tested in the independent validation cohort. Increased CD68 and CD163 expression was significantly associated with inferior failure-free survival and OS in the validation cohort. Increased CD68 and CD163 expression was associated with increased age, EBV-encoded RNA positivity, and mixed cellularity subtype of CHL. Multivariate analysis in the validation cohort showed increased CD68 or CD163 expression to be significant independent predictors of inferior failure-free survival and OS. We demonstrate the prognostic significance of TAMs in locally extensive and advanced-stage CHL in a multicenter phase 3 randomized controlled clinical trial.
Introduction
Despite advances in the treatment of classic Hodgkin lymphoma (CHL), current therapies fail to cure 10%-15% of patients, and a similar proportion of patients may be overtreated, resulting in both short-term and long-term treatment-related complications. The International Prognostic Factors Project Score (IPS) is the current gold standard used to risk-stratify patients with advanced-stage CHL, but its power to identify patients in whom treatment is likely to fail in the modern treatment era has weakened.1–3 Robust biomarkers are thus needed to better risk-stratify patients at diagnosis.
In CHL, the malignant Hodgkin-Reed-Sternberg (HRS) cells are greatly outnumbered by non-neoplastic cells in the tumor microenvironment, including macrophages, T cells, B cells, eosinophils, mast cells, and other stromal elements. Manipulation of the microenvironment by HRS cells through expression of a variety of cytokines and chemokines is thought to be the driving force for an abnormal immune response, perpetuated by additional factors secreted by recruited reactive cells in the microenvironment.4 Tumor-associated macrophages (TAMs) were shown to be associated with inferior outcomes in CHL.5 Steidl et al showed a macrophage gene expression signature to be associated with primary treatment failure in CHL and subsequently showed, using an independent validation cohort, that increased CD68 IHC expression was associated with inferior outcomes, including outcome after salvage treatment with autologous stem cell transplantation.6 Since then, most,7–18 but not all,12,18–20 subsequent studies have confirmed the inferior prognostic significance of TAMs in CHL using CD68 and/or CD163 IHC. In addition, early interim positron emission tomography analysis after 2 courses of chemotherapy has prognostic value in advanced-stage CHL, and increased CD68 IHC expression was recently shown to be associated with a higher rate of early positron emission tomography positivity.8 However, there has been variability in suggested threshold values for CD68 and CD163 IHC expression in the literature. This variability may reflect differences in IHC quantitation methodology between studies, the use of manual visual scoring techniques, and lack of subsequent validation of thresholds in their respective studies. In addition, studies thus far represent retrospective single institution experiences.
We address these current issues in our study by investigating the prognostic significance of TAMs using CD68 and CD163 IHC in the E2496 Intergroup trial, a large multicenter phase 3 randomized controlled clinical trial comparing ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and Stanford V (doxorubicin, vinblastine, bleomycin, vincristine, mechloroethamine, etoposide, and prednisone) chemotherapy. We use an objective method of quantitating CD68 and CD163 IHC expression with computer image analysis (Aperio Technologies) and establish optimum thresholds for CD68 and CD163 IHC expression using software X-tile (Version 3.6.1), which is based on the maximal χ2 value of the log-rank test for overall survival (OS) in a training cohort. These thresholds are then tested in a separate independent validation cohort.
Methods
Patients and samples
A total of 287 patients diagnosed with CHL according to the World Health Organization 2008 classification21 and with tissue available were included in this study, conducted in accordance with the Declaration of Helsinki. This represents a subset of the main clinical trial based on the availability of diagnostic paraffin blocks following central pathology review and patient consent for correlative studies (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These patients had locally extensive and advanced-stage CHL (stage 1 or 2 with bulky mediastinal disease, stage 3 and 4) enrolled in the E2496 ECOG/SWOG/NCIC/CALGB Intergroup trial, a phase 3 randomized controlled trial comparing ABVD and Stanford V chemotherapy treatment. All patients had complete data for CD68 and CD163 IHC, and in situ hybridization for EBV-encoded RNA (EBER ISH). The statistical software X-tile (Version 3.6.1)22 was used to randomly assign patients into training (n = 143) and validation (n = 144) cohorts. All participating sites received local institutional review board approval.
IHC
Tissue microarrays were constructed using duplicate 1.5-mm-diameter cores of formalin-fixed, paraffin-embedded tumor tissue. IHC performed on the tissue microarrays included CD68 (clone KP1, Dako North America; dilution 1:2000), CD163 (clone 10D6, Novocastra; dilution 1:100), and CD30 (clone BerH2, Dako North America; dilution 1:30). IHC stains were performed on a fully automated stainer (Ventana Benchmark XT) using a multimer detection kit (UltraView Universal DAB).
EBER ISH
EBER ISH was performed using the INFORM EBER probe (Ventana). Slides were also stained on an automated stainer (Ventana Benchmark XT) using the Ventana ISH/iView Blue detection kit. A known positive control was used. Nuclear staining in HRS cells was considered positive.
Immunohistochemistry scoring
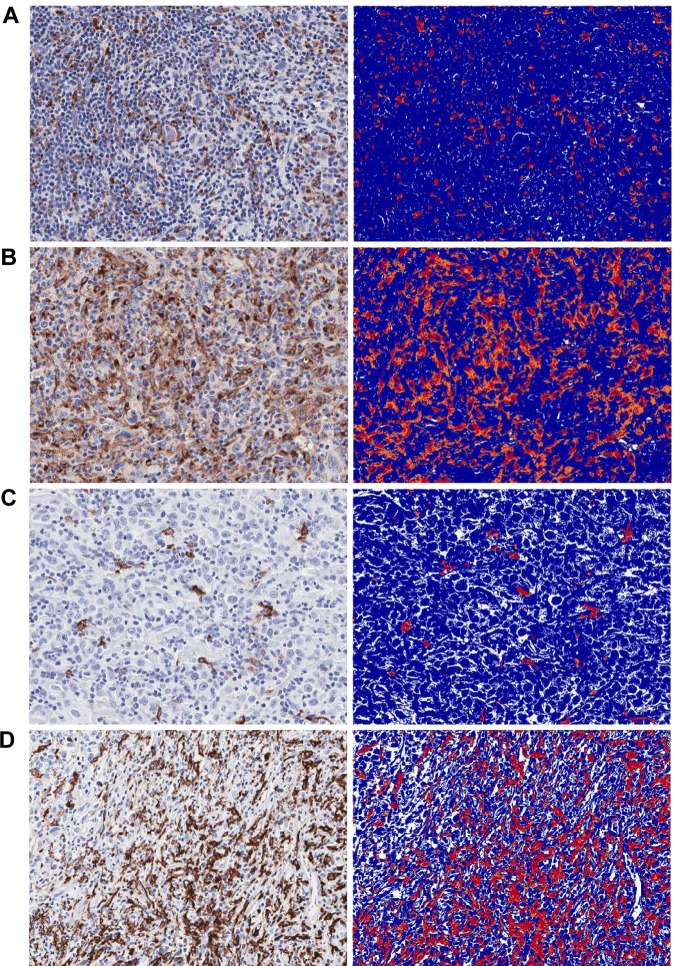
CD68 and CD163 IHCs were analyzed by computer image analysis (Aperio Technologies) and pathologist scoring (visual, K.L.T.). Immunostained slides were scanned by Aperio ScanScope XT at 20× magnification. CD68 and CD163 IHCs were analyzed using the Positive Pixel Count algorithm with the Aperio ImageScope (Version 11) viewer. Every core of tissue on the TMA was checked by a pathologist (K.L.T.) to ensure that computer image analysis was performed correctly. Aperio was able to analyze tissue cores in their entirety. Only areas containing tumor were analyzed for IHC expression. Areas without tumor (eg, fibrosis, medium to large blood vessels, residual reactive lymph node) and areas with necrosis or significant artifact (eg, tissue folding and crush artifact) were deselected and excluded from analysis. Cores lacking CD30+ HRS cells were also excluded from analysis. For the positive pixel count algorithm, hue value of 0.1 and hue width of 0.5 were used, and any intensity of staining was considered positive. A color saturation threshold of 0.1 was used for most cores. The color saturation threshold was rarely increased to 0.15 in cases with nonspecific background staining, to minimize analysis of nonspecific background staining. The number of positive pixels was divided by the total number of pixels (negative and positive) in the analyzed area, and multiplied by 100, to derive the percentage of positive pixels. Scores from both cores of the same patient were averaged when possible (Figure 1).
Figure 1.
CD68 and CD163 IHC expression and accompanying computer image analysis (original magnification ×10). (A) CD68low, 4.2%. (B) CD68high, 19.5%. (C) CD163low, 1.8%. (D) CD163high, 26.9%.
Visual scoring was performed by estimating relative percentages of CD68+ and CD163+ cells in relation to overall cellularity in both tissue cores from the same patient where possible; scores were recorded in 10% increments. Visual and Aperio scores showed excellent correlation (supplemental Figure 1).
Statistical analysis
Failure free survival (FFS) was defined as the time from randomization to treatment arm until progression, relapse, or death from any cause. OS was defined as the date of randomization to treatment arm to death from any cause. Correlation between variables was analyzed by Pearson correlation coefficient (R). Differences in variables between groups were analyzed by Pearson χ2 test, Student t test, and ANOVA. Survival estimates were calculated using the Kaplan-Meier method with differences assessed using the log-rank test. The Cox proportional hazards regression model was used for multivariate analysis. Statistical analyses were performed using SPSS software (Version 14.0). Two-sided P < .05 was considered statistically significant.
The statistical software X-tile (Version 3.6.1)22 was used to randomly assign patients into training and validation cohorts, as mentioned previously. X-tile was also used to determine the thresholds for CD68 and CD163 IHC expression, by selecting the maximal χ2 values of the log-rank test for OS between 2 groups, designated as low and high risk. These thresholds were then carried forward and tested in the independent validation cohort.
Results
Patient characteristics
There were no significant differences between the subset of cases available for correlative studies (n = 287) and those not available from the total clinical trial cohort (n = 507, giving a total of 794 patients; supplemental Table 1), suggesting that these cases were representative of the entire patient population. There were also no significant differences in patient characteristics between training and validation cohorts (Table 1).
Table 1.
Comparison of patient characteristics in training and validation cohorts
| Training |
Validation |
P | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 143 | 144 | |||
| Age (≥ 45 y) | |||||
| No | 118 | 83 | 122 | 85 | .61 |
| Yes | 25 | 17 | 22 | 15 | |
| Sex | |||||
| Female | 63 | 44 | 63 | 44 | .96 |
| Male | 80 | 56 | 81 | 56 | |
| Stage 4 disease | |||||
| No | 103 | 72 | 113 | 78 | .21 |
| Yes | 40 | 28 | 31 | 22 | |
| Albumin (< 4 g/dL)* | |||||
| No | 45 | 31 | 42 | 29 | .70 |
| Yes | 94 | 66 | 97 | 67 | |
| Unknown | 4 | 3 | 5 | 3 | |
| Hemoglobin (< 10.5 g/dL)* | |||||
| No | 117 | 82 | 109 | 76 | .15 |
| Yes | 18 | 13 | 27 | 19 | |
| Unknown | 8 | 6 | 8 | 6 | |
| WBC count (≥ 15 000/mm3)* | |||||
| No | 114 | 80 | 112 | 78 | .85 |
| Yes | 20 | 14 | 21 | 15 | |
| Unknown | 9 | 6 | 11 | 8 | |
| Lymphocyte count (< 600/mm3 or < 8%)* | |||||
| No | 126 | 88 | 125 | 87 | .81 |
| Yes | 9 | 6 | 10 | 7 | |
| Unknown | 8 | 6 | 9 | 6 | |
| IPS (≥ 3)* | |||||
| No | 99 | 69 | 90 | 63 | .26 |
| Yes | 44 | 31 | 53 | 37 | |
| Unknown | 0 | 0 | 1 | 1 | |
| Histologic subtype* | |||||
| Nodular sclerosis | 115 | 80 | 108 | 75 | .61 |
| Mixed cellularity | 18 | 13 | 20 | 14 | |
| Lymphocyte rich | 3 | 2 | 5 | 3 | |
| Lymphocyte depleted | 0 | 0 | 1 | 1 | |
| Unclassified | 7 | 5 | 10 | 7 | |
| Treatment received | |||||
| ABVD | 74 | 52 | 70 | 49 | .60 |
| Stanford V | 69 | 48 | 74 | 51 | |
| CD68 IHC expression | |||||
| ≤ 12.7% (CD68low) | 89 | 62 | 89 | 62 | .94 |
| > 12.7% (CD68high) | 54 | 38 | 55 | 38 | |
| CD163 IHC expression | |||||
| ≤ 16.8% (CD163low) | 90 | 63 | 78 | 54 | .13 |
| > 16.8% (CD163high) | 53 | 37 | 66 | 46 | |
| EBER ISH | |||||
| Positive | 25 | 17 | 24 | 17 | .85 |
| Negative | 118 | 83 | 120 | 83 | |
P values are for comparing training with validation cohorts.
Pearson χ2 test was performed with unknown or unclassified cases excluded.
CD68 expression
Using the optimum threshold of 12.7% obtained with X-tile, 89 patients had low CD68 expression (≤ 12.7%, CD68low) and 54 patients had high CD68 expression (> 12.7%, CD68high) in the training cohort. Carrying this threshold forward, the validation cohort consisted of 89 CD68low and 55 CD68high patients. There were no significant differences in CD68 expression (P = .91) or in proportions of CD68low and CD68high patients between training and validation cohorts (P = .94).
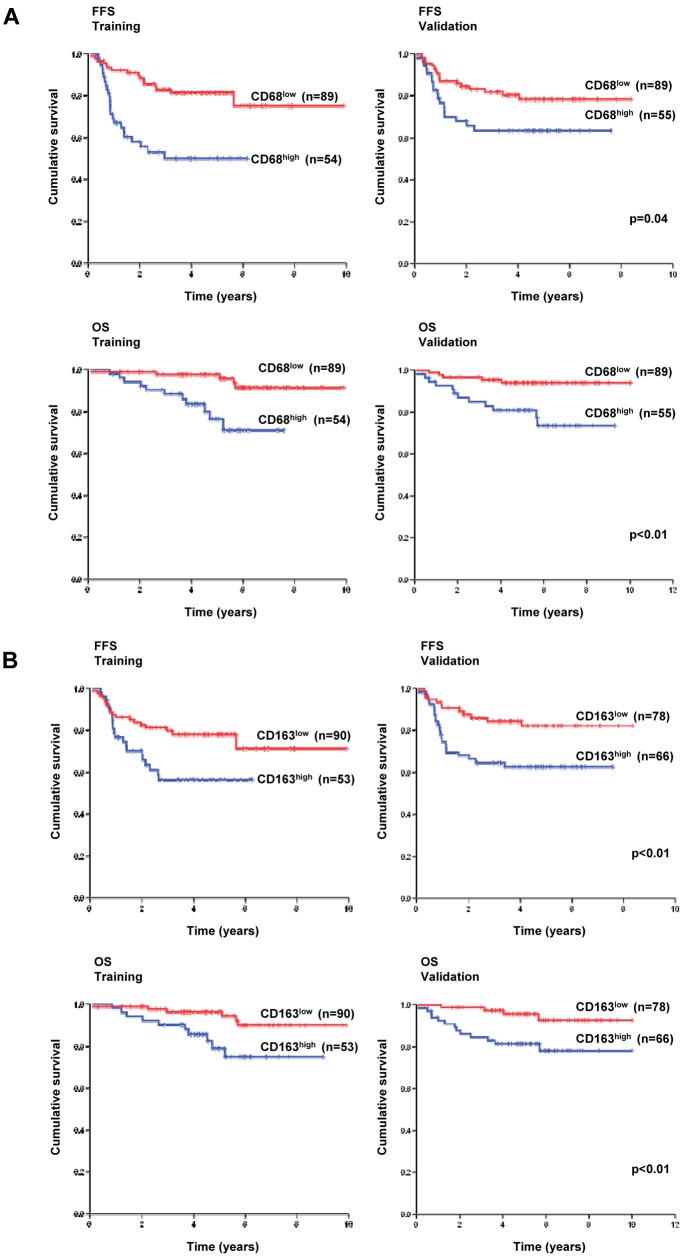
In the training cohort, CD68high patients had inferior outcomes, with the 5-year FFS rate being 50% versus 81% and 5-year OS rate being 76% versus 98%. In the validation cohort, CD68high patients also had significantly inferior outcomes, with the 5-year FFS rate being 64% versus 78% (P = .04) and 5-year OS rate being 81% versus 94% (P < .01; Figure 2).
Figure 2.
Survival analysis based on macrophage content. (A) CD68 and (B) CD163 IHC expression and survival in training and validation cohorts.
CD163 expression
Using the optimum threshold of 16.8% obtained with X-tile, 90 patients had low CD163 expression (≤ 16.8%, CD163low) and 53 patients had high CD163 expression (> 16.8%, CD163high) in the training cohort. Carrying this threshold forward, the validation cohort consisted of 78 CD163low and 66 CD163high patients. There were no significant differences in CD163 expression (P = .48) and proportions of CD163low and CD163high patients between training and validation cohorts (P = .13).
In the training cohort, CD163high patients had inferior outcomes, with the 5-year FFS rate being 56% versus 78% and the 5-year OS rate being 79% versus 96%. In the validation cohort, CD163high patients also had significantly inferior outcomes with the 5-year FFS rate being 63% versus 82% (P < .01) and 5-year OS rate being 81% versus 96% (P < .01; Figure 2).
Correlation of increased CD68 and CD163 expression with clinical and pathologic characteristics
When considering the entire cohort, patients with increased CD68 expression (CD68high) were significantly older (P < .01) and had increased proportions of mixed cellularity subtype of CHL (P < .01) and EBER+ cases (P < .01). Similarly, CD163high patients were also significantly older (P = .04) and had increased proportions of mixed cellularity subtype of CHL (P < .01) and EBER+ cases (P < .01; Table 2).
Table 2.
Comparison of patient characteristics with CD68 and CD163 IHC expression in the entire cohort
| CD68low |
CD68high |
P | CD163low |
CD163high |
P | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||
| Total | 178 | 109 | 168 | 119 | ||||||
| Age (≥ 45 y) | ||||||||||
| No | 157 | 88 | 83 | 76 | < .01 | 147 | 88 | 93 | 78 | .04 |
| Yes | 21 | 12 | 26 | 24 | 21 | 13 | 26 | 22 | ||
| Sex | ||||||||||
| Female | 82 | 46 | 44 | 40 | .35 | 76 | 45 | 50 | 42 | .59 |
| Male | 96 | 54 | 65 | 60 | 92 | 55 | 69 | 58 | ||
| Stage 4 disease | ||||||||||
| No | 132 | 74 | 84 | 77 | .58 | 129 | 77 | 87 | 73 | .48 |
| Yes | 46 | 26 | 25 | 23 | 39 | 23 | 32 | 27 | ||
| Albumin (< 4 g/dL)* | ||||||||||
| No | 56 | 31 | 31 | 28 | .62 | 45 | 27 | 42 | 35 | .11 |
| Yes | 117 | 66 | 74 | 68 | 118 | 70 | 73 | 61 | ||
| Unknown | 5 | 3 | 4 | 4 | 5 | 3 | 4 | 3 | ||
| Hemoglobin (< 10.5 g/dL)* | ||||||||||
| No | 140 | 79 | 86 | 79 | .97 | 136 | 81 | 90 | 76 | .39 |
| Yes | 28 | 16 | 17 | 16 | 24 | 14 | 21 | 18 | ||
| Unknown | 10 | 6 | 6 | 6 | 8 | 5 | 8 | 7 | ||
| WBC count (≥ 15 000/mm3)* | ||||||||||
| No | 135 | 76 | 91 | 83 | .10 | 129 | 77 | 97 | 82 | .18 |
| Yes | 30 | 17 | 11 | 10 | 28 | 17 | 13 | 11 | ||
| Unknown | 13 | 7 | 7 | 6 | 11 | 7 | 9 | 8 | ||
| Lymphocyte count (< 600/mm3 or < 8%)* | ||||||||||
| No | 157 | 88 | 94 | 86 | .19 | 148 | 88 | 103 | 87 | .32 |
| Yes | 9 | 5 | 10 | 9 | 9 | 5 | 10 | 8 | ||
| Unknown | 12 | 7 | 5 | 5 | 11 | 7 | 6 | 5 | ||
| IPS (≥ 3)* | ||||||||||
| No | 120 | 67 | 69 | 63 | .54 | 114 | 68 | 75 | 63 | .45 |
| Yes | 58 | 33 | 39 | 36 | 54 | 32 | 43 | 36 | ||
| Unknown | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | ||
| Histologic subtype* | ||||||||||
| Nodular sclerosis | 152 | 85 | 71 | 65 | < .01 | 141 | 84 | 82 | 69 | < .01 |
| Mixed cellularity | 11 | 6 | 27 | 25 | 13 | 8 | 25 | 21 | ||
| Lymphocyte rich | 5 | 3 | 3 | 3 | 6 | 4 | 2 | 2 | ||
| Lymphocyte depleted | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | ||
| Unclassified | 10 | 6 | 7 | 6 | 8 | 5 | 9 | 8 | ||
| Treatment received | ||||||||||
| ABVD | 88 | 49 | 56 | 51 | .75 | 82 | 49 | 62 | 52 | .58 |
| Stanford V | 90 | 51 | 53 | 49 | 86 | 51 | 57 | 48 | ||
| EBER ISH | ||||||||||
| Positive | 15 | 8 | 34 | 31 | < .01 | 20 | 12 | 29 | 24 | < .01 |
| Negative | 163 | 92 | 75 | 69 | 148 | 88 | 90 | 76 | ||
P values compare CD68low with CD68high, and CD163low with CD163high patients.
Pearson χ2 test was performed with unknown or unclassified cases excluded.
Both CD68high and CD163high were significantly associated with inferior outcomes in patients treated with either ABVD (CD68: FFS, P < .01; OS, P < .01; CD163: FFS, P = .03; OS, P = .04) or Stanford V chemotherapy (CD68: FFS, P < .01; OS, P = .02; CD163: FFS, P < .01; OS, P < .01; supplemental Figure 2).
EBER+ cases showed significantly higher CD68 and CD163 expression than EBER− cases (P < .01; Table 3). In addition, EBER+ patients were significantly older (P = .01), more often male (P = .02), with lower white blood cell counts (P = .02), IPS ≥ 3 (P = .04), and increased proportions of mixed cellularity subtype of CHL (P < .01), CD68high (P < .01), and CD163high (P < .01; supplemental Table 2). It was thus not surprising to observe significantly higher CD68 and CD163 expression in mixed cellularity subtype of CHL, compared with nodular sclerosis and lymphocyte rich subtypes (P < .01; Table 3).
Table 3.
Comparison of CD68 and CD163 IHC expression in training and validation cohorts, in EBER+ and EBER− cases, and selected subtypes of CHL in the entire cohort
| n | CD68 expression |
CD163 expression |
|||||
|---|---|---|---|---|---|---|---|
| Median | Range | P | Median | Range | P | ||
| Training | 143 | 10.6 | 2.7-57.4 | .91 | 13.0 | 0.4-79.5 | .48 |
| Validation | 144 | 10.5 | 2.4-51.8 | 13.5 | 0.4-79.1 | ||
| EBER+ | 49 | 15.7 | 3.1-57.4 | < .01 | 29.9 | 1.5-79.5 | < .01 |
| EBER− | 238 | 9.6 | 2.4-37.2 | 11.1 | 0.4-79.1 | ||
| Nodular sclerosis | 223 | 9.6 | 2.7-51.8 | < .01 | 10.9 | 0.4-79.1 | < .01 |
| Mixed cellularity | 38 | 16.1 | 2.8-57.4 | 28.9 | 0.4-79.5 | ||
| Lymphocyte rich | 8 | 7.1 | 2.8-26.7 | 8.9 | 1.4-41.4 | ||
No significant differences in outcome were seen between EBER+ and EBER− patients (FFS, P = .66; OS, P = .44). However, CD163high was significantly associated with inferior outcomes in both EBER+ (FFS, P < .01; OS, P = .02) and EBER− (FFS, P = .01; OS, P < .01) patients. CD68high was significantly associated with inferior outcomes in EBER− cases (FFS, P < .01; OS, P < .01) but not EBER+ cases (FFS, P = .34; OS, P = .33; supplemental Figure 3).
Increased CD68 or CD163 expression is a significant independent predictor of inferior outcome
Univariate and multivariate analyses were performed on the validation cohort. On univariate analysis, stage 4 disease, low lymphocyte count, and increased CD68 and CD163 expression were significantly associated with inferior FFS. Increased age and increased CD68 and CD163 expression were significantly associated with inferior OS (Table 4).
Table 4.
Univariate analysis in the validation cohort
| Factor | 5-y FFS, % | P | 5-y OS, % | P |
|---|---|---|---|---|
| Age (≥ 45 y) | ||||
| No | 74 | .49 | 91 | < .01 |
| Yes | 69 | 77 | ||
| Sex | ||||
| Female | 77 | .39 | 90 | .95 |
| Male | 70 | 88 | ||
| Stage 4 disease | ||||
| No | 77 | .01 | 91 | .12 |
| Yes | 57 | 84 | ||
| Albumin (< 4 g/dL) | ||||
| No | 74 | .96 | 87 | .9 |
| Yes | 73 | 89 | ||
| Hemoglobin (< 10.5 g/dL) | ||||
| No | 77 | .17 | 91 | .08 |
| Yes | 58 | 76 | ||
| WBC count (≥ 15 000/mm3) | ||||
| No | 73 | .85 | 89 | .67 |
| Yes | 76 | 86 | ||
| Lymphocyte count (< 600/mm3 or < 8%) | ||||
| No | 77 | .02 | 89 | .48 |
| Yes | 25 | 78 | ||
| IPS (≥ 3) | ||||
| No | 74 | .65 | 91 | .34 |
| Yes | 70 | 86 | ||
| CD68 | ||||
| ≤ 12.7% (CD68low) | 78 | .04 | 94 | < .01 |
| > 12.7% (CD68high) | 64 | 81 | ||
| CD163 | ||||
| ≤ 16.8% (CD163low) | 82 | < .01 | 96 | < .01 |
| > 16.8% (CD163high) | 63 | 81 | ||
| EBER | ||||
| Negative | 75 | .24 | 90 | .43 |
| Positive | 62 | 83 |
To determine whether CD68 or CD163 was independently associated with outcomes, respectively, 2 separate multivariate analyses were performed, including the factors significantly associated with FFS or OS in univariate analysis. These analyses demonstrated that increased CD68 or CD163 expression was a significant independent predictor of inferior FFS and OS (Table 5).
Table 5.
Multivariate analyses in the validation cohort
| Factor | HR | 95% CI | P |
|---|---|---|---|
| FFS | |||
| Lymphocyte count (< 600/mm3 or < 8%) | 2.1 | 0.8-5.8 | .14 |
| Stage 4 disease | 2.2 | 1.0-4.7 | .04 |
| CD68high | 2.1 | 1.1-4.2 | .04 |
| Lymphocyte count (< 600/mm3 or < 8%) | 2.1 | 0.8-5.9 | .14 |
| Stage 4 disease | 1.8 | 0.8-3.9 | .13 |
| CD163high | 2.5 | 1.2-5.3 | .02 |
| OS | |||
| Age (≥ 45 y) | 2.5 | 0.9-7.1 | .08 |
| CD68high | 3.5 | 1.2-10.2 | .02 |
| Age (≥ 45 y) | 3.4 | 1.3-9.2 | .02 |
| CD163high | 3.9 | 1.3-11.9 | .02 |
HR indicates hazard ratio; and CI, confidence interval.
Discussion
We confirm the prognostic significance of TAMs in CHL in the E2496 ECOG/SWOG/NCIC/CALGB Intergroup trial, a multicenter phase 3 randomized controlled trial comparing ABVD and Stanford V chemotherapy. Increased CD68 and CD163 IHC expression was significantly associated with inferior outcomes in locally extensive and advanced-stage CHL. Multivariate analysis in the validation cohort showed CD68 or CD163 expression to be significant independent predictors of FFS and OS. All previous studies on TAMs and outcome in CHL have been based on retrospective single institution experiences. This work represents the first confirmation of the prognostic role of TAMs in CHL in a multicenter randomized controlled clinical trial setting.
Although most studies in the literature demonstrate an association between increased TAMs and inferior outcome in CHL, there has been variability in suggested threshold values for CD68 and/or CD163 IHC expression. This probably relates to differences in IHC quantitation methodology, with most using manual visual scoring techniques, and a lack of subsequent validation of thresholds in these studies. The lack of reproducibility and inconsistency of manual or visual IHC scoring has been identified as a potential pitfall regarding the use of IHC biomarkers in routine clinical practice.5 Azambuja et al showed poor interobserver agreement for low scores with CD68 IHC.20 These issues are potentially overcome through use of computer image analysis to produce greater objectivity in scoring. Kamper et al used computer-assisted stereologic analysis and point grid counting methodology in assessing CD68 and CD163 IHC, and showed increased CD68 and CD163 IHC expression to be associated with inferior outcome in CHL.16 However, they reported this method to be labor intensive.16 We used Aperio Technologies for computer image analysis to assess CD68 and CD163 IHC. In addition, we attempted to produce robust thresholds for CD68 and CD163 IHC expression by developing optimum thresholds based on the maximal χ2 values of the log-rank test for OS in a training cohort, and then testing these thresholds in a separate independent validation cohort. Aperio was able to analyze tissue cores in their entirety, with scores averaged from both cores of the same patient to provide a more representative score for each case, within the limitations of a tissue microarray. In addition, Aperio is a user friendly system and shows potential for application on whole tissue sections. Compared with CD68, CD163 appeared to show an overall crisper and stronger intensity of staining and a cleaner background in our hands, making it more ideal for analysis by Aperio's Positive Pixel Count algorithm. This experience with the quality of CD163 IHC is in agreement with other investigators.19,20 In addition, the KP1 clone for CD68 has been reported to be a less specific marker for macrophages, as it is also known to react with myeloid and fibroblastic cells,23 whereas clone 10D6 for CD163 has been reported to be more specific than both KP1 and PGM1 clones for CD68 in identifying macrophages.24 For these reasons, CD163 may be a better marker for identifying TAMs than CD68.
Expression of CD163 is restricted to cells of the monocyte/macrophage lineage and is reported to be a more specific marker for alternatively activated (M2) macrophages in the M1/M2 macrophage polarization model.24–27 We showed a correlation between CD68 and CD163 expression, suggesting that TAMs in CHL show features described for M2 macrophages, including promoting tumor growth and angiogenesis, tissue remodeling, and suppression of adaptive immune responses, contributing to immune evasion by tumor cells, and thus associated with poor prognosis.28–32 However, the macrophage polarization model based on in vitro experiments is probably an oversimplification of macrophages in vivo, which probably display a spectrum of activated phenotypes and with the ability to switch from one functional phenotype to another in response to varied local microenvironmental signals.30,33,34
We also showed increased CD68 and CD163 expression to be associated with increased age, EBER positivity, and mixed cellularity subtype of CHL.16,20,35 Indeed, a gene expression profiling study showed overexpression of genes associated with either histiocytes or T cells, including CD68 and CD163, in EBV+ CHL compared with EBV− CHL.36 Despite an association with increased CD68 and CD163 expression in EBER+ cases, no survival differences were seen with regards to EBER status in our study. However, TAMs remained predictive of inferior outcomes in both EBER+ and EBER− cases, suggesting that there are probably other mechanisms responsible for the recruitment and the poor prognostic impact of increased TAMs in the CHL microenvironment. CD163 may be a better marker than CD68 in predicting inferior outcomes in EBER+ cases; however, numbers of EBER+ patients in our study were small, and this requires further study. The precise biologic mechanisms underlying TAMs and the relationship between TAMs with EBV and tumor cells are currently not well understood, and further functional studies are required.
In conclusion, we confirm the prognostic significance of TAMs using CD68 and CD163 IHC in CHL in the E2496 Intergroup trial, a multicenter phase 3 randomized controlled clinical trial comparing ABVD and Stanford V chemotherapy. We demonstrate an objective method of quantitating CD68 and CD163 IHC expression using computer image analysis (Aperio Technologies) and established robust thresholds for CD68 and CD163 IHC expression that is trained and validated in independent cohorts. Our findings, in conjunction with other previous studies, firmly establish TAMs to be important in the CHL tumor microenvironment. Further functional studies are required to determine the precise biologic mechanisms associated with increased numbers of TAMs in the tumor microenvironment and the relationship with EBV in CHL. Evaluation of TAMs should be considered in prospective clinical trials, and patients with increased TAMs may benefit from more intensive chemotherapy or novel agents designed to disrupt the crosstalk between HRS cells and benign macrophages.
Supplementary Material
Acknowledgments
The authors thank Lynda Bell, Sylvia Lee, and Julie Lorette at the Center for Translational and Applied Genomics for technical support.
This work was coordinated by the Eastern Cooperative Oncology Group (Dr Robert L. Comis, Chair) and supported in part by Public Health Service (grants CA21115, CA23318, CA66636, CA17145, CA11083, CA32102, CA38926, CA77202, CA21076, and CA77470), the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Biospecimens were provided by the ECOG Pathology Coordinating Office and Reference Laboratory. K.L.T and D.W.S were supported by the Terry Fox Foundation Strategic Health Research Training Program in Cancer Research at Canadian Institutes of Health Research (postdoctoral fellowships grant TGT-53912). C.S. was supported by postdoctoral fellowships of the Cancer Research Society (Steven E. Drabin Fellowship) and the Michael Smith Foundation for Health Research.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.L.T. performed the research, analyzed data, and prepared the manuscript; D.W.S. analyzed data; F.H., B.S.K., R.I.F., N.L.B., R.H.A., R.B., L.M.R., J.M.C., C.S., L.I.G., and S.J.H. contributed data and materials; and R.D.G. designed the research, analyzed data, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Randy D. Gascoyne, British Columbia Cancer Agency & British Columbia Cancer Research Centre, 675 W 10th Ave, Room 5-113, Vancouver, BC, V5Z 1L3, Canada; e-mail: rgascoyn@bccancer.bc.ca.
References
- 1.Moccia AM, Donaldson J, Chhanabhai M, et al. The International Prognostic Factor Project Score (IPS) in advanced stage Hodgkin lymphoma has limited utility in patients treated in the modern era. J Clin Oncol. 2012;30(27):3383–3388. doi: 10.1200/JCO.2011.41.0910. [DOI] [PubMed] [Google Scholar]
- 2.Guisado-Vasco P, Arranz-Saez R, Canales M, et al. Stage IV and age over 45 years are the only prognostic factors of the International Prognostic Score for the outcome of advanced Hodgkin lymphoma in the Spanish Hodgkin Lymphoma Study Group series. Leuk Lymphoma. 2012;53(5):812–819. doi: 10.3109/10428194.2011.635861. [DOI] [PubMed] [Google Scholar]
- 3.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease: International Prognostic Factors Project on Advanced Hodgkin's Disease. N Engl J Med. 1998;339(21):1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 4.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin's lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol. 2011;29(14):1812–1826. doi: 10.1200/JCO.2010.32.8401. [DOI] [PubMed] [Google Scholar]
- 5.Steidl C, Farinha P, Gascoyne RD. Macrophages predict treatment outcome in Hodgkin's lymphoma. Haematologica. 2011;96(2):186–189. doi: 10.3324/haematol.2010.033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362(10):875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casulo C, Arcila M, Teruya-Feldstein J, Maragulia J, Moskowitz CH. Tumor-associated macrophages are predictive of survival in relapsed and refractory Hodgkin lymphoma [abstract]. Blood (ASH Annual Meeting Abstracts) 2011;118(21):2630. [Google Scholar]
- 8.Touati M, Delage-Corre M, Monteil J, et al. CD68+ tumor-associated macrophages predict unfavorable treatment outcomes in classic Hodgkin's lymphoma in correlation with early FDG-PET assessment results [abstract]. Blood (ASH Annual Meeting Abstracts) 2011;118(21):1558. [Google Scholar]
- 9.Farinha P, Rodrigues S, Fernandes T, et al. Lymphoma associated macrophages predict survival in uniformly treated patients with classical Hodgkin lymphoma [abstract]. Mod Pathol. 2011;24(1 Suppl):1257. [Google Scholar]
- 10.Greaves P, Clear A, Coutinho R, et al. Expression of FOXP3, CD68 and CD20 at diagnosis in the microenvironment of classical Hodgkin lymphoma is predictive of outcome [prepublished online ahead of print October 8, 2012]. J Clin Oncol. doi: 10.1200/JCO.2011.39.9881. doi: 10.1200/JCO.2011.39.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deau B, Bachy E, Ribrag V, et al. Macrophages and mast cells infiltration are biomarkers of primary refractory Hodgkin's lymphoma [abstract]. Blood (ASH Annual Meeting Abstracts) 2010;116(21):3881. [Google Scholar]
- 12.Sánchez-Espiridión B, Martin-Moreno AM, Montalban C, et al. Immunohistochemical markers for tumor associated macrophages and survival in advanced classical Hodgkin lymphoma. Haematologica. 2012;97(7):1080–1084. doi: 10.3324/haematol.2011.055459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzankov A, Matter MS, Dirnhofer S. Refined prognostic role of CD68-positive tumor macrophages in the context of the cellular micromilieu of classical Hodgkin lymphoma. Pathobiology. 2010;77(6):301–308. doi: 10.1159/000321567. [DOI] [PubMed] [Google Scholar]
- 14.Jakovic LR, Mihaljevic BS, Perunicic Jovanovic MD, Bogdanovic AD, Andjelic BM, Bumbasirevic VZ. The prognostic relevance of tumor associated macrophages in advanced stage classical Hodgkin lymphoma. Leuk Lymphoma. 2011;52(10):1913–1919. doi: 10.3109/10428194.2011.580026. [DOI] [PubMed] [Google Scholar]
- 15.Yoon DH, Koh YW, Kang HJ, et al. CD68 and CD163 as prognostic factors for Korean patients with Hodgkin lymphoma. Eur J Haematol. 2012;88(4):292–305. doi: 10.1111/j.1600-0609.2011.01731.x. [DOI] [PubMed] [Google Scholar]
- 16.Kamper P, Bendix K, Hamilton-Dutoit S, Honore B, Nyengaard JR, d'Amore F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin's lymphoma. Haematologica. 2011;96(2):269–276. doi: 10.3324/haematol.2010.031542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaki MA, Wada N, Ikeda J, et al. Prognostic implication of types of tumor-associated macrophages in Hodgkin lymphoma. Virchows Arch. 2011;459(4):361–366. doi: 10.1007/s00428-011-1140-8. [DOI] [PubMed] [Google Scholar]
- 18.Agostinelli C, Gallamini A, Rigacci L, et al. The prognostic value of biologic markers in classical Hodgkin lymphoma (CHL) patients [abstract]. Ann Oncol. 2011;22(Suppl 4):153–154. [Google Scholar]
- 19.Harris JA, Jain S, Ren Q, Zarineh A, Liu C, Ibrahim S. CD163 versus CD68 in tumor associated macrophages of classical Hodgkin lymphoma. Diagn Pathol. 2012;7:12. doi: 10.1186/1746-1596-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azambuja D, Natkunam Y, Biasoli I, et al. Lack of association of tumor-associated macrophages with clinical outcome in patients with classical Hodgkin's lymphoma. Ann Oncol. 2012;23(3):736–742. doi: 10.1093/annonc/mdr157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swerdlow SH, Harris NL, Jaffe ES, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. [Google Scholar]
- 22.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 23.Kunisch E, Fuhrmann R, Roth A, Winter R, Lungershausen W, Kinne RW. Macrophage specificity of three anti-CD68 monoclonal antibodies (KP1, EBM11, and PGM1) widely used for immunohistochemistry and flow cytometry. Ann Rheum Dis. 2004;63(7):774–784. doi: 10.1136/ard.2003.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122(5):794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- 25.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambarus CA, Krausz S, van Eijk M, et al. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods. 2012;375(2):196–206. doi: 10.1016/j.jim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47(8):1650–1660. doi: 10.1016/j.molimm.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol. 2011;41(9):2522–2525. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
- 30.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167(2):195–205. doi: 10.1111/j.1365-2249.2011.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukuda K, Kobayashi A, Watabe K. The role of tumor-associated macrophage in tumor progression. Front Biosci (Schol Ed) 2012;4(Suppl 4):787–798. doi: 10.2741/s299. [DOI] [PubMed] [Google Scholar]
- 33.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stables MJ, Shah S, Camon EB, et al. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118(26):e192–e208. doi: 10.1182/blood-2011-04-345330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hohaus S, Santangelo R, Giachelia M, et al. The viral load of Epstein-Barr virus (EBV) DNA in peripheral blood predicts for biological and clinical characteristics in Hodgkin lymphoma. Clin Cancer Res. 2011;17(9):2885–2892. doi: 10.1158/1078-0432.CCR-10-3327. [DOI] [PubMed] [Google Scholar]
- 36.Chetaille B, Bertucci F, Finetti P, et al. Molecular profiling of classical Hodgkin lymphoma tissues uncovers variations in the tumor microenvironment and correlations with EBV infection and outcome. Blood. 2009;113(12):2765–3775. doi: 10.1182/blood-2008-07-168096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.