Abstract
A three-year-old boy presented with community-acquired pneumonia complicated by empyema. Streptococcus pyogenes (group A streptococcus) was identified on culture of the pleural fluid. The patient improved with antibiotic therapy and drainage of the empyema.
During his convalescence, the patient developed persistent fever, lethargy and anorexia. His inflammatory markers were elevated, and repeat cultures were negative. Although the patient had none of the classical mucocutaneous features of Kawasaki disease, an echocardiogram was performed, which revealed coronary artery dilation.
The patient was diagnosed with incomplete Kawasaki disease and treated with intravenous immunoglobulin and high-dose acetylsalicylic acid. The fever subsided within 48 h.
To the authors’ knowledge, the present report is the first report of Kawasaki disease associated with complicated S pyogenes pneumonia. It emphasizes the importance of considering incomplete Kawasaki disease among children with persistent fever, the role of echocardiography in diagnosis, and the potential link between Kawasaki disease and superantigen-producing organisms such as S pyogenes.
Keywords: Coronary arteritis, Group A streptococcus, Intravenous immunoglobulin, Kawasaki disease, Streptococcus pyogenes
Abstract
Un garçon de trois ans a consulté à cause d’une pneumonie non nosocomiale compliquée par un empyème. On a décelé un Streptococcus pyogenes (streptocoque du groupe A) à la culture du liquide pleural. L’état du patient s’est amélioré au moyen d’une antibiothérapie et d’un drainage de l’empyème.
Pendant sa convalescence, le patient a présenté une fièvre persistante, une léthargie et une anorexie. Ses marqueurs inflammatoires étaient élevés, et les nouvelles cultures étaient négatives. Même s’il ne présentait aucune des caractéristiques mucocutanées classiques de la maladie de Kawasaki, on a effectué un échocardiogramme, qui a révélé une dilatation des artères coronaires.
Le patient a reçu un diagnostic de maladie de Kawasaki incomplète et a été traité au moyen d’immunoglobuline intraveineuse et d’acide acétylsalicylique à forte dose. La fièvre a disparu en 48 heures. En autant que le sache les auteurs, c’est le premier rapport de cas de maladie Kawasaki incomplète compliquée par une pneumonie à S pyogenes, ce qui fait ressortir l’importance d’envisager une maladie de Kawasaki chez les enfants atteints de fièvre persistante, le rôle de l’échocardiographie dans le diagnostic et le lien potentiel entre la maladie de Kawasaki et les organismes producteurs de superantigène comme le S pyogenes.
Kawasaki disease (KD) is an acute childhood illness that presents with high spiking fever and a constellation of five classical clinical criteria: a polymorphous exanthem, bilateral nonpurulent bulbar conjunctivitis, erythema or swelling of hands/feet, lip and other oral mucosal changes, and cervical lymphadenopathy (1). It is currently the most common cause of acquired childhood heart disease in the developed world (2). Patients with fewer than four clinical criteria associated with KD, but clinical or laboratory features suggestive of the illness, are said to have ‘incomplete’ KD.
The etiology of KD, despite almost four decades of research, remains unknown. The epidemiological and clinical features of KD are suggestive of an infectious source (3). Many researchers have linked Streptococcus pyogenes (group A streptococcus [GAS]) infection to KD. Evidence to support this link has come from case reports and case series of KD in the setting of recent or coincident GAS infection, especially pharyngitis (4–8). We describe what we believe to be the first reported case of Kawasaki disease in association with complicated GAS pneumonia.
CASE PRESENTATION
A previously healthy three-year-old boy was admitted to The Hospital for Sick Children (Toronto, Ontario) with a four-day history of fever and pharyngitis, and a one-day history of lethargy, vomiting, dyspnea and cough. He was tachycardic (heart rate 190 beats/min) and normotensive, with a prolonged capillary refill time (4 s). He was in respiratory distress on admission, and a chest radiograph revealed large right-sided, lower-lobe pneumonia with associated loculated pleural effusion, from which 200 mL of purulent material was subsequently drained. His respiratory distress improved with empyema drainage and supplemental oxygen. His tachycardia resolved and peripheral perfusion improved with fluid resuscitation; inotropic support was not required. Initially, he was treated with an antibiotic combination of vancomycin (45 mg/kg/day), cefuroxime (150 mg/kg/day) and clindamycin (30 mg/kg/day), but this regimen was simplified to penicillin (400,000 U/kg/day) and clindamycin (30 mg/kg/day) when the culture of his pleural fluid grew GAS. Clindamycin was continued for its potential benefit in toxin-mediated disease. On day 3 of his admission, his chest drain was removed.
The patient continued to experience daily fever, and suffer from anorexia and lethargy. He had no localizing symptoms, lymphadenopathy, rash or mucocutaneous changes. On day 10 of his admission, a full blood count revealed a leukocyte count of 30.1×109/L, a neutrophil count of 23.1×109/L and a platelet count of 804×1012/L. The patient’s erythrocyte sedimentation rate reached 103 mm/h, and his C-reactive protein was 214 mg/L; the platelet count and erythrocyte sedimentation rate had increased gradually from his date of admission. His albumin level remained low (31 g/L) and his γ-glutamyltransferase level was elevated (53 U/L); his liver transaminase and alkaline phosphatase levels were normal. Repeat blood, urine and stool cultures were negative. A bone scan revealed no evidence of osteomyelitis. An abdominal ultrasound was normal, aside from mild dilation of the intrahepatic bile ducts and splenomegaly. A chest ultrasound revealed a small, localized pleural collection with internal septations and debris (4 cm × 2 cm), but radiological improvement from the previous appearance. In view of the persistent fever and elevated inflammatory markers, the diagnosis of incomplete KD was considered and an echocardiogram was performed on day 10 of the patient’s admission, with particular emphasis on his coronary vasculature (Figure 1). It revealed prominence of the left anterior descending artery (0.30 cm, z-score 4.7) and left main coronary artery (0.35 cm, z-score 3.2). No vegetations were observed. The patient received 2 g/kg of intravenous immunoglobulin, and was started on acetylsalicylic acid based on the notion that he had incomplete KD. His fever subsided within 48 h. The patient developed desquamative changes on his hands and feet on day 15. Follow-up echocardiography on day 18 revealed ongoing dilation of the left anterior descending artery (0.26 cm, z-score 3.2) and the left main coronary artery (0.35 cm, z-score 3.2). He was well when discharged home, and remained on acetylsalicylic acid for an additional six weeks. Repeat echocardiogram performed on day 64 revealed no evidence of coronary aneurysms, but persistent dilation of the left anterior descending artery (0.23 cm, z-score 1.7) and left main coronary artery (0.38 cm, z-score 3.7). The patient had otherwise made a full clinical recovery.
Figure 1).
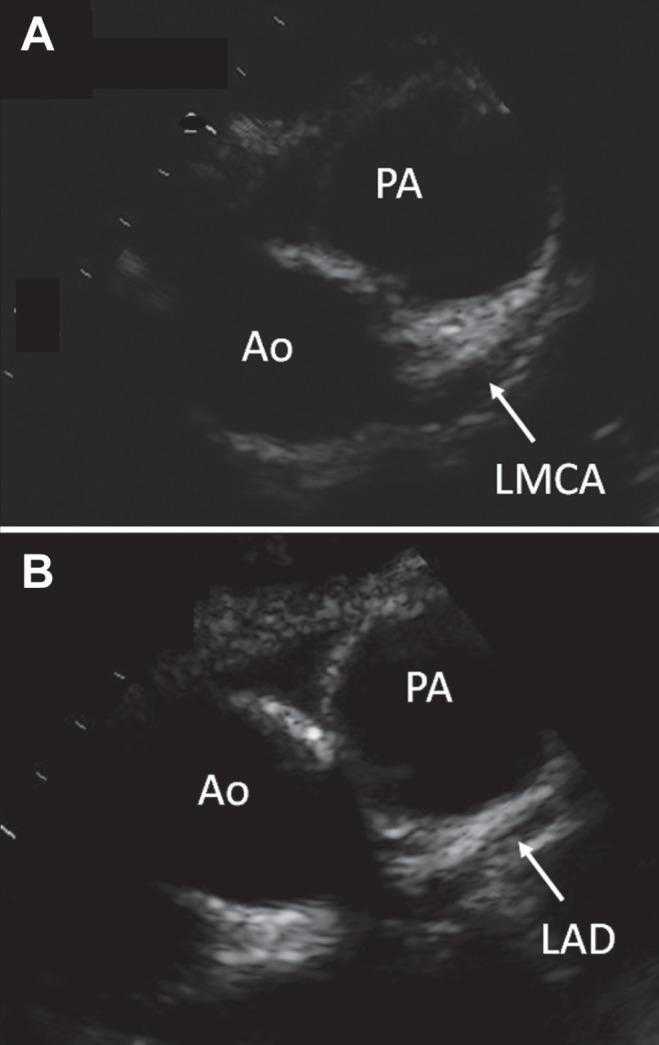
A Dilated left main coronary artery (LMCA). B Dilated left anterior descending artery (LAD) beyond the bifurcation of the LMCA. Ao Aorta; PA Pulmonary artery
DISCUSSION
The present case of incomplete KD is noteworthy for a number of reasons. The first noteworthy feature of the case is the close temporal relationship of the illness with complicated GAS pneumonia. While many attempts have been made to link KD with infection, the link with GAS is especially compelling. In some instances, children with proven GAS infection have met the clinical criteria for KD diagnosis, most notably in a study by Benseler et al (9), in which 16 of 134 patients with KD experienced recent GAS pharyngitis. In particular, there is considerable clinical overlap (ie, persistent fever, desquamative rash and mucous membrane erythema) between KD and streptococcal toxic shock syndrome. Many studies have suggested that KD, similar to streptococcal toxic shock syndrome, is a superantigen-mediated process (10). Superantigens are a group of proteins that interact intact, and relatively nonspecifically, with major histocompatibility complex class II molecules and the T cell receptor at the variable region of the β-chain (Vβ). They can stimulate up to 30% of the naive lymphocyte pool, leading to exaggerated release of cytokines by both T lymphocytes and macrophages, and producing an uncoordinated inflammatory response (10).
Evidence to support the ‘superantigen hypothesis’ for KD comes from a variety of sources: case reports of individuals with both KD and GAS infection, elevated serological response (both immunoglobulin M and immunoglobulin G) to superantigens, and skewed T cell receptor Vβ distribution among KD patients (10–13). Other sources, however, dispute the findings of these studies, and propose a conventional antigenic process. Recent studies hypothesize the involvement of an unknown respiratory pathogen, possibly an RNA virus (14). Therefore, the link with GAS, while compelling, remains subject to debate as long as we lack a clear understanding of the pathogenesis of KD.
The second noteworthy feature of the case was the absence of classical clinical diagnostic criteria of KD. The diagnosis of incomplete KD was initially suspected because of the child’s age, ethnicity, persistent fever, suggestive changes in laboratory markers, recent infection with a superantigen-producing organism and, ultimately, coronary artery changes on echocardiogram. The decision to treat with intravenous immunoglobulin was particularly influenced by the echocardiogram findings, and later vindicated by the patient’s clinical response.
There is much evidence to suggest that incomplete KD is as likely to be associated with coronary artery abnormalities as complete KD, and may, in fact, be a risk factor for delayed diagnosis and consequently poor outcome (15–17). This has led many to suggest that a ‘screening’ echocardiogram may be helpful in ambiguous/incomplete KD cases (1,18). Such an approach may lead to overdiagnosis because coronary artery dilation is not entirely specific to KD, and is present in a variety of childhood inflammatory disorders including polyarteritis nodosa and systemic-onset juvenile idiopathic arthritis (15,19). The consequences of unnecessarily treating a child with intravenous immunoglobulin are minimal in comparison with the potential preventable cardiac complications of a missed KD diagnosis. More research needs to be performed to examine the specificity of coronary artery abnormalities for KD in the setting of acute febrile illness during childhood.
CONCLUSION
The present case emphasizes the importance of considering incomplete KD in children with persistent fever due to an organism known to be associated with KD (such as superantigen-producing bacteria), and in those who have not responded to antibiotics and/or are not following the expected clinical course, especially in the setting of elevated inflammatory markers and thrombocytosis. It also emphasizes the evolving role of echocardiography, not only as an adjunct in management, but also as a diagnostic tool in ambiguous cases.
Acknowledgments
The authors thank the patient’s parents for consenting to publication of this case report. They also thank Dr Fraser Golding from the Division of Cardiology, The Hospital for Sick Children, for providing the images for Figure 1.
Footnotes
FINANCIAL SUPPORT/CONFLICTS OF INTEREST: The authors have no financial support or conflicts of interest to declare.
REFERENCES
- 1.Newburger J, Takahashi M, Gerber M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 2.Taubert K, Rowley A, Shulman S. Nationwide survey of Kawasaki disease and acute rheumatic fever. J Pediatr. 1991;119:279–82. doi: 10.1016/s0022-3476(05)80742-5. [DOI] [PubMed] [Google Scholar]
- 3.Burns JC, Cayan DR, Tong G, et al. Seasonality and temporal clustering of Kawasaki syndrome. Epidemiology. 2005;16:220–5. doi: 10.1097/01.ede.0000152901.06689.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson D, Warner G, Barlow E. Kawasaki disease associated with streptococcal infection within a family. J Paediatr Child Health. 1995;31:355–7. doi: 10.1111/j.1440-1754.1995.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 5.Barton M, Melbourne R, Morais P, Christie C. Kawasaki syndrome associated with group A streptococcal and Epstein-Barr virus co-infections. Ann Trop Paediatr. 2002;22:257–60. doi: 10.1179/027249302125001543. [DOI] [PubMed] [Google Scholar]
- 6.Cox F, Foshee W, Miller J, Jr, Moore S. Simultaneous Kawasaki disease and group A streptococcal pharyngitis. Clin Pediatr (Phila) 1993;32:48–50. doi: 10.1177/000992289303200109. [DOI] [PubMed] [Google Scholar]
- 7.Hoare S, Abinun M, Cant AJ. Overlap between Kawasaki disease and group A streptococcal infection. Pediatr Infect Dis J. 1997;16:633–4. doi: 10.1097/00006454-199706000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Rider LG, Mendelman PM, French J, Sherry DD. Group A streptococcal infection and Kawasaki syndrome. Lancet. 1991;337:1100–1. doi: 10.1016/0140-6736(91)91751-f. [DOI] [PubMed] [Google Scholar]
- 9.Benseler S, McCrindle B, Silverman E, Tyrrell P, Wong J, Yeung RS. Infections and Kawasaki disease: Implications for coronary artery outcome. Pediatrics. 2005;116:e760–6. doi: 10.1542/peds.2005-0559. [DOI] [PubMed] [Google Scholar]
- 10.Matsubara K, Fukaya T. The role of superantigens of group A streptococcus and Staphylococcus aureus in Kawasaki disease. Curr Opinion Infect Dis. 2007;20:298–303. doi: 10.1097/QCO.0b013e3280964d8c. [DOI] [PubMed] [Google Scholar]
- 11.Abe J, Kotzin BL, Jujo K, et al. Selective expansion of T cells expressing T-cell receptor variable regions V beta 2 and V beta 8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–70. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung DY, Meissner HC, Shulman ST, et al. Prevalence of superantigen-secreting bacteria in patients with Kawasaki disease. J Pediatr. 2002;140:742–6. doi: 10.1067/mpd.2002.123664. [DOI] [PubMed] [Google Scholar]
- 13.Matsubara K, Fukaya T, Miwa K, et al. Development of serum IgM antibodies against superantigens of Staphylococcus aureus and Streptococcus pyogenes in Kawasaki disease. Clin Exp Immunol. 2006;143:427–34. doi: 10.1111/j.1365-2249.2006.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowley A, Baker S, Orenstein J, Shulman S. Searching for the cause of Kawasaki disease – cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol. 2008;6:394–401. doi: 10.1038/nrmicro1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cimaz R, Sundel R. Atypical and incomplete Kawasaki disease. Best Pract Res Clin Rheum. 2009;23:689–97. doi: 10.1016/j.berh.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Minich L, Sleeper L, Atz A, et al. Delayed diagnosis of Kawasaki disease: What are the risk factors? Pediatrics. 2007;120:e1434–e40. doi: 10.1542/peds.2007-0815. [DOI] [PubMed] [Google Scholar]
- 17.Sonobe T, Kiyosawa N, Tsuchiya K, et al. Prevalence of coronary artery abnormality in incomplete Kawasaki disease. Pediatr Int. 2007;49:421–6. doi: 10.1111/j.1442-200X.2007.02396.x. [DOI] [PubMed] [Google Scholar]
- 18.Baer A, Rubin L, Shapiro C, et al. Prevalence of coronary artery lesions on the initial echocardiogram in Kawasaki syndrome. Arch Pediatr Adolesc Med. 2006;160:686–90. doi: 10.1001/archpedi.160.7.686. [DOI] [PubMed] [Google Scholar]
- 19.Binstadt B, Levine J, Nigrovic P, et al. Coronary artery dilation among patients presenting with systemic-onset juvenile idiopathic arthritis. Pediatrics. 2005;116:e89–e93. doi: 10.1542/peds.2004-2190. [DOI] [PubMed] [Google Scholar]


