Abstract
B lymphocytes producing antiplatelet autoantibodies play a major role in autoimmune thrombocytopenia (ITP). However, certain B cells, including the human CD19+CD24hiCD38hi subpopulation, possess regulatory functions mediated partly by IL-10. In a cohort of chronic ITP patients with low platelet counts who consisted of patients off treatment, we found a lower frequency of CD19+CD24hiCD38hi in the peripheral compartment of nonsplenectomized patients (P = .03). IL-10 expression after activation was decreased in all ITP circulating CD19+ subpopulations (P < .03), and inhibition of monocyte TNF-α expression by activated B cells was reduced in patients with platelet numbers of < 50 × 109 cells/L (P = .001), indicating that regulatory B cells of patients with ITP are functionally impaired in their ability to dampen monocyte activation. Interestingly, in nonsplenectomized patients whose platelet counts were elevated after treatment with thrombopoietic agents, the frequency of CD19+CD24hiCD38hi B cells was increased compared with those before treatment (P = .02). Altogether, these data indicate a compromised regulatory B-cell com-partment as an additional defect in immune regulation in patients with chronic ITP that may be restored in responders to thrombopoietic treatment.
Introduction
B lymphocytes participate in immune responses through production of antibodies, antigen presentation to T cells, and cytokine secretion. Animal studies suggest that naive B cells can also regulate autoimmune responses through secretion of anti-inflammatory IL-101 and control proinflammatory differentiation of other antigen-presenting cells (APCs).2 More recently, human B cells with regulatory functions mediated in part by IL-10 and/or through inhibitory interactions with effector T cells and monocytes have been described.3–5 In the original report of human B-regulatory cells (Bregs), the CD19+CD24hiCD38hi human B-cell subpopulation, that included immature transitional B cells, was shown to possess regulatory activity by reducing CD4+ T-cell activation at least in part via IL-10 secretion.3 Interestingly, in patients with systemic lupus erythematosus (SLE), the CD19+CD24hiCD38hi subset produced less IL-10 production and had reduced suppressive activity, suggesting that altered cellular function of the Breg compartment in SLE may impact the immune effector responses in this autoimmune disease.3 Furthermore, in renal transplantation patients, increased frequency of CD19+CD24hiCD38hi was associated with positive outcome.6 Although human studies have yet to be performed, various mouse disease models indicate that IL-10 produced by Bregs is important for control and maintenance of regulatory T cells (Tregs),7 and promote their differentiation8 or their recruitment,9 thus further expanding the role of Bregs in immunoregulation.
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder because of immune destruction of platelets and insufficient platelet production. Antiplatelet autoantibodies are responsible for platelet destruction by the reticuloendothelial system and probably for inhibition of megakaryopoiesis.10 Marked reduction of memory B cells and high plasma levels of B-cell activating factor BAFF that may affect B cell compartment size and subset distribution have been reported in patients with ITP.11–13 Furthermore, a shift toward stimulatory monocytes with enhanced FcγR-mediated phagocytic capacity may exacerbate platelet destruction, and also contribute to the autoimmune response to platelet antigens.14 A generalized altered immune regulation in ITP is further supported by presence of platelet-autoreactive T cells, cytokine imbalance,15–19 and altered regulatory T-cell (Treg) numbers and function.20–25 Several treatment options are available to ITP patients, including the use of thrombopoietic (TPO) agents, which have yielded overall safe and durable responses while treatment continues in patients with chronic and refractory ITP.26–31 Interestingly, various therapies that increase platelet counts, such as rituximab, thrombopoietic agents, or treatment with high-dose dexamethasone are also associated with improved Treg function or numbers.22,23,32
Given that ITP pathogenesis is in part related to defective Treg, T helper, and monocyte functions and because Bregs are important for controlling effector T-cell and monocyte responses and possibly Tregs, we initiated studies to characterize the Breg compartment in ITP patients. Because we had observed an improvement in Treg activity in patients on TPO agents with increased platelet counts, we studied a cohort of patients on TPO agents with various platelet count responses. Our studies are consistent with a disturbed B-cell regulatory function in patients with ITP as with other autoimmune diseases with the potential to improve after treatment with TPO agents that increase platelet counts, similar to the findings with improved Tregs in responders to treatment.
Methods
Patients and controls
All the studies were approved by the institutional review boards of the Weill Medical College of Cornell University and of the New York Blood Center. Patients with chronic ITP (> 1 year since diagnosis, 17 male and 19 females, n = 36) were enrolled in the study (see Tables 1–3 for patient clinical profiles) after informed consent. Closely age-matched, healthy subjects were recruited as controls. Although not indicated, most had been refractory to previous treatments. Some patients had received rituximab several years before the study and, with only 1 exception (no. 29), were considered nonresponsive. Patients listed as being “no treatment” in Tables 1–3 had been without ITP treatment regimen for at least 2 weeks before the study. Patients “On TPO agents” included those who were on promacta and romiplostim as well as the investigational small-molecule thrombopoietin receptor (TPO-R) agonist Shionogi S-888711. Unless indicated in Tables 1–3, patients were not on any other ITP treatment at the time of the blood draw. Patients 30 and 35 are considered in remission since both had been without a need for treatment for more than 1 year since their last treatment: patient 30 was last treated with a TPO agent a year and a half before the study, and patient 36 had been treated with non-TPO agents 5 years before the study. Patient 29, who had a platelet count of 54 × 109/L at the time of the study visit, had been treated with rituximab and had since been without a need for treatment for 9 years when she was enrolled in the study and is considered partially in remission.
Table 1.
Clinical characteristics of ITP patient cohort: B-cell surface phenotypic analysis
| B-cell surface phenotypic analysis, patient no. | Sex | Age, y | Treatment | Platelet count, 109/L |
|---|---|---|---|---|
| Off treatment, nonsplenectomized, platelet counts < 50 × 109/L | ||||
| 1 | M | 50 | No treatment | 24 |
| 2 | M | 56 | No treatment | 37 |
| 31 | M | 37 | No treatment | 5 |
| 42 | M | 69 | No treatment | 11 |
| 5 | M | 29 | No treatment | 38 |
| 6 | F | 67 | No treatment | 11 |
| 7 | F | 29 | No treatment | 13 |
| 8 | F | 18 | No treatment | 17 |
| 9 | F | 66 | No treatment | 38 |
| On treatment, nonsplenectomized, platelet counts > 50 × 109/L | ||||
| Same as 1 | M | 50 | On TPO agent | 94 |
| Same as 2 | M | 56 | On TPO agent | 64 |
| Same as 4 | M | 69 | On TPO agent plus danzol | 66 |
| Same as 5 | M | 29 | On TPO agent | 91 |
| Same as 9 | F | 66 | On TPO agent | 101 |
| 10 | F | 20 | On TPO agent | 181 |
| 11 | F | 54 | On TPO agent | 142 |
| 12 | F | 25 | On TPO agent | 102 |
| Off treatment, splenectomized, platelet counts < 50 × 109/L | ||||
| 133 | M | 54 | No treatment | 5 |
| 14 | M | 16 | No treatment | 27 |
| 15 | M | 15 | No treatment | 7 |
| 16 | F | 23 | No treatment | 32 |
| 17 | F | 38 | No treatment | 13 |
| 18 | M | 81 | No treatment | 35 |
| 19 | M | 57 | No treatment | 20 |
| 20 | M | 45 | No treatment | 21 |
| 21 | F | 31 | No treatment | 20 |
| On treatment, splenectomized, platelet counts > 50 × 109/L | ||||
| Same as 15 | M | 15 | On TPO agent | 340 |
| Same as 16 | F | 23 | On TPO agent | 204 |
| Same as 17 | F | 38 | On TPO agent | 89 |
| Same as 184 | M | 81 | On TPO agent | 52 |
| Same as 195 | M | 57 | On TPO agent | 133 |
| 22 | F | 20 | On TPO agent | 108 |
| 236 | F | 69 | On TPO agent | 126 |
| 24 | F | 73 | On TPO agent | 166 |
| 25 | M | 46 | On TPO agent | 214 |
| 26 | M | 20 | On TPO agent | 138 |
Patients are subdivided based on their splenectomy status and their platelet counts (less than or more than 50 × 109/L). Some patients were also analyzed for B-cell surface phenotypic expression as well as functional studies on the same visit and they are indicated by the number followed by a numerical superscript. Patients' age and sex, as well as their platelet counts and the ITP treatment type on the day of the blood draw, are indicated. The exact B-cell subset frequencies for each patient are shown in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
ITP indicates immune thrombocytopenia; and TPO, thrombopoietic.
Table 2.
Clinical characteristics of ITP patient cohort: intracellular B-cell IL-10 production assay
| Intracellular B-cell IL-10 production assay, patient no. | Sex | Age, y | Treatment | Platelet count, 109/L |
|---|---|---|---|---|
| Platelet count < 50 × 109/L (only patients 13, 14, and 20 were splenectomized) | ||||
| Same as 31 | M | 37 | No treatment | 5 |
| Same as 42 | M | 69 | No treatment | 11 |
| Same as 7 | F | 29 | On TPO agent | 12 |
| Same as 13 | M | 54 | On TPO agent | 4 |
| Same as 14 | M | 16 | On TPO agent | 13 |
| Same as 20 | M | 45 | On TPO agent | 40 |
| 27 | M | 14 | On TPO agent | 21 |
| Platelet count > 50 × 109/L (patients 18, 23, and 28 were splenectomized) | ||||
| 28 | F | 31 | No treatment | 112 |
| 29 | F | 55 | Partially in remission | 54 |
| 30 | F | 21 | In remission | 165 |
| Same as 184 | M | 81 | On TPO agent | 52 |
| Same as 236 | F | 69 | On TPO agent | 123 |
Patients are grouped according to platelet counts of below 50 × 109/L or above 50 × 109/L. In many cases, the same patient's blood was drawn for the study on more than one visit and this has been indicated by referring to such patients as “same as no.” Some patients were also analyzed for B-cell surface phenotypic expression as well as functional studies on the same visit and they are indicated by the number followed by a numerical superscript. Patients' age and sex, as well as their platelet counts and the ITP treatment type on the day of the blood draw, are indicated. The exact B-cell subset frequencies for each patient are shown in supplemental Table 1.
Table 3.
Clinical characteristics of ITP patient cohort: inhibition of monocyte cytokine assay
| Inhibition of monocyte cytokine assay, patient no. | Sex | Age, y | Treatment | Platelet count, 109/L |
|---|---|---|---|---|
| Platelet counts < 50 × 109/L (patients 13, 33, and 35 were splenectomized) | ||||
| Same as 133 | M | 54 | No treatment | 5 |
| Same as 7 | F | 29 | On TPO agent | 17 |
| 31 | F | 72 | On TPO agent | 29 |
| 32 | M | 19 | On TPO agent | 29 |
| 33 | F | 59 | On TPO agent | 17 |
| 34 | M | 20 | On TPO agent | 33 |
| 35 | F | 63 | On TPO agent plus IVIg | 33 |
| Platelet counts > 50 × 109/L (patients 19, 25, and 36 were splenectomized) | ||||
| 36 | M | 58 | In remission | 372 |
| Same as 8 | F | 18 | On TPO agent | 68 |
| Same as 195 | M | 57 | On TPO agent | 133 |
| Same as 25 | M | 46 | On TPO agent | 93 |
B-cell surface and cytoplasmic expression analysis
B-cell phenotypic analysis was performed using whole blood (100 μL) using fluorescently conjugated antibodies against human CD19, CD24, CD38, and CD27 (all from BD Biosciences). For intracellular IL-10 expression studies, PBMCs were resuspended (2 × 106 cells/mL) in media containing 10% fetal calf serum, 4mM l-glutamine, and antibiotics (all from Invitrogen Life Technologies) before stimulation with 100nM CpG ODN2006 (Invivogen) for 72 hours. PMA (50 ng/mL; Sigma-Aldrich), ionomycin (750 ng/mL; Sigma-Aldrich), and GolgiPlug (Brefeldin A; BD Biosciences) were added 5.5 hours before the end of the cultures. After B-cell surface staining, cytoplasmic IL-10 expression was analyzed using a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's instructions and staining with anti–human IL-10 or an isotype-matched control.
Functional assay of monocyte cytokine expression
CD19+ and CD14+ cells from PBMCs were enriched using microBeads according to the manufacturer's instructions (Miltenyi Biotec). Purified (> 90%) CD19+ cells (2 × 106/mL) were cultured for 24 hours with mega-sCD40L (50 μg/mL; Enzo Life Sciences) plus CpG ODN2006 (InVivogen; 200nM) while purified CD14+ (> 90%) cells were cultured alone. Washed 2 × 105 CD19 cells were then added to equal numbers of CD14+ monocytes and cocultured for 16-20 hours. LPS (1 μg/mL) together with GolgiPlug (BD Biosciences) was added for 4 hours followed by flow cytometric analysis of surface CD14 and intracellular TNF-α expression. Inhibition of monocyte cytokine expression was calculated as 1 − (% TNF-α expressed in cocultures of activated B cells and monocytes/% TNF-α expressed in monocytes cultured alone) × 100, where 100% was considered complete inhibition and 0% no inhibition.
Statistical analysis
Statistical analyses were performed using the Mann-Whitney U test for all comparisons described in the study, and differences were considered significant at P < .05.
Results
Altered peripheral B-cell compartment in patients with ITP
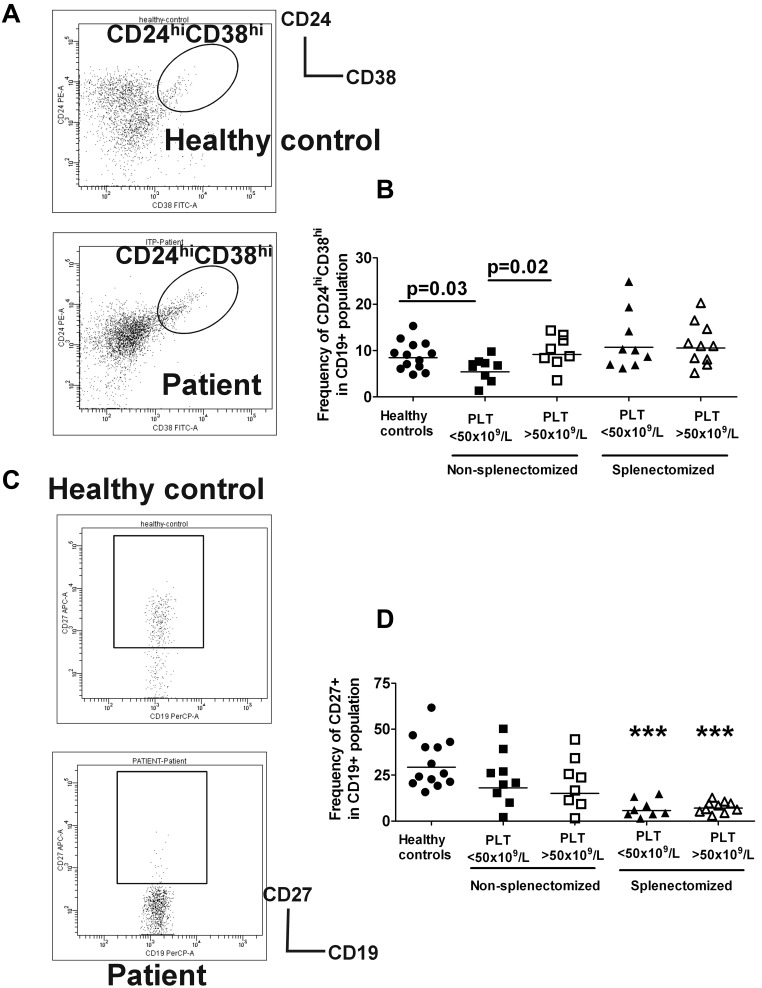
As a first step to analyze the regulatory B-cell compartment, we divided our chronic ITP patient cohort based on platelet counts of either below or above 50 × 109/L because this platelet count is a clinical indicator of patients' disease severity. Given the role of the spleen in B-cell development, we further divided the patients for the phenotypic analysis into 2 groups consisting of splenectomized and nonsplenectomized patients. Despite comparable proportions of CD19+ B cells in nonsplenectomized patients with ITP (supplemental Figure 1A), the frequency of circulating CD19+CD24hiCD38hi B cells, previously shown to have regulatory function,3 was decreased in patients with low platelet counts who were off ITP treatment (n = 8, filled squares) compared with healthy controls (n = 13, filled circles, P = .03, Figure 1A-B); however, it was normal in the group on treatment with TPO agents with increased platelet counts (open squares, P = .02, Figure 1B, and Table 1 and supplemental Table 1). In contrast to the CD19+CD24hiCD38hi B cells, the frequency of CD19+CD24intCD38int (mostly mature B cells) and CD19+CD24+CD38− (primarily memory B cells) subsets in nonsplenectomized patients were not statistically different compared with healthy controls, and did not appear to be affected by platelet counts (supplemental Figure 2B-C).
Figure 1.
Phenotypic analysis of peripheral B-cell subpopulations in patients with ITP. Peripheral blood from patients and healthy controls were stained with CD19, CD24, CD38, and CD27. Patients listed in Tables 1 through 3 were analyzed: nonsplenectomized ITP cohort without treatment with low platelet counts, nonsplenectomized group on treatment with TPO agents with increased platelet counts, splenectomized patients with platelet counts of less than 50 × 109/L without ITP treatment, and splenectomized patients on treatment with TPO agents with platelet counts of more than 50 × 109/L compared with healthy controls (n = 13; median age, 40 years; range, 24-66; 8 males and 5 females). (A) CD24 and CD38 expression patterns of CD19+ lymphocyte gated cells are shown in the representative dot plots of healthy controls and ITP patients. Three distinct subpopulations have previously been described in human peripheral blood3: CD19+CD24hiCD38hi cells that include immature B cells, CD19+CD24intCD38int consisting primarily of mature B cells and CD19+CD24+CD38− with mostly memory B cells. The gating strategy for analysis of the CD24hiCD38hi B cells is indicated. In supplemental Figure 2, the gating strategy for the other 2 subpopulations are shown. (B) Frequency of CD24hiCD38hi of CD19+ subset in healthy controls and nonsplenectomized or splenectomized ITP patients with platelet counts of less or more than 50 × 109/L is indicated. P values shown highlight the statistically significant reduction in CD19+CD24hiCD38hi subpopulation frequency in nonsplenectomized ITP patients with platelet counts less than 50 × 109/L compared with healthy controls, and also indicate a significant increase in this B-cell population in patients on treatment with TPO agents whose platelet counts are above 50 × 109/L. Although the P value is not shown, splenectomized patients with platelet counts less than 50 × 109/L have increased frequency of CD19+CD24hiCD38hi compared with nonsplenectomized patients off treatment with low platelet counts (P = .02). (C) Representative dot plots of CD19 and CD27 memory surface marker expression pattern in healthy controls and patients are shown with gating strategy used to determine the proportion of CD27+ cells in B cells. (D) Frequency of CD19+CD27+ in the controls and patient groups in panel B are shown in various cohorts. *Statistically significant differences were observed in splenectomized patients regardless of platelet counts compared with controls.
Splenectomized patients, irrespective of platelet counts, had a higher frequency of CD19+CD24hiCD38hi cells compared with nonsplenectomized patients (Figure 1B, Table 1, and supplemental Table 1) and the frequencies of CD19+CD24intCD38int and CD19+CD24+CD38− subsets in splenectomized patients were altered in comparison to those of healthy controls (supplemental Figure 2B-C). A marked deficiency of memory CD19+CD27+ B cells in some ITP patients was noted which as previously described was particularly pronounced in the splenectomized cohort33,34 (Figure 1C, supplementary Figure 2).
Reduced IL-10 expression in stimulated B cells of ITP patients
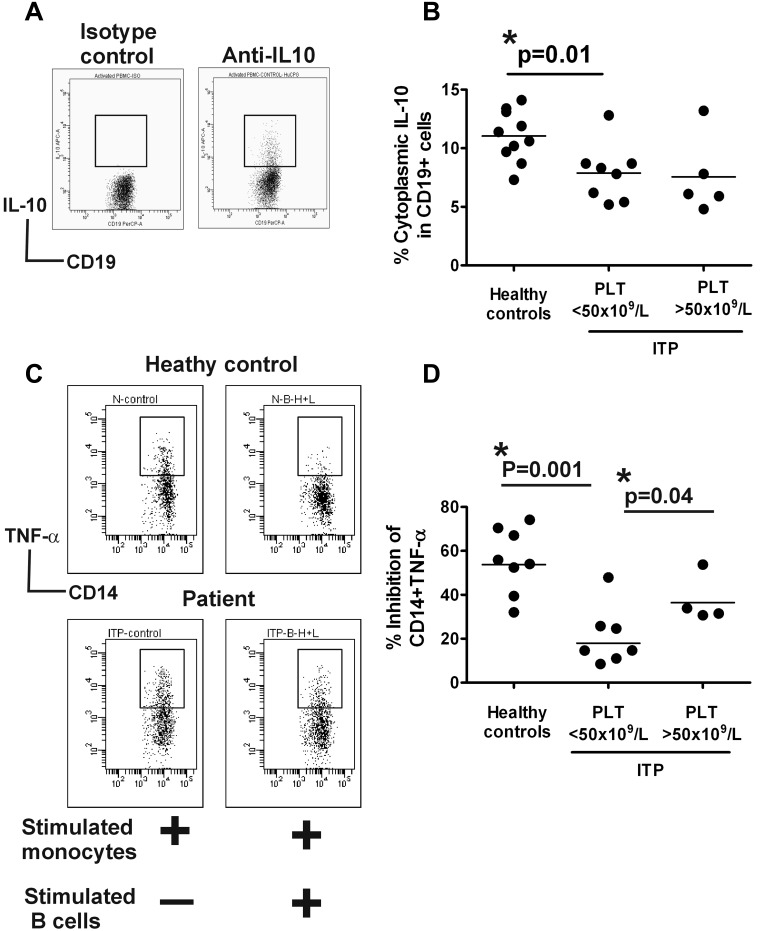
Human Bregs mediate regulatory activity partly via IL-10–dependent mechanisms.3,5 To determine whether B-cell IL-10 expression levels differ in ITP patients, we grouped the patients based on platelet counts of above or below 50 × 109/L and compared the 2 groups to B-cell IL-10 expression levels in healthy controls. Some of the patients who were analyzed for phenotypic expression of B-regulatory markers were also assayed for B-cell intracellular IL-10 expression (see Table 2). In addition, patients on treatment with TPO agents who did not have a platelet response were included in the B-cell IL-10 expression analysis of the group with lower (< 50 × 109/L) platelet counts, while patients in remission or off treatment with higher (> 50 × 109/L) platelet counts were analyzed with the group that had elevated (> 50 × 109/L) platelet numbers (see Table 2). As previously reported,5 we found rare IL-10–producing human B cells before stimulation (data not shown). Although cytoplasmic IL-10 expression was dramatically increased after stimulation with a B-cell activating agent, Toll-like receptor 9 (TLR9) agonist CpG oligonucleotides (CpG), the levels in total as well as CD19+ subsets were significantly less in ITP patients with low platelet counts than in healthy controls (Figure 2A-B, supplementary Figure 3, P < .03). IL-10 expression was equally low in splenectomized (n = 3) and nonsplenectomized patients (n = 4) with low platelet counts and did not appear to improve in patients with elevated platelet counts (n = 6, Figure 2B); secreted IL-10 levels in culture supernatants were not tested.
Figure 2.
Altered B-regulatory activity in ITP patients. (A) PBMCs from healthy controls and patients with ITP (see Tables 1–3) were stimulated with CpG and following short stimulation with PMA and ionomycin, intracellular expression of IL-10 relative to isotype-matched control antibody in various B-cell subsets was analyzed. Representative dot blot of CD19+ cells positive for IL-10 expression is shown and the gating strategy based on isotype control for anti–IL-10 antibody is indicated. (B) Frequency of IL-10–positive CD19 cells in healthy controls versus ITP patients whose platelet counts were below or above 50 × 109/L are shown. As indicated by the P value, patients with less than 50 × 109/L counts have reduced levels of CD19+ IL-10 expression. Similar data were obtained for comparison of IL-10 expression in various B cells subsets based on CD24 and CD38 staining as shown in supplemental Figure 3. (C) Purified CD14+ monocytes from ITP patients (see Table 1) and healthy controls were cultured either alone or with purified CD19+ cells that had been previously stimulated (for 24 hours) with sCD40L plus CpG. After a further stimulation with LPS for 4 hours in presence of brefeldin A, the cells were stained for surface CD14 and cytoplasmic TNF-α expression. Representative dot blots of TNF-α in CD14+ monocytes when stimulated CD14+ monocytes were cultured alone or with equal numbers of activated CD19 cells for healthy controls and patients are shown. Differences in levels of TNF-α produced by stimulated monocytes between healthy and ITP patients when cultured alone did not reach significance (see supplemental Figure 4, P = .07). (D) The percentage of inhibition of CD14+ TNF-α expression in cocultures of monocytes plus activated B cells relative to CD14+ TNF-α expressed when monocytes were cultured alone is shown. As indicated, monocyte cytokine inhibition was significantly lower in ITP patients with low platelet counts compared with controls. In patients with higher platelet counts, the inhibitory activity was increased, although it did not reach the levels found in healthy controls (P value not shown in figure, but the difference between healthy controls and patients with increased platelet counts at P = .04 is still significant).
Compromised B cell–mediated monocyte suppressive activity in patients with ITP
B-cell subpopulations activated through TLR and CD40 engagement can negatively regulate monocyte cytokine production during in vitro functional assays.5 Because of the low numbers of peripheral CD19+CD24hiCD38hi B cells that make their purification in sufficient numbers difficult, we analyzed the regulatory function of bulk B-cell populations in ITP patients (n = 7, see Table 3). Purified CD19+ cells activated using a previously described5 combination of CpG and sCD40L were cultured with monocytes that were stimulated with LPS to secrete TNF-α. Although TNF-α levels in stimulated monocytes from healthy controls (mean, 44% ± 2%) and ITP patients (mean, 53% ± 6%) when cultured alone were not significantly different (supplemental Figure 4, P = .07), suppression of monocyte TNF-α expression by activated B cells was less in patients with low platelet counts (mean, 56% ± 5%) compared with controls (mean, 21% ± 5%; P = .001; Figure 2C-D), suggesting that the ability of ITP B-cell compartment to control proinflammatory activity of other APCs is compromised. We also measured monocyte inhibitory activity of bulk B cells in a small number of patients (n = 4), one in remission and 3 on treatment whose platelet counts were above 50 × 109/L and found an improvement in TNF-α inhibition (mean, 37% ± 5%; P = .04; Figure 2D), although it did not reach the levels found in healthy controls (P = .04).
Discussion
In summary, we have observed a significant distortion of B-cell homeostasis in our cohort of patients with ITP which consisted mostly of patients on treatment with thrombopoietic agents but with various platelet count responses. Phenotypically, the frequency of peripheral CD19+ subsets based on CD24 and CD38 expression was significantly altered in ITP patients. We found impaired IL-10 response in stimulated B cells and a reduced B-cell suppressive activity with less dampening of monocyte activation in ITP patients with low platelet counts, consistent with a generalized immune-regulatory cell defect in ITP.20–25 Importantly, an improvement, specifically in the frequency of CD19+CD24hiCD38hi subpopulation, previously described as Bregs,3 in nonsplenectomized ITP patients with increased platelet counts on treatment with TPO agents compared with those with low platelet counts, but off treatment, was observed. Although the observation that Bregs may improve in responders to TPO treatment needs to be confirmed in larger longitudinal studies, we were able to analyze 5 nonsplenectomized patients before and after therapy at the phenotypic level and found that indeed their CD19+CD24hiCD38hi frequency did improve as their platelet counts increased after receiving treatment (patients 1, 2, 4, 5, and 9, see Table 1). It should be noted that we cannot exclude an effect of drugs, namely the TPO agents, on the Breg compartment and studies that compare Bregs in TPO treatment responders versus nonresponders are needed to address this. In a small group of nonsplenectomized patients (n = 5) who were off treatment and had > 50 × 109/L platelet counts, we also found a trend toward an increase in CD19+CD24hiCD38hi frequency compared with the cohort (n = 9) off treatment with low platelet counts (6.1% ± 0.8% to 9.4% ± 1.6%, P = .06). In renal transplantation patients, higher CD19+CD24hiCD38hi frequencies were associated with positive outcomes.6 One hypothesis is that increased frequency of CD19+CD24hiCD38hi subpopulation in ITP patients with higher platelet counts can lead to improvement in B cell–mediated immunoregulation, although direct proof of this will have to await the isolation and characterization of this subset in patients with low-versus-high platelet counts in patients off treatment. Nevertheless, we observed an increased B cell–mediated monocyte suppressive activity in 4 patients with platelet counts of > 50 × 109/L compared with the group with lower counts (< 50 × 109/L), indicating an improved B-regulatory compartment in ITP patients with elevated platelet numbers which we believe is independent of the effect of drug since there was roughly equal representation of patients on TPO agents in the group with low (< 50 × 109/L) and high (> 50 × 109/L) platelet counts. It should be stressed that future studies with larger and more focused patient groups are needed to confirm these findings and exclude the possible effect of the drug on the Breg compartment. Because CD40L is expressed on and in platelets,35 and B-cell regulatory activity in vitro requires activation through CD40 engagement,3 one may speculate that platelets may directly contribute to improved Breg activity in vivo in ITP patients with increased platelet counts, although this may imply that up-regulation of CD40L, normally associated with activated platelets,36 would stimulate Breg activity.
A deficiency of memory B cells has been previously reported in patients with ITP,13 and similar low numbers of peripheral memory B cells have been reported in patients with primary Sjogren syndrome,37 as well as in patients with severe chronic sarcoidosis,38 and in a subgroup of patients with common variable immunodeficiency with autoimmune manifestations.13 The underlying explanation for decreased memory B cells remains unknown, but it may be that because of continuous stimulation with platelet autoantigens, memory cells differentiate into plasma cells. Because naive B cells differentiate into either memory or plasma cells,39 another possibility may be that there is more plasma cell differentiation and therefore less memory cells in ITP patients. The disturbances in the B-cell compartment were particularly pronounced in our splenectomized ITP cohort, possibly caused by impaired B-cell differentiation resulting from loss of germinal centers of secondary lymphoid organs.33,34 Our splenectomized cohort were refractory ITP patients and had all been nonresponders to splenectomy. Most were being treated with TPO agents at the time of this study and yet regardless of platelet numbers, all had elevated frequency of the CD19+CD24hiCD38hi subset. It remains unknown whether the quality of transitional cells, including the CD19+CD24hiCD38hi subset, differ in splenectomized versus nonsplenectomized individuals and whether these cells are functionally active in our splenectomized patients. Interestingly, splenectomy responders have normalized T-cell receptor V-β repertoires, whereas nonresponders have abnormally expanded T-cell clones,40 consistent with a state of immune dyregulation in nonresponders. In addition, some of our patient cohort, regardless of splenectomy status, had been treated several years before this study with the anti-CD20 monoclonal antibody, rituximab, which also depletes Bregs, and all but one had been nonresponsive. Based on studies with patients with SLE and rheumatoid arthritis, clinical remission with rituximab treatment is thought to be associated with preferential repopulation of Breg compartment relative to pathogenic B cells.41 We therefore speculate that long-term ITP responders to rituximab will have an early and persistent repopulation of Bregs and a delay in pathogenic B-cell reconstitution after rituximab therapy, whereas in nonresponders there will be a delay in reconstitution of Breg compartment. Interestingly, ITP rituximab responders were reported to have reduced T-cell clonality42 and improvement in Treg numbers and function,23 although their Breg compartment was not analyzed. It remains to be determined whether ITP rituximab nonresponders have particularly lower numerical or functional Bregs compared with responders or even those not previously treated with rituximab. Furthermore, future studies that analyze both the Treg as well as the Breg compartments of patients with ITP are needed to systematically address the relative importance of these regulatory cells in disease pathogenesis and response to treatment.
The potential clinical importance and utility of the findings reported here will have to await confirmation by completion of ongoing prospective, longitudinal studies. Combined with previous reports of defects in Treg compartment in ITP,20–25 our data suggest that a generalized dysregulation in immune-regulatory networks may be present in ITP as in other autoimmune diseases. In mouse models, IL-10–secreting regulatory B cells promote differentiation of Tregs8 or their recruitment.9 Although the ability of human Bregs on Treg differentiation has not yet been demonstrated, it may be that altered Bregs in ITP patients are responsible for the compromised Treg compartment.20–25 Decreased IL-10 secretion (Figure 2B) may affect Treg differentiation. Alternatively, because direct engagement of Bregs with Tregs was reported to be required for up-regulation of Foxp3 and CTLA-4, 2 molecules critical for Treg activity,43 a reduced Breg compartment may directly impact Treg activity. Based on the current data we cannot distinguish whether the dysregulation is the trigger for autoimmunity or secondary to the ITP disease process. Longitudinal studies that monitor patients as the disease progresses are needed to address the in vivo relevance of Breg dysregulation in ITP. Nevertheless, our preliminary observation that as with Tregs,23,32 the B-regulatory compartment has the potential to be normalized after therapy raises the possibility that analysis of regulatory B-cell subpopulations could serve as cellular biomarkers for specific defects in patients with ITP as with Treg-associated markers that might guide therapy in ITP patients in the future. This raises an important issue that manipulations that only increase Treg activity may be insufficient for treatments of ITP that hope to have lasting effects. It also brings up the question of how these 2 immunoregulatory cell types interact with each other and indicates that there may be a hierarchy among these regulatory compartments. Future studies are needed to analyze possible interactions of different immunoregulatory compartments and determine their relevance to disease and therapy in ITP.
Supplementary Material
Acknowledgments
This work was supported in part by National Heart, Lung, and Blood Institute grant HL096497-01 (K.Y.).
Footnotes
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.L. conceived the idea, performed research, analyzed data, and drafted the manuscript; H.Z. performed research, and analyzed and interpreted data; W.B. performed research, analyzed data, and edited the manuscript; N.B. and J.E. recruited patients and obtained their consent; M.A.H. compiled clinical data; J.B. selected and recruited patients and edited the manuscript; and K.Y. designed, directed, and wrote the manuscript.
Conflict-of-interest disclosure: J.B. receives clinical research support from the following companies: Amgen, Cangene, GlaxoSmithKline, Genzyme, IgG of America, Immunomedics, Ligand, Eisai Inc, Shionogi, and Sysmex. The family of J.B. owns stock in Amgen and GlaxoSmithKline. J.B. has participated in Advisory Boards for Amgen, GlaxoSmithKline, Ligand, Shionogi, and Eisai, and has also had a 1-day consult with Portola. The remaining authors declare no competing financial interests.
Correspondence: Karina Yazdanbakhsh, PhD, Laboratory of Complement Biology, New York Blood Center, 310 E 67th St, New York, NY 10065; e-mail: kyazdanbakhsh@nybloodcenter.org.
References
- 1.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 2.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192(4):475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair PA, Norena LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Bouaziz JD, Calbo S, Maho-Vaillant M, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010;40(10):2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 5.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauri C, Bosma A. Immune regulatory function of B cells. Ann Rev Immunol. 2012;30:221–41. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 8.Carter NA, Vasconcellos R, Rosser EC, et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186(10):5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 9.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125(5):1114–1124. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Gernsheimer T. Chronic idiopathic thrombocytopenic purpura: mechanisms of pathogenesis. Oncologist. 2009;14(1):12–21. doi: 10.1634/theoncologist.2008-0132. [DOI] [PubMed] [Google Scholar]
- 11.Zhu XJ, Shi Y, Peng J, et al. The effects of BAFF and BAFF-R-Fc fusion protein in immune thrombocytopenia. Blood. 2009;114(26):5362–5367. doi: 10.1182/blood-2009-05-217513. [DOI] [PubMed] [Google Scholar]
- 12.Emmerich F, Bal G, Barakat A, et al. High-level serum B-cell activating factor and promoter polymorphisms in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2007;136(2):309–314. doi: 10.1111/j.1365-2141.2006.06431.x. [DOI] [PubMed] [Google Scholar]
- 13.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99(5):1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 14.Liu XG, Ma SH, Sun JZ, et al. High-dose dexamethasone shifts the balance of stimulatory and inhibitory Fcgamma receptors on monocytes in patients with primary immune thrombocytopenia. Blood. 2011;117(6):2061–2069. doi: 10.1182/blood-2010-07-295477. [DOI] [PubMed] [Google Scholar]
- 15.Semple JW, Freedman J. Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood. 1991;78(10):2619–2625. [PubMed] [Google Scholar]
- 16.Semple JW, Milev Y, Cosgrave D, et al. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87(10):4245–4254. [PubMed] [Google Scholar]
- 17.Ogawara H, Handa H, Morita K, et al. High Th1/Th2 ratio in patients with chronic idiopathic thrombocytopenic purpura. Eur J Haematol. 2003;71(4):283–288. doi: 10.1034/j.1600-0609.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuwana M, Kaburaki J, Ikeda Y. Autoreactive T cells to platelet GPIIb-IIIa in immune thrombocytopenic purpura. Role in production of anti-platelet autoantibody. J Clin Invest. 1998;102(7):1393–1402. doi: 10.1172/JCI4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwana M, Kaburaki J, Kitasato H, et al. Immunodominant epitopes on glycoprotein IIb-IIIa recognized by autoreactive T cells in patients with immune thrombocytopenic purpura. Blood. 2001;98(1):130–139. doi: 10.1182/blood.v98.1.130. [DOI] [PubMed] [Google Scholar]
- 20.Sakakura M, Wada H, Tawara I, et al. Reduced CD4+CD25+ T cells in patients with idiopathic thrombocytopenic purpura. Thromb Res. 2007;120(2):187–193. doi: 10.1016/j.thromres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Zhao H, Poon MC, et al. Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol. 2007;789(2):139–143. doi: 10.1111/j.1600-0609.2006.00780.x. [DOI] [PubMed] [Google Scholar]
- 22.Ling Y, Cao X, Yu Z, Ruan C. Circulating dendritic cells subsets and CD4+Foxp3+ regulatory T cells in adult patients with chronic ITP before and after treatment with high-dose dexamethasome. Eur J Haematol. 2007;79(4):310–316. doi: 10.1111/j.1600-0609.2007.00917.x. [DOI] [PubMed] [Google Scholar]
- 23.Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147–1150. doi: 10.1182/blood-2007-12-129262. [DOI] [PubMed] [Google Scholar]
- 24.Yu J, Heck S, Patel V, et al. Defective circulating CD25 regulatory T cells in patients with chronic immune thrombocytopenic purpura. Blood. 2008;112(4):1325–1328. doi: 10.1182/blood-2008-01-135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Audia S, Samson M, Guy J, et al. Immunologic effects of rituximab on the human spleen in immune thrombocytopenia. Blood. 2011;118(16):4394–4400. doi: 10.1182/blood-2011-03-344051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355(16):1672–1681. doi: 10.1056/NEJMoa054626. [DOI] [PubMed] [Google Scholar]
- 27.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–2247. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 28.Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113(10):2161–2171. doi: 10.1182/blood-2008-04-150078. [DOI] [PubMed] [Google Scholar]
- 29.Bussel JB. Update on eltrombopag for ITP. Oncology. 2009;23(13):1177–1178. [PubMed] [Google Scholar]
- 30.Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]
- 31.Kuter DJ, Rummel M, Boccia R, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med. 2010;363(20):1889–1899. doi: 10.1056/NEJMoa1002625. [DOI] [PubMed] [Google Scholar]
- 32.Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639–4645. doi: 10.1182/blood-2010-04-281717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez-Gamboa L, Mei H, Loddenkemper C, et al. Role of the spleen in peripheral memory B-cell homeostasis in patients with autoimmune thrombocytopenia purpura. Clin Immunol. 2009;1309(2):199–212. doi: 10.1016/j.clim.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Wasserstrom H, Bussel J, Lim LC, Cunningham-Rundles C. Memory B cells and pneumococcal antibody after splenectomy. J Immunol. 2008;181(5):3684–3689. doi: 10.4049/jimmunol.181.5.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solanilla A, Pasquet JM, Viallard JF, et al. Platelet-associated CD154 in immune thrombocytopenic purpura. Blood. 2005;105(1):215–218. doi: 10.1182/blood-2003-07-2367. [DOI] [PubMed] [Google Scholar]
- 36.May AE, Kalsch T, Massberg S, et al. Engagement of glycoprotein IIb/IIIa (alpha(IIb)beta3) on platelets upregulates CD40L and triggers CD40L-dependent matrix degradation by endothelial cells. Circulation. 2002;106(16):2111–2117. doi: 10.1161/01.cir.0000033597.45947.0f. [DOI] [PubMed] [Google Scholar]
- 37.Bohnhorst JO, Bjorgan MB, Thoen JE, Natvig JB, Thompson KM. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjogren's syndrome. J Immunol. 2001;167(7):3610–3618. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- 38.Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol. 2001;167(4):2361–2369. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- 39.Liu YJ, Banchereau J. Regulation of B-cell commitment to plasma cells or to memory B cells. Semin Immunol. 1997;99(4):235–240. doi: 10.1006/smim.1997.0080. [DOI] [PubMed] [Google Scholar]
- 40.Fogarty PF, Rick ME, Zeng W, Risitano AM, Dunbar CE, Bussel JB. T cell receptor VB repertoire diversity in patients with immune thrombocytopenia following splenectomy. Clin Exp Immunol. 2003;133(3):461–466. doi: 10.1046/j.1365-2249.2003.02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6(6):326–337. doi: 10.1038/nrrheum.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stasi R, Del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110(8):2924–2930. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 43.Kessel A, Haj T, Peri R, et al. Human CD19(+)CD25(high) B regulatory cells suppress proliferation of CD4(+) T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev. 2012;11(9):670–677. doi: 10.1016/j.autrev.2011.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.