CASE PRESENTATION
A 54-year-old man from Guyana presented with a painful, nonerythematous, subcutaneous nodule on his right patella at the site of a remote injury. The patient fell while climbing a coconut tree at 10 years of age, 44 years before presentation. The fall caused him to land on the stump of a ‘black sage tree’ injuring his right patella. Multiple small splinters from the stump penetrated his skin over and around his patella, which were removed shortly after the initial injury. The patient reported a significant amount of purulent discharge that eventually subsided. He was never treated with antibiotics. For approximately the next 40 years, the patient reported a history of knee swelling that dramatically increased in recent years. He reported no recent trauma. He experienced no fever, chills, sweats or recent weight loss. The patient was a nonsmoker, did not drink alcohol and had no history of injection drug use.
On examination, he was afebrile and looked well. There was no lymphadenopathy. There was a 4 cm × 4 cm nonerythematous, indurated nodule on the anterior aspect of the right patella. There was no joint effusion; however, there was a prepatellar bursitis. The lesion was not adherent to the patella and moved freely. Complete blood count, glucose level, renal function, hepatic transaminases and serum protein electrophoresis were all normal. He was HIV seronegative. A radiograph of the right knee did not demonstrate any foreign body or bone destruction. The prepatellar fluid was aspirated, producing thick fluid, tan-red in colour. Cytological analysis of the fluid showed histiocytes with inflammatory cells.
A Gram stain of the fluid did not demonstrate any pus cells or bacteria. Routine aerobic and anaerobic bacterial cultures were negative for bacterial growth. Mycobacterial smears and cultures were also negative.
A direct mycology smear using calcofluor white was negative, but two colonies of a filamentous fungus were noted both on the routine sheep blood agar and chocolate agar culture plates after four days of incubation.
Diagnosis
A second review of the mycology cultures after 17 days of incubation revealed a filamentous fungus. The fungus was identified as Pleurostomophora richardsiae based on gross and microscopic morphology (Figure 1). Its identification was confirmed with DNA sequencing.
Figure 1).
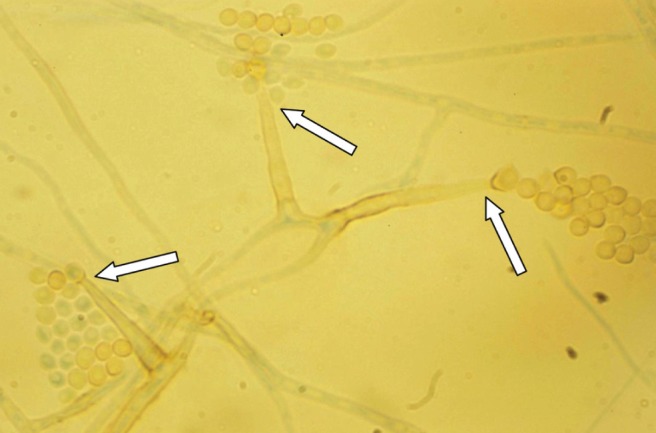
A lactophenol aniline blue stain of Pleurostomophora richardsiae on potato dextrose agar incubated for 10 days. Prominent solitary phialides (arrows) with flared tips producing oval to round conidia were noted, typical of P richardsiae (original magnification ×400)
The patient declined antifungal medications and surgical removal. He was subsequently lost to follow-up but then presented again one year later. On re-examination, the lesion remained unchanged. Fluid was aspirated and culture again produced P richardsiae. Again he declined therapy, and in follow-up surveillance, stated “I’ve lived with it this long, why do anything now?”
DISCUSSION
P richardsiae is a rare dematiaceous (dark-walled) fungus that was previously known as Phialophora richardsiae but has been recently renamed (1). It was first isolated from a patient with a phaeomycotic cyst in 1968 (2). It is found in soil, decaying wood and vegetation. Although rare, infection has been reported worldwide but is believed to be more common in tropical climates (2,3). Similar to other dematiaceous fungi, disease can follow traumatic inoculation.
In general, dematiaceous fungi are associated with three disease states: phaeohyphomycosis, eumycotic mycetoma and chromoblastomycosis, which differ in their clinical presentation and histopathology. All three diseases are usually a result of traumatic introduction of the fungi into the skin and subcutaneous tissue. Host immunity and the specific fungal species both play a role in determining the clinical presentation. As in our patient, P richardsiae usually presents as phaeohyphomycosis, with skin and subcutaneous encapsulated cystic granulomas with central suppuration. Multiple abscesses may occur early in infection and disseminated disease primarily occurs in immunocompromised hosts. In addition to P richardsiae, subcutaneous phaeohyphomycosis may be due to a wide variety of other dematiaceous fungi such as Phaeoacremonium parasiticum, Exophiala jeanselmei and Wangiella dermatitidis (1,3). Therefore, it is important for clinicians to recognize the necessity of ordering mycology smears and cultures in persons who present with chronic cystic subcutaneous abscesses. In chromoblastomycosis, patients typically present with chronic localized skin and subcutaneous verrucoid, ulcerated or crusted lesions (1,4,5). Pseudoepitheliomatous hyperplasia with hyperkeratosis and parakeratosis and sclerotic bodies are seen on histopathology (1,5). One case of P richardisae presenting in this way was recently reported (6). Eumycotic mycetomas are midway in the spectrum of disease between phaeohyphomycosis and chromoblastomycosis. In eumycotic mycetoma, patients typically present with multiple draining sinus tracts and abscesses with granules containing septate hyphae seen on histopathology. The skin and subcutaneous tissues, primarily involving the lower leg and foot, are involved, but long-standing infections may extend to muscle, fascia and bone (3,4).
To our knowledge, this is the first documented case of P richardsiae to present after such a long period from initial inoculation (44 years). Because his initial trauma and likely inoculation occurred in 1964, four years before the organism was first described (1), this likely represents both the first and most recently recorded human case. The persistence of the organism in two aspirates one year apart confirms the chronicity of the infection.
An Ovid MEDLINE and PubMed search revealed 22 other human cases of infection with P richardsiae in the world literature since 1968 (Table 1). Only cases identified to the species level according to culture were included. Given the preponderance of cases in the tropics and subtropics, limiting the search to the English language could have excluded other reports.
TABLE 1.
Case reports of Pleurostomophora richardsiae
| Author (reference), year | General condition of patient (country, if specified) | History and symptoms* | Treatment | Outcome |
|---|---|---|---|---|
| Schwartz and Emmons (2), 1968 | 80-year-old white man; healthy, mild heart failure | Cystic mass with yellow viscous contents; no adenopathy; fungal elements present | Excision | Complete recovery |
| Listemann (16), 1975 | 50-year-old white woman; healthy | Small nodule in lacrymoidal duct; yellowish-brown contents; Pseudomonas aeruginosa also isolated | Excision | Complete recovery |
| Torstrick et al (11), 1979 | 79-year-old black man; 30-year history of thorn puncture | Extra-articular, cystic mass of the elbow; no erythema; no adenopathy; extracapsular granuloma; viscous yellowish fluid; fibrous capsule; some inflammation; hyphal fungal elements present | Excision | Complete recovery |
| Corrado et al (17), 1980 | 65-year-old Puerto Rican man; diabetes | Painful swelling on sole; yellow purulent fluid; no granules; Staphylococcus epidermidis also isolated | Open and drained | Complete recovery |
| 43-year-old Jamaican man; type-2 diabetes | Pedal edema; ulcerative lesion of the leg drained with multiple tracts; no granules; nonfibrillar; seropurulent material; hyphal fragments present | None | Death from nonfungal causes | |
| Moskowitz et al (18), 1983 | 52-year-old white man; healthy; wood splinter six months before first noticing mass (Miami, Florida, USA) | Five-month history of nonpainful smooth subcutaneous mass on left index finger; no drainage; yellow contents; slight inflammation; fibrous capsule; no fever or adenopathy; fungal elements present; Enterobacter species also isolated | Excision | Complete recovery |
| Yangco et al (14), 1984 | 70-year-old white woman; hepatosplenomegaly, recurrent fever | Pedal infiltrate draining thick yellowish-brown material from the dorsal aspect of the right foot; some local bone inflammation; fungal elements present; Enterobacter species also isolated | Debridement and left open to drain; surgery and intravenous antimycotic therapy (separate courses of amphotericin B [1 week, 161 mg total] and 5-fluorocytosine [2.5 weeks, 150 mg/kg/day]), the wound continued to drain purulent material but resolved spontaneously three weeks later | Death from nonfungal causes |
| Reyes and Buchman (19), 1985 | 54-year-old white man; received depomedrol injection for extensor tendinitis (Miami, Florida, USA) | Three-month history of mass over the dorsum of the middle phalanx of the right index finger; physical examination revealed a firm, nontender subcutaneous mass, 0.7 cm in diameter, without local erythema, fever or adenopathy | Excision | Complete recovery |
| Suppiah et al (20), 1987 | 30-year-old Indian man; previously healthy, sustained motor vehicle accident; fractured left femur, splinted and intramedullary nail inserted (Malaysia) | Nine-month history of pruritic, moist, crusty, eruptions over the lower tibia and left ankle (the area that had rested on the splint); hyphal fragments and yeast cells seen | Oral griseofulvin 500 mg daily; topical naftifine | Recurred after three weeks; subsequently excised; lost to follow-up |
| Ikai et al (10), 1988 | 30-year-old Japanese man; non-Hodgkin’s lymphoma and diabetes; treated with immunosuppressive agents for his primary cancer | Subcutaneous encapsulated abscess; yellow fluid; no inflammation; fungal elements present | 5-fluorocytosine and topical injection of amphotericin B in combination with surgical excision of the subcutaneous abscess. | Lesion immediately recurred; death from primary disease |
| Pitrak et al (13), 1988 | 60-year-old woman; hypertension, type-2 diabetes and coronary artery disease | Six-week history of lump (hyperpigmented) on medial aspect of left foot; physical examination revealed a subcutaneous cystic nodule (1.0 cm in diameter); no adenopathy | Excision | Complete recovery |
| de Hoog et al (5), 1989 | 47-year-old man from Senegal; healthy (Paris, France) | Nodule at knee; no drainage; yellow excision fluid; slight inflammation; fibrous capsule; no adenopathy; fungal elements present; Pseudomonas stutzeri also isolated | Excision | Complete recovery |
| de Hoog et al (5), 1989 | 39-year-old white man; renal transplant; corticotherapy (Paris, France) | Bursitis of the knee; yellow fluid; fungal elements present (unknown duration) | Open and drained; oral antimycotic therapy (not specified) | Lesion immediately recurred; followed by excision |
| Tam and Freedman (21), 1989 | 64-year-old man; boat builder; history of polymyalgia rheumatica treated with prednisone and azathioprine | Slowly enlarging plaques on right knee with occasional exudation; within three months multiple nodules developed on elbow; culture grew Exophiala jeanselmei and P richardsiae | Itraconazole 100 mg/day | Resolution within four weeks |
| Singh et al (22), 1992 | 15-year-old girl and her 50-year-old mother, both bidi makers at Majholi, a suburb of Jabalpur, India; presented with similar skin lesions of the upper gluteal region near the waist line | 6-month history of multiple grey lesions averaging 3 cm to 4 cm in diameter. They appeared hypopigmented, and slightly hyperkeratotic with irregular borders. The lesions were puritic with frequent watery discharge. | Topical application of clotrimazole-betamethasone-gentamicin cream (Gentalene-C, Crosland Research Laboratories, India) twice a day | Lesions resolved within 15 days; No further evidence of P richardsiae on direct microscopy and culture |
| Juma et al (8), 1993 | 52-year-old man; noninsulin-dependent diabetes mellitus; mitral valve replacement in 1986 | 10-day history of redness in the right eye and loss of vision with increasing shortness of breath; both blood cultures revealed P richardsiae; aqueous humour culture was sterile | Amphotericin B at 0.1 mg/kg/day was titrated to a final dose of 0.5 mg/kg/day; aortic and mitral valve were replaced | Death 10 weeks postoperation due to nonfungal complications; necropsy results not available |
| Das et al (23), 1995 | 54-year-old Afro-Carribean man; cadaveric renal transplant secondary to hypertensive nephropathy two years before presentation; maintenance of immunosuppressive therapy; steroid-induced diabetes mellitus (London, United Kingdom) | 12-month history of an increasing fluctuant swelling over the left knee and proximal interphalangeal joint of his right fourth digit secondary to trauma; no systemic signs of infection; negative Gram stain; P richardsiae grew from both lesions; sensitive to amphotericin B and itraconazole | Excision; opaque, brown pus | Complete recovery |
| Apetrei et al (7), 1995 | 28-year-old man; HIV positive, hepatitis C and tuberculous meningitis; intravenous drug use | Three-month history of fever and pain in proximal part of right tibia and left foot; radiological changes of osteomyeltitis present; technetium-99 scan positive | Amphotericin B and flucytosine followed by 200 mg/day itraconazole (duration not specified) | Complete recovery; decrease in technetium-99 uptake |
| Lieb et al (9), 2003 | 36-year-old otherwise healthy man sustained an intraocular metallic foreign body | One-month history of an acute onset, painful loss of vision in right eye; RAPD present; normal visual acuity; right conjunctiva showed 1+ injection; fibrotic plaque covered the anterior surface of the posterior chamber; intraocular lens was noted to be enveloped in a cocoon of fibrinous material; undiluted vitreous fungal culture was positive for P richardsiae. | Vitrectomy with intraocular lens removal and an intravitreal injection of amphotericin B; miconazole 200 mg daily (duration not specified) | Total retinal detachment with severe pain and blindness in right eye; patient considering enucleation |
| Yehia et al (24), 2004 | 45-year-old man with ESRD secondary to interstitial nephritis; maintenance immunosuppression, cyclosporine and mycophenolate mofetil | Two-month history of painful lump on the medial aspect of his left foot (1.5 cm in diameter) | Debridement and liposomal amphotericin B for two weeks followed by itraconazole (200 mg twice/day) for six weeks | Complete recovery |
| Yiel-Hea Seo, et al (25), 2010 | 43-year-old Korean construction worker | Two-month history of a slowly enlarging pruritic lesion on his right shin; right shin showed a dark-brownish, hyperkeratotic, erythematous, fluctuant plaque with focal purulent discharge, crust formation, and several conglomerated firm nodules (chromoblastomycosis) | Three months of oral itraconazole (200 mg/day); followed by three months of combination therapy: oral itraconazole (200 mg/day) and terbinafine (500 mg/day) | Partial response after six months of oral therapy |
All cases isolated P richardsiae. ESRD End-stage renal disease; RAPD Relative afferent pupillary defect
The mean age of affected individuals was 49.3 years (range 15 to 80 years) with a male:female ratio of 3:1, possibly reflecting increased occupational and recreational exposure to wood chips and soil in men. Four of 12 (33%) cases that specified the patient’s origin were inoculated and had presented in the developing world. Eight of 12 cases (67%), however, presented in the developed world. This disparity likely reflects a combination of the organism’s long latency period and lack of diagnostic laboratory facilities in the developing world.
The most common manifestation of P richardsiae infection is a subcutaneous phaeohyphomycosis, with a well-circumscribed subcutaneous cyst filled with pus or serous material (17 of 22 cases). Histology usually consists of a pus- or serous-filled central cavity, surrounded by a fibrous wall with the appearance of a typical foreign body granuloma. In these 17 cases, infection remained localized, and regional adenopathy was not observed. Two patients with positive blood cultures developed disseminated, severe infection with this organism (7,8). One of these disseminated cases was in an HIV-positive, injection drug user (7) and the second case involved prosthetic valve endocarditis (8). Two cases involved endopthalmitis (8,9). Two cases of osteomyelitis occurred, one from hematogenous dissemination and one via local extension. A very indolent and prolonged course was documented in two previous cases that had 12-year (10) and 30-year (11) lag periods between injury and presentation, respectively. This suggests that this organism should be suspected in chronic, pus-filled, subcutaneous cysts with a history of localized trauma with soil or wood contamination, irrespective of how remote the trauma. The lack of history of trauma in some cases in the literature may be due to a long lag period between the time of injury and presentation with the lesion and, thus, a lack of recognition of the importance of the remote exposure. Therefore, it is important for clinicians to recognize the necessity of ordering mycology smears and cultures in persons who present with chronic, cystic, subcutaneous abscesses.
Pleurostomophora have been reported to have in vitro susceptibility to systemic antifungal agents such as amphotericin B, itraconazole, terbinafine, voriconazole and posaconazole (4,5,7–10). However, it was previously believed that dematiaceous fungi such as Pleurostomophora do not respond well to systemic antifungal therapy alone, making surgical excision the treatment of choice (12,13). Our literature review demonstrates that of the 17 cases of P richardsiae presenting as localized subcutaneous phaeohyphomycosis, the 10 treated with excision alone had successful outcomes. Of the three cases that were treated with both antimycotic agents and surgery, the only failure was in a highly immunocompromised patient (10). Three of four patients treated with antifungal agents alone had successful outcomes (7,11,12). The one case that failed antifungal therapy was treated with oral griseofulvin and topical naftifine and required subsequent excision; therefore, it is possible that antimycotic agents are more effective than previously believed. In cases with hematogenous dissemination, antimycotic agents remain central to therapy, although the small number of cases makes it difficult to clarify the clinical efficacy of different antifungal regimens.
REFERENCES
- 1.St-Germain G, Summerbell R. Identifying Fungi: A Clinical Laboratory Handbook. 2nd edn. Belmont: Star Publishing Company, Inc; 2011. p. 212. [Google Scholar]
- 2.Schwartz IS, Emmons CW. Subcutaneous cystic granuloma caused by a fungus of wood pulp (Phialophora richardsiae) Am J Clin Pathol. 1968;49:500–5. doi: 10.1093/ajcp/49.4.500. [DOI] [PubMed] [Google Scholar]
- 3.Mostert L, Groenewald J, Summerbell R, et al. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. J Clin Microbiol. 2005;43:1752–67. doi: 10.1128/JCM.43.4.1752-1767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier L, Balows A, Sussman M. Topley and Wilson’s Microbiology and Microbial Infections, Vol 4, Medical Mycology. 9th edn. Oxford: Oxford University Press; 1998. [Google Scholar]
- 5.de Hoog GS, Guarro J, Gene J, Figueras MJ. Atlas of Clinical Fungi. 3rd edn. Utrecht: CBS; 2011. [Google Scholar]
- 6.Son YM, Kang HK, Na SY, et al. Chromoblastomycosis caused by Phialophora richardsiae. Ann Dermatol. 2010;22:362–6. doi: 10.5021/ad.2010.22.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apetrei C, Descamps D, Panzaru C, Duc MC, Simon F, Brun-Vézinet F. First case of osteomyelitis due to Phialophora richardsiae in a patient with HIV infection. AIDS. 1995;9:975–6. [PubMed] [Google Scholar]
- 8.Juma A. Phialophora richardsiae endocarditis of aortic and mitral valves in a diabetic man with a porcine mitral valve. J Infect Dis. 1993;27:173–5. doi: 10.1016/0163-4453(93)94782-7. [DOI] [PubMed] [Google Scholar]
- 9.Lieb D, Smiddy W, Miller D, Cooperman E. Case report: Fungal endophthalmitis caused by Phialophora richardsiae. Retina. 2003;23:406–7. doi: 10.1097/00006982-200306000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Ikai K, Tomono H, Watanabe S. Phaeohyphomycosis caused by Phialophora richardsiae. J Am Acad Dermatol. 1988;19:478–81. doi: 10.1016/s0190-9622(88)70200-5. [DOI] [PubMed] [Google Scholar]
- 11.Torstrick F, Harrison K, Heckman J, Johnson J. Chronic bursitis caused by Phialophora richardsiae. J Bone Joint Surg Am. 1979;61:772–4. [PubMed] [Google Scholar]
- 12.Guého E, Bonnefoy A, Luboinski J, Petit JC, de Hoog GS. Subcutaneous granuloma caused by Phialophora richardsiae: Case report and review of the literature. Mycoses. 1989;32:219–23. doi: 10.1111/j.1439-0507.1989.tb02235.x. [DOI] [PubMed] [Google Scholar]
- 13.Pitrak DL, Koneman EW, Estupinan RC, Jackson J. Phialophora richardsiae infection in humans. Rev Infect Dis. 1988;10:1195–1203. doi: 10.1093/clinids/10.6.1195. [DOI] [PubMed] [Google Scholar]
- 14.Yangco BG, TeStrake D, Okafor J. Phialophora richardsiae isolated from infected human bone: Morphological, physiological and antifungal susceptibility studies. Mycopathologia. 1984;86:103–11. doi: 10.1007/BF00436495. [DOI] [PubMed] [Google Scholar]
- 15.Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–6. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Listemann H. Die kulturelle Untersuchung eines Tranesteines mit Isolierung des Pilzes Phialophora richardsiae. E Rodenwaldt Archiv. 1976;2:45–52. [Google Scholar]
- 17.Corrado ML, Weitzman I, Stanek A, et al. Subcutaneous infection with Phialophora richardsiae and its susceptibility to 5-fluorocytosine amphotericin-B and miconazole. Sabouraudia. 1980;18:97–104. [PubMed] [Google Scholar]
- 18.Moskowitz LB, Cleary TJ, McGinnis MR, Thomson CB. Phialophora richardsiae in a lesion appearing as a giant cell tumor of the tendon sheath. Arch Pathol Lab Med. 1983;107:374–6. [PubMed] [Google Scholar]
- 19.Reyes FA, Buchman MT. Phialophora richardsiae infection mimicking a soft tissue mass of a finger. J Hand Surg Br. 1986;11B:174. doi: 10.1016/0266-7681(86)90282-2. [DOI] [PubMed] [Google Scholar]
- 20.Suppiah M, Chin CS, Keah KC. Philaphora richardsiae isolated from a cutaneous lesion. Med J Malaysia. 1987;2:306–8. [PubMed] [Google Scholar]
- 21.Tam M, Freeman S. Phaeohyphomycosis due to Phialophora richardsiae. Australas J Dermatol. 1989;30:37–40. doi: 10.1111/j.1440-0960.1989.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 22.Yehia M, Thomas M, Helen P, Walter M, Dittmer I. Subcutaneous black fungus (phaeohyphomycosis) infection in renal transplant recipients: Three cases. Transplantation. 2004;77:140–2. doi: 10.1097/01.TP.0000107287.70512.E7. [DOI] [PubMed] [Google Scholar]
- 23.Singh S, Agrawal A, Naidu J, Hoog G, Figueras M. Cutaneous phaeohyphomycosis caused by Phialophora richardsiae and the effect of topical clotrimazole in its treatment. Antonie van Leeuwenhoek. 1992;61:51–5. doi: 10.1007/BF00572122. [DOI] [PubMed] [Google Scholar]
- 24.Son YM, Kang HK, Na SY, et al. Chromoblastomycosis caused by Phialophora richardsiae. Ann Dermatol. 2010;22:362–6. doi: 10.5021/ad.2010.22.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jumaa EA, Lightowler C, Baker LRI, Das SS. Cutaneous infection caused by Phialophora richardsiae treated successfully by surgical excision in an immunocompromised patient. J Infect. 1995;30:261–7. doi: 10.1016/s0163-4453(95)90877-3. [DOI] [PubMed] [Google Scholar]


