Abstract
“Spinal instrumentation without fusion” techniques, which do not interfere with spinal growth, have been used extensively in the treatment of progressive spinal scoliosis in very young children. Due to subperiosteal exposure, the process of spinal instrumentation may induce spontaneous bony fusion. Instrumentation and surgical techniques have been modified in order to prevent spontaneous posterior fusion from occurring in children. An absorbable ADCON-L gel has been shown to inhibit scar and epidural adhesions following spinal surgeries. However, little is known about its influence on spinal fusion. In the present study, a single-level intertransverse arthrodesis at L4-5 on both sides was performed on each of nine pigs. Each side was randomly designed to receive autogenous bone graft with or without ADCON-L gel. The animals were followed for 10 weeks postoperatively. A fusion rate of 78% (7/9) was obtained in the autograft treatment by plain X-ray and CT evaluation, while the autograft/ADCON-L treatment yielded a 0% (0/9) fusion rate (p=0.001). Histomorphometric evaluation revealed that the addition of ADCON-L gel to bone graft decreased bone and bone marrow formation and significantly increased fibrous tissue formation. No statistical difference between the two treatments was found in cartilage, bone surface density, osteoid surfaces or osteoclast-covered surfaces in any zone. We conclude that ADCON-L gel mixed into autogenous bone graft can delay or decrease bone formation at spinal arthrodesis sites, thus influencing the extent of spinal fusion. This accords with our hypothesis that the use of ADCON-L gel can prevent not only the occurrence of spontaneous fusion in very young scoliosis patients after instrumentation without fusion, but also re-ossification of a decompressed spinal canal.
Keywords: Spinal fusion, ADCON-L, Bone regeneration, Pig
Introduction
Because of the associated disturbance of spinal growth, surgery for progressive spinal deformities in very young children is a challenging task. Most foregoing studies report having used a “growing spinal instrumentation without fusion” technique in order to avoid interference with spinal growth. Harrington first described the procedure, which used a Harrington rod [10]. The “Luque trolley” technique of segmental spinal instrumentation without fusion has been used with some success [13, 14, 18]. Blakemore et al [1] and Morin [15] introduced segmental spinal instrumentation systems (Cotrel-Dubousset, Isola, etc) for severe spinal deformities in young children. However, in all of these procedures, spontaneous bony fusion can occur before the patients reach maturity [7, 14], also causing posterior ankylosis and crankshaft [5].
Takaso et al designed a remote-controlled growing rod instrumentation system to reduce the number of procedures required in young children with severe scoliosis and postoperative complications [23]. Other authors designed an operative technique to prevent the spontaneous posterior fusion from occurring [1]. Prevention of bone growth in relation to a decompressed spinal canal also has clinical implications, since re-stenosis has occurred in up to 20% of patients who have received decompression.
The intent of the present study was to pursue a biological method for the inhibition of spontaneous posterior spinal fusion by use of a tissue ingrowth inhibitor, ADCON-L Anti-Adhesion Barrier Gel (Gliatech, Cleveland, OH, USA), which is a resorbable gel designed to inhibit scar and peridural adhesions following laminectomy, laminotomy, and discectomy [6, 8, 12, 20]. To realize this aim, the efficiency of ADCON-L gel in preventing spinal fusion in an experimental pig model was tested.
Materials and methods
Animal model and surgical preparation
An un-instrumented posterolateral lumbar spinal fusion at L4-L5 level served as the experimental model to assess the influence of ADCON-L gel on bone growth. All animal surgeries and experiments complied with the Danish Law on Animal Experimentation and were approved by the Danish Ministry of Justice Ethical Committee, J.no.1998-561-67. A total of nine female 12-week-old Danish landrace pigs, weighing 46.4 kg (SD 3.6 kg), were involved in the investigation. The pigs served as their own controls.
After normal health status determination, each animal was premedicated with 25 mg midazolam and 200 mg azaperon intramuscularly. Orotracheal intubation was set up after intravenous injection of 20 mg etomidate. Anesthesia was maintained by inhalation of isoflurane (1.5%) and intravenous injection of ketamin (10 ml/h). With the animal in prone position, the bilaterial iliac crest and posterior lumbar region was shaved, aseptically prepared and draped in a sterile fashion. Prophylactic ampicillin and analgesic buprenorphine were given before and immediately after surgery, and twice a day during the following 3 days. The pigs were killed 10 weeks after surgery.
Surgical technique
Under fluoroscopic control, the L4-L5 vertebral level was identified before surgical intervention. The bilateral L4-L5 transverse processes were exposed through posterior midline incision and paraspinal intramuscular approach. After exposure, the posterior cortices of the L4-L5 transverse processes were decorticated using a rongeur until punctuate bleeding was observed, irrespective of the subsequent treatment group. One single-level posterolateral arthrodesis at the L4-L5 transverse level was then performed. For the autograft treatment, 4.5 g of autologous iliac bone was randomly placed to one side of the decorticated arthrodesis site beside the facet joint. For the autograft/ADCON-L treatment, 4.5 g of autologous iliac bone mixed with 3 g ADCON-L gel was implanted to another side of the decorticated arthrodesis site beside the facet joint.
The iliac crest bone graft was harvested from bilateral iliac crest using a sharp curette, and prepared as morselized cancellous chips for re-implantation at the graft site. After the surgical procedure, the fascia of the back and the incision were carefully closed in an interrupted fashion. Blood loss, operative times and intraoperative and perioperative complications were quantified. Observations of ambulatory activities and wound healing were monitored daily, and all animals received analgesics and prophylactic antibiotics for the first 3 days postoperatively. All the pigs were kept in single boxes and killed by intravenous injection of pentobarbital (0.4 mg/kg) under general anesthesia. The spinal column from L1 to L7 with the sacrum was then carefully dissected en bloc, stripped of soft tissue and frozen at −20°C in double-layer plastic bags, pending examination.
Plain X-ray and CT analyses
After the pigs were killed, the status of the posterolateral spinal fusion was evaluated on plain posteroanterior radiographs and computed tomography (CT) images using a modified Lenke et al grading scale [11]. The modified grading scale was:
(A) Solid fusion: clearly solid transverse process fusion with confluent trabeculated bone extending from transverse process to transverse process. (B) Partial union: evidence of bone growth between the transverse processes, but with lucency indicative of nonconfluent trabeculation. (C) Nonunion: no evidence of bone growth between the transverse processes.
The percentage of successful fusions (grade A) or pseudoarthrosis (Grade B or C) was determined on the basis of film and CT images by two independent observers blinded to treatment groups.
Histopathological and histomorphometric evaluation
Upon completion of the X-ray and CT analysis, the posterolateral fusion mass, including the transverse processes, was cut parasagittally beside the facet joint with a water-cooled diamond blade (EXAKT Apparatebau, Nordenstedt, Germany). These posterolateral specimens were fixed for 1 week in 70% ethanol. After fixation, the specimens were dehydrated in 96% ethanol followed by 100% ethanol, and cleared in xylene. The undecalcified specimens were all embedded in methylmethacrylate (MMA) and sectioned sagittally on a Polycut-E sawing microtome (Reichert-Jung, Heidelberg, Germany) to a thickness of 7 µm.
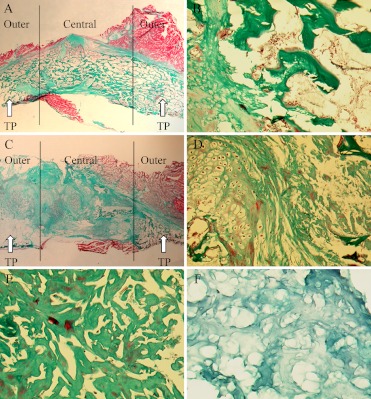
The first section was cut beside the facet joint. The sections were collected at 500 µm-intervals between each section and stained with Goldner Trichrome. To serve as tissue control, fresh ADCON-L gel was embedded and processed for histological analysis similar to the specimens. Histomorphometric measurements were made on a microscope (LEITZ-DIALUX 20, Wetzlar, Germany) using an Olympus grid (100) in the eyepiece of the microscope. Each grid comprised 100 equal points of 10 lines with 10 points on each line in a 10×10 pattern. At low magnification (26.5×), the entire fusion mass was visualized and identified for tissue type. Figure 3B and Fig. 3D depicts bone, cartilage, marrow, fibrous tissues, and residual ADCON-L gel and bone graft.
Fig. 3A–F.
Parasagittal sections through the fusion mass with Goldner Trichrome staining. A Autologous bone graft fusion showing mature cortical rim in some areas and medullary cavity (×2). B Magnified view (×50) of the autologous fusion mass showing mature trabecular bone and marrow elements (right) and endochondral bone formation (left). C The autograft/ADCON-L treatment showing fibrous nonunion (×2). D Magnified view (×50) at the intertransverse fusion mass and the matrix showing a limited osteogenesis and chondrogenesis (left), the residual ADCON-L gel (right) and bone graft (upper right corner). E Magnified view (×100) showing the residual ADCON-L gel in the tissue. F Magnified view (×100) showing the construct of the original ADCON-L gel. It is similar to the construct of the residual ADCON-L gel, but the different colors are due to different pH values in the tissue and ADCON-L gel. TP Transverse process (arrow)
In each specimen, a total of 4 sections with 500 µm-intervals were chosen for bone histomorphometry. Bone volume (BV) was defined as the amount of mineralized bone in percentage of the total volume. A total of 5,000–8,000 points were counted approximately in fields of vision equally divided between the four sections using a point-counting technique. At high magnification (125×), the bone surface (BS) was estimated and expressed in relation to BV as surface density (BS/BV), which is the percentage of bone surface by total bone volume. The parameter BS/BV is an indicator of trabecular thickness, with lower values for thicker trabeculae. Osteoid surfaces (OS) and osteoclast-covered surfaces (Oc.S) were presented as a percentage of BS.
All surface parameters were counted by line-intercept technique at at least a 200–2,000 intersection with the bone surface on the four sections. The fusion mass between the two transverse processes was divided spatially into three zones. The two “outer” zones were adjacent to the upper and lower transverse processes, and the “central” zone was located between them (Fig. 3A and 3C) [2]. One independent observer evaluated all the sections blindly. To evaluate the accuracy of the method, an interobserver error of 4.6% (CV) on volume fractions was calculated after double counting of nine representative sections on two occasions.
Statistics
The chi-square test was used for nonparametric values in radiographs and CT images. Histomorphometric parameters are presented as mean values and standard error of mean (SEM). A paired T-test was done to compare the two treatments; p values less than 0.05 were considered significant.
Results
Surgical procedures
All nine animals survived the surgery and postoperative period without incidence of vascular, neurologic or infectious complications. A mean operative time of 55 min (SD 7 min) and average blood loss of 24 cm3 (SD 5 cm3) were recorded. All animals were ambulatory after 24 h and experienced normal wound healing. The total body weight of the pigs increased from 46.4 kg (SD 3.6 kg) at the beginning of the observation to 57.1 kg (SD 7.0 kg) at the time of being killed.
Plain X-ray and CT analyses
Table 1 shows the grading results of the posterolateral fusion mass on plain X-ray and CT analyses. In radiographic fusion, the autograft alone reached a 77.8% (7/9) fusion in the course of the 10-week time period. The autograft/ADCON-L treatments, in contrast, showed no successful posterolateral fusion (0/9) at the conclusion of the 10-week time period (Fig. 1).
Table 1.
Plain X-ray radiographic and CT analyses
| Grade | Autograft/ADCON-L (n=9) |
Autograft (n=9) |
|---|---|---|
| A | 0% (0/9)* | 77.8% (7/9)* |
| B | 88.9% (8/9) | 22.2% (2/9) |
| C | 11.1% (1/9) | 0% (0/9) |
* Fisher Exact Test, p =0.001
Fig. 1.
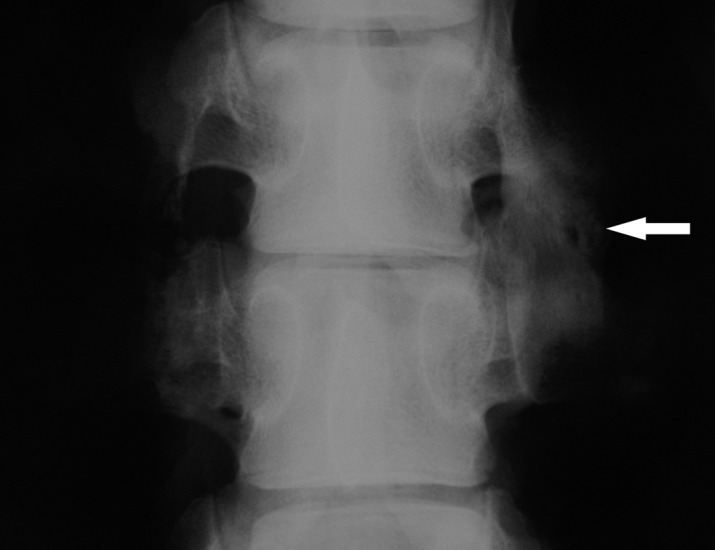
Posteoanterior radiograph of intertransverse process fusion of porcine lumbar spine, 10 weeks after operation. The autograft treatment (L4/5, right side, arrow) with posterolateral fusion mass created by autogenous iliac crest graft; the autograft/ADCON-L mixture (L4/5, left side) with nonunion after iliac crest grafting
Coronal CT images clearly showed the confluent trabeculated bone across the two transverse processes in the autograft treatment (7/9), while the autograft/ADCON-L treatment showed lucency indicative of nonconfluent trabeculation (Fig. 2). The results demonstrated that posterolateral fusion healing was significantly reduced in the presence of ADCON-L gel compared to autograft alone (p<0.001).
Fig. 2.
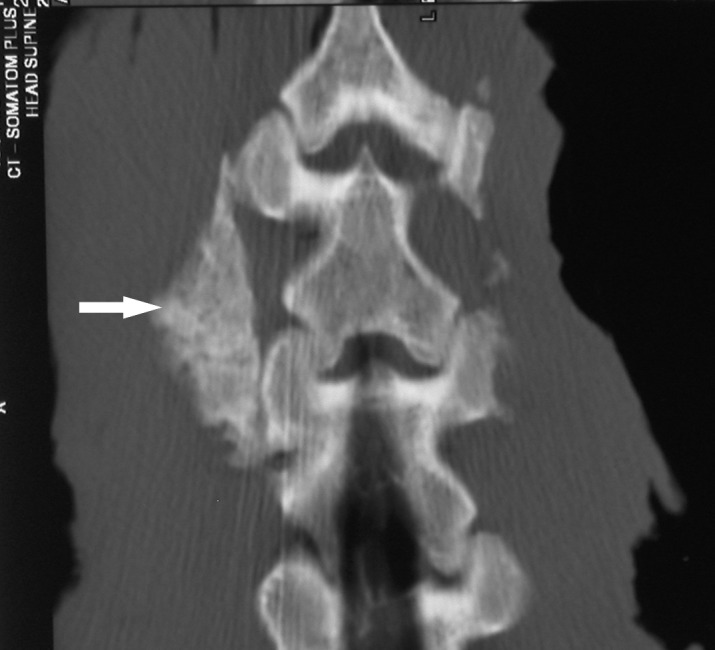
Coronal CT image of intertransverse process fusion of porcine lumbar spine, 10 weeks after operation. The autograft treatment (L4/5, left side, arrow) with a solid fusion mass of intertransverse process; the autograft/ADCON-L mixture (L4/5, right side) with few new bone formation between the transverse processes
Histopathological and histomorphometric evaluation
Undecalcified bone histomorphology
In most of the pure autograft locations, histological characterization of the 10-week iliac crest autograft treatments revealed maturation of intertransverse fusion bridges (Fig. 3A), containing not only lamellar and woven trabeculae, but also presenting dramatic remodeling as evidenced by a peripheral cortical rim around the fusion in some areas. The iliac crest autograft was resorbed completely and replaced by new bone and bone marrow. Moreover, there was still the presence of connective tissues, and minor evidence of an endochondral bone formation (Fig. 3B).
In contrast, the autograft/ADCON-L treatment showed no solid intertransverse fusion bridge (Fig. 3C). In one case, there was no new bone formation. We found that 89% (8/9) of the specimens contained residual ADCON-L gel and 44% (4/9) of the specimens contained residual bone graft (Fig. 3D). The residual ADCON-L gel was partially degradable or non-degradable in comparison to the ex vivo analysis of fresh ADCON-L gel (Fig. 3E).
Bone histomorphometry
Table 2 shows the bone histomorphometric results. Histomorphometric analysis demonstrated that bone and bone marrow in the autograft/ADCON-L treatment were significantly lower than that in the autograft treatment in each zone (p=0.005, p=0.02 in the central zone and p=0.006, p=0.03 in the outer zone, respectively). Fibrous tissue was statistically significantly more abundant in the autograft/ADCON-L treatment when compared to the exclusively autograft treatment in both zones: in the central zone (p=0.001) and in the outer zone (p=0.02). No statistical differences were found in cartilage, bone surface density (BS/BV), osteoid surface (OS/BS) or osteoclast-covered surface (Oc.S/BS) between the two treatments in each zone.
Table 2.
Bone histomorphometric results in both the two treatments ( n =9)
| Zones | BV/TV | CV/TV | MV/TV | FV/TV | BS/BV | OS/BS | Oc.S/BS |
|---|---|---|---|---|---|---|---|
| Central zone | |||||||
| Autograft/ADCON-L | 5.5±1.8 | 3.1±0.3 | 6.6±1.7 | 82.4±3.6 | 2.7±0.2 | 9.2±1.5 | 4.5±0.9 |
| Autograft | 19.9±3.4 | 3.7±0.6 | 26.4±7.1 | 43.4±9.6 | 1.9±0.3 | 12.0±2.9 | 3.6±0.5 |
| p values | p =0.005 | p =0.1 | p =0.02 | p =0.001 | p =0.2 | p =0.5 | p =0.3 |
| Outer zone | |||||||
| Autograft/ADCON-L | 4.9±1.3 | 2.1±0.6 | 9.5±3.5 | 79.9±5.5 | 3.1±0.3 | 8.2±1.2 | 6.8±1.8 |
| Autograft | 17.5±3.9 | 1.5±0.7 | 27.0±7.9 | 50.1±11.5 | 2.0±0.2 | 9.5±1.9 | 3.4±0.5 |
| p values | p =0.006 | p =0.4 | p =0.03 | p =0.02 | p =0.5 | p =0.2 | p =0.1 |
Data are presented as mean ± SEM. BV/TV = Bone volume (%), CV/TV = Cartilage volume (%), MV/TV = Bone marrow volume (%), BS/BV = Bone surface density (%), OS/BS = Osteoid surface (%), Oc.S/BS = Osteoclast covered surface (%).
Discussion
ADCON-L gel is a biocompatible, resorbable antiadhesion barrier specifically designed for use in lumbar spine surgery. It consists of a small carbohydrate combined with other components to form a gel. The gel formulation is designed to act as a physical barrier and to facilitate both anterior and posterior placement around the dura and nerve root [8]. Cell analysis demonstrated that ADCON-L gel blocked the ingrowth of fibroblasts [8]; animal laminectomy, laminotomy, and discectomy models have demonstrated a major decrease in the amount of peridural fibrosis [6, 8, 12, 20].
Clinically, ADCON-L gel has been used extensively to inhibit postoperative peridural scarring and adhesions [3, 4, 16, 17, 21, 22]. The results of using the gel clinically have been positive, however, there is controversy over the patient-oriented endpoints realized. No positive effect of treatment with ADCON-L gel has been found in patients for whom one-level lumbar microdiscectomy was performed in a multicentral, randomized, controlled clinical study [19]. There have not yet been any reports of inhibition of bone formation in that connection.
The present study is the first to document inhibition of bone growth in a spinal fusion model by a tissue ingrowth inhibitor, ADCON-L Anti-Adhesion Barrier Gel. The radiographic and histological findings of the present study suggest that ADCON-L gel can delay or decrease bone formation at the spinal arthrodesis sites, thus influencing iatrogenic spinal fusion or re-growth of the spinal skeleton. The effects of ADCON-L gel might be regarded as that of physically blocking communication between osteoblasts and osteoclasts, resulting in reduced new bone formation and consolidation.
In the current investigation, the difference in radiographs and CT images for establishing successful fusion rates was highlighted due to complete fusion failure in the autograft/ADCON-L treatment compared with the autograft alone within the 10-week time period. An interesting observation in this study is that none of the treatments produced a rate of 100% successful spinal arthrodesis. This is most likely attributable to the short observation time and the biomechanically challenging animal model which did not employ stabilizing spinal instrumentation or spinal brace immobilization. To overcome the problem of the “worst case scenario” in the animal model used in the current study, a study allowing fusion maturation accompanied by interference of ADCON-L treatment under a stabilizing biomechanical environment is needed.
Histological analysis provided the most distinctive findings of this investigation. At the end of the 10-week time period, based on plain and polarized light microscopy, the autograft/ADCON-L-treated fusion mass displayed the remaining presence of ADCON-L gel, bone graft fragments embedded in connective tissue, and a minor cartilage island within the intertransverse space or transverse process areas. In contrast, the autograft treatment showed remodeled bone tissue only at the fusion site. This finding indicates significant inhibition of the osteoinductive properties of the bone graft as well as inhibition of other healing processes. ADCON-L gel prevented the influx of nutrients and osteoprogenitor cells from the decorticated host bone during the healing of an intertransverse process spinal fusion when autogenous bone was mixed with ADCON-L gel. This resulted in fusion failure over a 10-week period.
Previous studies have reported that ADCON-L gel is for the most part absorbed 4 weeks after administration [12, 21]. In our animal study, we found that ADCON-L gel still remained at the conclusion of a 10-week time period. Natural, physiological dissipation or absorption of ADCON-L gel might therefore be prolonged when in contact with bone graft material. This prolonged absorption might account for the strong inhibitory effect on spinal fusion in this study. The persistently present cartilage in the intertransverse space and transverse processes would be transformed to mineralized bone during the absorption of ADCON-L, and spinal fusion would probably follow with delay.
Histomorphometric analysis demonstrated that bone and bone marrow in the autograft/ADCON-L treatment were significantly reduced within the intertransverse space or transverse process areas, when compared to the autograft treatment. Although bone and bone marrow were reduced in the autograft/ADCON-L treatment, compared with the autograft treatment, the endochondral bone formation evident by the end of the 10-week time period in both treatment groups had resulted from an ongoing ossification process. The cartilage tissue observable within the intertransverse space or transverse process areas was not different between the two treatment groups. Bone remodeling activity at the fusion sites was not influenced by ADCON-L gel, as indicated by histomorphometric data showing that the osteoid surfaces (OS/BS), bone surface densities (BS/BV) and osteoclast-covered surfaces (Oc.S/BS) within the intertransverse space and transverse process areas were not different between the two treatment groups.
The use of ADCON-L gel might act as a physical barrier to block the influx of nutrients and osteoprogenitor cells from the decorticated host bone (transverse processes), and so inhibit new bone formation at the intertransverse arthrodesis sites. The consistent presence of cartilage in the intertransverse space and transverse process areas may be explained by lower oxygen saturation. There was no rapid healing in the transverse processes via membranous bone formation in the healing process of a spinal fusion. Thus, ADCON-L gel interrupted the normal biological healing process in the intertransverse arthrodesis. That is, the rapid membranous bone formation response near the transverse processes contrasted with the delayed reparation response seen in the intertransverse space, where there was endochondral bone formation [2].
It is speculated that ADCON-L gel could be used advantageously to prevent spontaneous spinal fusion from occurring after a violation of the periosteum during a surgical intervention of scoliosis during childhood, which would preserve longitudinal spinal growth. Posterior instrumentation of the spine after subperiostal or supraperiostal preparation with and without the use of ADCON-L gel is therefore recommendable for future animal and human studies.
Studies have shown that ADCON-L gel can prevent epidural fibrosis and adhesions by means of blocking the ingrowth of fibroblasts from the surgically detached muscle in several animal models of laminectomy and discectomy [6, 12, 20]. Our results showed that there was more fibrous tissue in the intertransverse space or transverse process areas of the autograft/ADCON-L treatment, when compared to the treatment exclusively using autograft. This inconsistent result indicated that a normal biological healing response between the graft and graft bed was hindered when the autograft was mixed with ADCON-L, which might (1) reduce haematoma formation around the graft with release of cytokines and growth factors; (2) block migration and proliferation of mesenchymal cells; (3) decrease fibrovascular tissue formation in and around the graft. The effect could constitute favorable conditions for fibroblastic differentiation from marrow cells in the autograft and formation of more fibrous tissue at the fusion sites.
In conclusion, this study showed that ADCON-L gel significantly reduced new bone formation and spinal fusion at the spinal arthrodesis sites. ADCON-L gel might act as a physical barrier to block the ingrowth of osteoblasts and inhibit new bone formation at the intertransverse arthrodesis sites. The use of ADCON-L gel does not seem to change bone remodeling during intertransverse fusion. It is speculated that ADCON-L gel could be used advantageously to prevent spontaneous bony fusion from occurring after surgical treatment (growing instrumentation without arthrodesis) in very young scoliosis patients. This finding could also be important for the safety of clinical usage of ADCON-L gel applied locally to peridural areas when posterolateral spinal fusion is necessary.
Acknowledgements
The authors would like to thank Anette Milton and Anette Baatrup for their technical assistance in histological analysis. They would also like to thank the Institute of Experimental Clinical Research at the University of Aarhus and the Aarhus Spine Research Foundation in Denmark for their financial support.
Footnotes
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. This paper was presented at the SSE 2001 annual meeting, Göteberg, Sweden
References
- 1.Blakemore Spine. 2001;26:2044. doi: 10.1097/00007632-200109150-00021. [DOI] [PubMed] [Google Scholar]
- 2.Boden Spine. 1995;20:2626. doi: 10.1097/00007632-199512150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Brotchi J, Pirotte B, De Witte O, Levivier M (1999) Prevention of epidural fibrosis in a prospective series of 100 primary lumbo-sacral discectomy patients: follow-up and assessment at re-operation. Neurol Res 21 [Suppl 1]:S47–50 [DOI] [PubMed]
- 4.DeAm J Orthop 1998271119506196 [Google Scholar]
- 5.Dubousset J Pediatr Orthop. 1989;9:541. doi: 10.1097/01241398-198909010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Einhaus Spine. 1997;22:1440. doi: 10.1097/00007632-199707010-00003. [DOI] [PubMed] [Google Scholar]
- 7.Fisk J Pediatr Orthop. 1995;15:182. [PubMed] [Google Scholar]
- 8.Frederickson RC (1996) ADCON-L: a review of its development, mechanism of action, and preclinical data. Eur Spine J 5 [Suppl 1]:S7–9 [DOI] [PubMed]
- 9.Geisler FH (1999) Prevention of peridural fibrosis: current methodologies. Neurol Res 21 [Suppl 1]:S9–22 [DOI] [PubMed]
- 10.Harrington J Bone Joint Surg Am. 1962;44:591. [PubMed] [Google Scholar]
- 11.Lenke J Spinal Disord. 1992;5:433. doi: 10.1097/00002517-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Lo H, Frederickson RC (1999) Use of ADCON in neurosurgery: preclinical review. Neurol Res 21 [Suppl 1]:S27–32 [DOI] [PubMed]
- 13.LuqueClin Orthop 19821632027067255 [Google Scholar]
- 14.MoeClin Orthop 1984185356705397 [Google Scholar]
- 15.Morin C (1991) Pediatric Cotrel-Dubousset instrumentation system. In: Bridwell KH, Dewald RL et al, The textbook of spinal deformity, Lippincott, Philadelphia, pp 212–217
- 16.Petrie JL, Ross JS (1996) Use of ADCON-L to inhibit postoperative peridural fibrosis and related symptoms following lumbar disc surgery: a preliminary report. Eur Spine J 5 [Suppl 1]:S10–17 [DOI] [PubMed]
- 17.Porchet F, Lombardi D, de Preux J, Pople IK (1999) Inhibition of epidural fibrosis with ADCON-L: effect on clinical outcome one year following re-operation for recurrent lumbar radiculopathy. Neurol Res 21 [Suppl 1]:S51–60 [DOI] [PubMed]
- 18.Pratt Spine. 1999;24:1538. doi: 10.1097/00007632-199908010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Richter HP, Kast E, Tomczak R, Besenfelder W, Gaus W (2001) Results of applying ADCON-L gel after lumbar discectomy: the German ADCON-L study. J Neurosurg 95 [Suppl 2]:179–89 [DOI] [PubMed]
- 20.Robertson JT, Maier K, Anderson RW, Mule JL, Palatinsky EA (1999) Prevention of epidural fibrosis with ADCON-L in presence of a durotomy during lumbar disc surgery: experiences with a pre-clinical model. Neurol Res 21 [Suppl 1]:S61–66 [DOI] [PubMed]
- 21.Robertson JT, Petrie JL, Frederickson RC, de Tribolet N, Hardy R (1996) ADCON-L symposium. Round table discussion. Eur Spine J 5 [Suppl 1]:S26–28 [DOI] [PubMed]
- 22.Schwicker D (1996) Cost effectiveness of lumbar disc surgery and of a preventive treatment for peridural fibrosis. Eur Spine J 5 [Suppl 1]:S21–25 [DOI] [PubMed]
- 23.Takaso J Orthop Sci. 1998;3:336. doi: 10.1007/s007760050062. [DOI] [PubMed] [Google Scholar]