Abstract
The recommended surgical options for postoperative wound infections after instrumented spine surgery include a wide debridement and irrigation with antibiotics. In most cases, implant removal is not recommended for a solid fusion. However, there are few reports on the treatment choices for persistent postoperative wound infections following a posterior lumbar interbody fusion (PLIF) using cages. This paper reviewed ten patients referred to our department, who underwent revision surgery for a postoperative, deep infection after a PLIF with cages. The surgery included an anterior radical debridement and interbody fusion with removal of all implants. The clinical and laboratory results, including a bacteriologic study for the causative organism and the radiological changes, were analyzed. All patients complained of persistent severe back pain after the primary surgery. MRSA was the main organism found in these patients (five cases). Complete bony fusion was obtained in nine patients (90%). In one patient, back pain and radiating pain prevented him from returning to his original work. Despite the anterior interbody fusion with an autogenous iliac bone graft, all cases had a complete collapse of the intervertebral disc space, without a dislodgement or collapse of the graft bone. The mean loss of the height and lordosis in the involved segment was 12.7 mm (range 4–46 mm) and 5.6° (range 0–15°), respectively. Anterior radical debridement with the removal of all implants would be an effective way to manage patients with postoperative spondylitis after a PLIF using cages.
Keywords: Spondylitis, Posterior lumbar interbody fusion, Cage
Introduction
Posterior lumbar interbody fusion (PLIF) using cages is a technically demanding procedure for arthrodesis in the treatment of unstable lumbar segments. Numerous techniques have been developed, including the use of autologous posterior iliac crest bone, allografts and xenografts. Recently various cages have been developed to prevent a collapse of the graft bones and increase the fusion rate [8, 11]. The combination of a rigid cage filled with autologous cancellous bone is an attractive concept for preventing a collapse of the intervertebral disc space. This cage has many advantages in improving the sagittal curvature of the lumbar curve and widening of the intervertebral foramen [8, 11]. However, it requires more operative time than a conventional posterolateral fusion if used with pedicular instrumentation. Therefore, the concurrent use of implants might incur additional risk of wound infection.
The increased infection rates are likely to be the result of procedure-related increases in operative time, blood loss, and tissue damage [1, 2, 6, 9, 17]. In order to manage a postoperative infection of an instrumented spine, most surgeons agree that removal of implants might be unnecessary, particularly in the early stages of a postoperative infection. A wide debridement and irrigation-suction system should first be attempted to eradicate the infection [1, 7, 9, 10, 13, 17, 20]. However, in the case of an uncontrolled, persistent postoperative infection, especially one associated with a PLIF using cages, a different approach is needed.
At present, there are few reports regarding treatment for a deep infection after the use of cages for a lumbar fusion. Therefore, the aim of this study is to analyze the surgical results of a postoperative spondylitis after a PLIF using cages.
Materials and methods
The series included ten patients referred to our department, from October 1998 to November 1999, for a postoperative, uncontrolled wound infection. All patients had undergone a PLIF with cages (at other institutes) to treat various back problems, including spinal stenosis and spondylolisthesis. Prior to referral, several debridements and irrigations of the infection, with administration of sensitive antibiotics, had been done. Three patients were referred after removal of the pedicular implants. We removed the cages from all ten patients. We first attempted a posterior approach. However, epidural scarring and a hindrance to a firm grip on instruments made this difficult. Consequently, the anterior retroperitoneal approach was used for cage removal and anterior debridement of infected tissue, simultaneously. However, care should be taken because the cages are very mobile and difficult to grip.
Preservation of the end plates of the infected vertebrae was barely possible. Destruction of the infected vertebral body was inevitable. The large anterior iliac strut bone graft was positioned into the intervertebral disc space for stability and bony union. Postoperatively, ambulation was permitted with a custom-made brace for 3 months. In most patients, parenteral antibiotics were administrated for 6 weeks and oral antibiotics were given for a minimum of 8 weeks. Radiologically, bony fusion was considered to be present if there was no radiolucency around the graft and vertebral body and no motion of more than 3° on the dynamic radiographs [8].
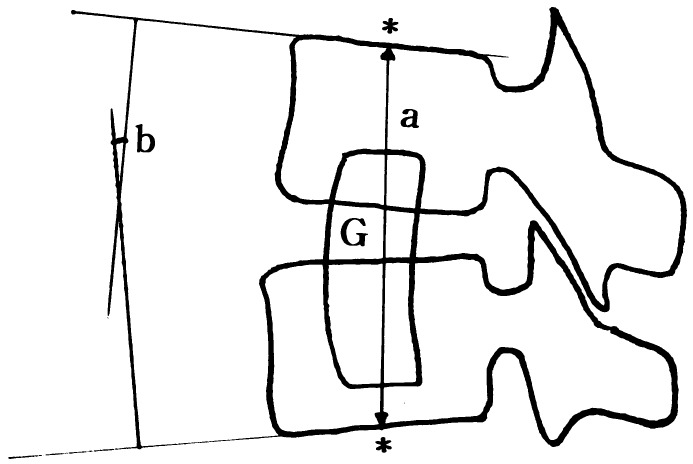
Laboratory results, including the erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), were used to modulate the period of antibiotic use. In order to evaluate the fate and result of the grafted bone, serial roentgenograms were examined and the involved segment lordotic angle and height were measured (Fig. 1). A culture study of the causative organisms and serial laboratory tests for ESR and CRP were reviewed.
Fig. 1.
Measurement of height and local lordosis at the involved segment (a height of involved segment (mm), b lordosis of involved segment, G graft bone
Results
Patient demographics
Ten patients (six men and four women) were included in this study. The mean age was 59.2 years (range 38–74 years). The initial diagnoses for a spine fusion were seven cases of isthmic spondylolisthesis and three cases of spinal stenosis with segmental instability. In nine cases, the posterior pedicular screw fixation was done with a PLIF using cages. An additional posterolateral autogenous bone graft was done in one patient. The fusion levels involved were: L4–5 for five patients; L3–4 for two patients; L5–S1 for one patient; and for two patients two levels were involved, L3–L4, and L4–5. At referral, all patients complained of unmanageable back pain and, in three cases, pain radiating to the thigh and buttocks. Two patients had fevers before the infection was discovered. Wound discharge and erythema were noted in three patients. Patients had undergone surgery for the deep wound infection an average of 2.4 times (range 1–5 times) prior to presentation. The mean interval for the detection of the first wound infection was 38.1 days (range 4–150 days). In one patient, the infection was found 5 months after the initial operation, and Aspergillus fumigatus was isolated (Table 1).
Table 1.
Summary of patient data (SS spinal stenosis, SPL spondylolisthesis; PS pedicular screw, PLF posterolateral fusion; SA Staphylococcus aureus, MRSA methicillin-resistant Staphylococcus aureus, MRCNS methicillin-resistant coagulase-negative staphylococcus, AF Aspergillus fumigatus)
| Case no. | Age(years)/sex | Diagnosis for surgery | Fusion level (cage) | Instrumentation | Days after surgery before diagnosis | No. of revisions | Organ-ism | Total lympho-cyte count | Serum albumin (Nl: 3.8–5.3 g/dl) | Additional risks |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67/F | SS | L3, 4, 5 | PS+PLF+cage | 60 | 2 | MRSA | 2,200 | 3.8 | Hepatitis |
| 2 | 38/M | SS | L4, 5 | PS+PLF+cage | 150 | 5 | AF | 2,300 | 3.5 | None |
| 3 | 62/M | SPL | L4, 5 | PS+PLF+cage | 30 | 3 | MRCNS | 3,150 | 4.2 | None |
| 4 | 56/F | SPL | L3, 4 | PS+cage | 30 | 3 | SA | 1,287 | 3.6 | None |
| 5 | 61/M | SPL | L4, 5 | PS+PLF+cage | 35 | 1 | SA | 1,510 | 4.2 | High blood pressure |
| 6 | 60/M | SPL | L5, S1 | Cage | 4 | 2 | MRSA | 2,749 | 4.1 | None |
| 7 | 66/F | SPL | L3, 4, 5 | PS+PLF+cage | 30 | 1 | SA | 1,062 | 2.7 | None |
| 8 | 53/M | SS | L4, 5 | PS+PLF+cage | 14 | 3 | MRSA | 1,596 | 3.1 | None |
| 9 | 52/M | SPL | L3, 4 | PS+PLF+cage | 7 | 2 | MRSA | 1,625 | 4.3 | None |
| 10 | 65/F | SPL | L4, 5 | PS+cage | 21 | 2 | MRSA | 2,540 | 3.8 | None |
Laboratory results
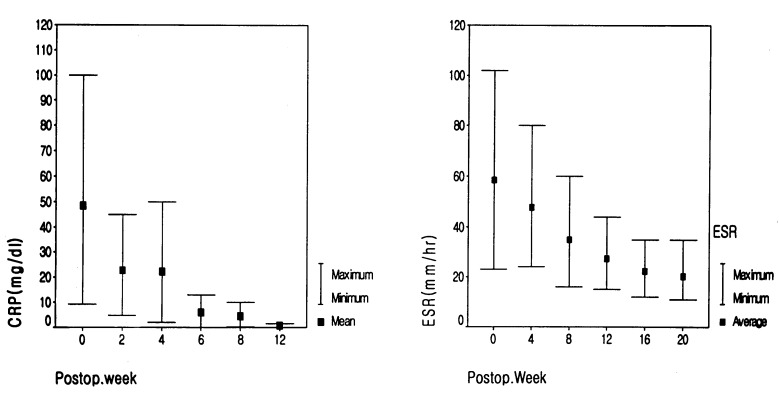
Although the initial preoperative laboratory results checked at other institutes could not be obtained, our results showed a low total serum albumin in three patients and a low total lymphocyte count in five patients (Table 1). Both ESR and CRP were serially checked in all cases. The ESR was elevated over a relatively long period, even 12 months later. However, the CRP was mostly normalized at 8 weeks after the last surgery (Fig. 2). Methicillin-resistant Staphylococcus aureus (MRSA) was isolated in five cases. Staphylococcus aureus was isolated in three patients and methicillin-resistant coagulase-negative Staphylococcus (MRCNS) was detected in one patient.
Fig. 2.
Serial average results of ESR and CRP. ESR is elevated over a long period, unlike CRP. Although patient has solid fusion and is symptom-free, ESR is elevated for a postoperative 12 months
Clinical and radiological results
The mean follow-up period was 28.1 months (range 24–38 months) after anterior removal of the cages. At the last follow-up, radiological and laboratory results were used to determine if the infection was eradicated. A solid bony fusion and complete eradication of the infection were achieved in nine patients. Clinically, three patients complained of residual back pain, but only two could tolerate active daily life. In one patient, persistent back pain and radiating pain to both buttocks forced him to limit his active daily life and original work. However, in this patient, although a radiolucency around the grafted bone remained at the last follow-up, motion at the fused segment could not be observed (Fig. 3). Although bony union and eradication of the infection were achieved in most cases, the normal lumbar lordosis could not be reconstructed. Comparing the immediate postoperative films of the revision and the last follow-up films, the mean height loss of the involved segment was 12.7 mm (range 4–46 mm) and the mean lordotic angle loss was 5.6° (range 0–15°) (Table 2). The loss of height was mainly attributed not to a collapse of the graft bone but to the collapse of the intervertebral disc space. Instead of a dislodgment of the graft bone, an impaction of the graft into the vertebral bodies was noted (Fig. 4).
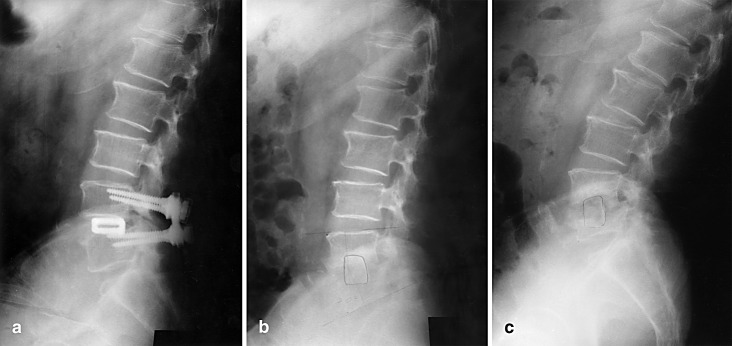
Fig. 3a–c.
Case 3. This 74-year-old man presented persistent low back pain and intermittent low-grade fever. a Wide decompression and pedicular screw and PLIF with cages were performed. b After trying unsuccessfully to remove the cage posteriorly, we did anterior cage removal and anterior autogenous iliac bone graft. c At last follow-up, the patient complained of persistent back and buttock pain. Radiographs showed radiolucency around the graft bone
Table 2.
Follow-ups on height and lordosis of involved segment
| Height of involved segments (mm)a | Lordosis of involved segments (°)b | |
|---|---|---|
| Immediate postop. | 96.4±34.5 | 0.9±19.6 |
| Last follow-up | 83.7±29.1 | −4.7±15.1 |
aMean height loss of involved segment: 12.7 mm (range 4–46 mm)
bMean loss of lordosis: 5.6° (range 0–15°); − means kyphosis
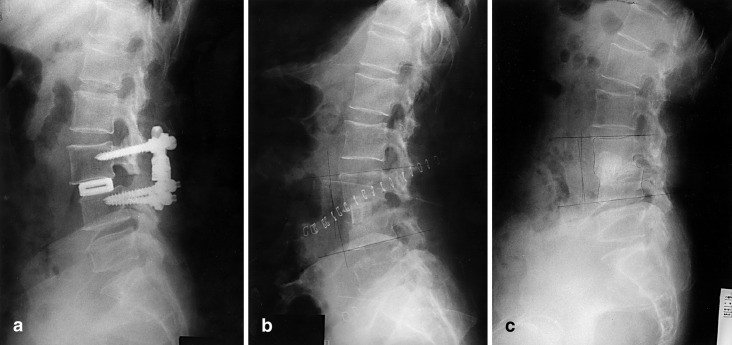
Fig. 4a–c.
. Case 4. a This 56-year-old woman presented persistent deep wound infection detected 12 days postoperatively. Initially, irrigation and drainage were done several times. b Anterior removal of cages and autogenous iliac bone graft, performed separately with posterior removal of instrument. c At last follow-up, complete bony union and collapse of disc space was found
Discussion
In order to increase fusion rate, correct deformity and regain normal lordosis, PLIF with cages has become popular [6, 8, 11]. Perioperative or postoperative complications have been reported for a spinal fusion with transpedicular screws and cages [6, 11]. Of the many possible complications, a postoperative infection is potentially devastating. The reported rates of a postoperative wound infection for a spinal fusion with instrumentation range from 2.6% to 10% [1, 3, 9, 13, 15, 17]. The increased infection rates are likely to be due to procedures that increase surgical time, blood loss, and tissue damage [2, 10]. There are few reports on the incidence of infections following a PLIF. Hee et al [5] reported five cases (5%) of infection in 111 transforaminal interbody fusions, suggesting that adjunctive treatment such as an internal bone stimulator or a demineralized bone matrix was highly associated with the occurrence of infection (four of five cases). Many risk factors, including the underlying disease, drug or alcohol abuse, malnutrition and smoking, have been demonstrated to be related to the incidence of postoperative wound infection after spinal surgery [2, 7, 9, 13]. This study analyzed the risk factors for malnutrition and the underlying disease (Table 1).
Staphylococcus aureus is known to be the most common organism in a postoperative spine infection [17, 20]. However, because the use of prophylactic antibiotics for spine surgery is common, the number of infections by MRSA and other gram-negative bacilli are increasing [7, 13]. Postoperative spine-wound infections can be classified according to when they appear: early (earlier than 20 weeks postoperatively) or late (later than 20 weeks) [17]. Uncommon, low-virulence organisms such as Propiniobacterium acnes or Staphylococcus epidermidis have been reported in late postoperative infections [14,20]. In this study, one patient with an Aspergillus infection was recognized as a postoperative infection 5 months later.
In the management of a postoperative deep wound infection after instrumentation, implant removal is not recommended, particularly in early active infection [1, 9, 10, 15, 17]. The first choice to eradicate the infection would be a debridement and irrigation only, while maintaining implants and using antibiotics. The premature removal of the implants would result in spinal instability and pseudarthrosis, compounding the deep wound infection. Implants can be removed in patients with a late presentation, who had a solid fusion. Therefore, the implants may be safely left in situ to provide stability for the fusion, until the solid fusion is evident on follow-up. However, in the current cases, several wound debridement and irrigation steps were mostly unsuccessful. Therefore, these infections were treated more aggressively with a wide anterior debridement, implant removal, and an anterior interbody fusion with an autogenous graft bone. The posterior removal of the cages was a highly complicated procedure, due to postoperative and post-infection epidural fibrosis. Although the successful removal of a cage with a special device was described [11], the anterior approach was chosen to remove the cages and position the strut iliac bone graft.
After removing cages, a bony fusion was attempted by a primary autogenous iliac strut bone graft. Fang et al. [14] reported that bony fusion occurred in 93% of pyogenic spondylitis cases (average time to fusion, 6.8 months), and 90% of patients were able to return to their original work 4 to 20 months after surgery. They concluded that a primary bone graft could be successful, despite the presence of infection. Graziano and Sidhu [5] reported that a reconstruction of the spine with an anterior autogenous fibula graft after a debridement is an effective procedure for pyogenic spondylitis. In our cases, a solid bony union was obtained in nine cases (90%), but some loss of intervertebral height and lordosis was noticed, despite sufficient immobilization. This phenomenon resulted from a sunken graft into the vertebral bodies rather than absorption or dislodgement of the graft. The end-plate is a very important structure to prevent graft collapse into the vertebral body [19]. However, destruction of the end-plate was inevitable due to the infection and the removal of the cages. Therefore, stability was not obtained with the end-plate used for anterior graft bone. After revision surgery, nine patients were satisfied and returned to their normal daily activity and original employment. One patient complained of radiating pain to lower extremities. However, his symptoms were tolerable after administering nonsteroidal anti-inflammatory drugs. A repeat decompressive laminectomy was not recommended.
Therefore, in postoperative uncontrolled deep infections following a PLIF with cages, solid fusion and eradication of the infection could not be expected without removal of the instrumentation. In this situation, early removal of cages might be the treatment of choice.
Several investigators have demonstrated a concurrent elevation in CRP in postoperative spinal infections [12, 16]. After successful treatment of the infection, the normalization time for CRP is much shorter than that for ESR in cases of septic arthritis [12]. Thelander and Larson [18] demonstrated that CRP peak values occur within 2–3 days postoperatively, and normalization occurs in all spinal procedures within 2 weeks. In our cases, all patients had markedly higher ESR (up to 100 mm) and CRP values. Even if back pain was resolved after the revision procedure, ESR was not easily normalized. Therefore, it was very difficult to determine when the antibiotic should be stopped. One patient showed elevated ESR until 12 months after the operation. However, CRP was mostly normalized within the postoperative 8 weeks. Therefore, CRP levels may be more useful than ESR in the early detection of a postoperative infection.
Conclusion
In compound cases of postoperative infection after PLIF using cages, anterior debridement, cage removal and autogenous strut iliac bone graft are one treatment option. However, the collapse of the disc space and loss of normal lordosis are inevitable complications.
References
- 1.Abbey J Spinal Disord. 1995;7:278. doi: 10.1097/00002517-199508040-00003. [DOI] [PubMed] [Google Scholar]
- 2.Capen Clin Orthop. 1996;27:83. [Google Scholar]
- 3.Davne Spine. 1992;17:184. [Google Scholar]
- 4.Fang J Spinal Disord. 1994;7:173. [PubMed] [Google Scholar]
- 5.Graziano J Spinal Disord. 1993;6:199. doi: 10.1097/00002517-199306030-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hee J Spinal Disord. 2001;14:533. doi: 10.1097/00002517-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Klekamp J Spinal Disord. 1999;12:187. [PubMed] [Google Scholar]
- 8.Kuslich Spine. 2000;25:2656. doi: 10.1097/00007632-200010150-00018. [DOI] [PubMed] [Google Scholar]
- 9.Levi J Neurosurg. 1997;86:975. doi: 10.3171/jns.1997.86.6.0975. [DOI] [PubMed] [Google Scholar]
- 10.Lonstein Clin Orthop. 1973;96:222. [PubMed] [Google Scholar]
- 11.McAfee Spine. 1999;24:2147. doi: 10.1097/00007632-199910150-00015. [DOI] [PubMed] [Google Scholar]
- 12.Peltola J Pediatr Orthop. 1984;4:179. [Google Scholar]
- 13.Rechtine J Orthop Trauma. 2001;15:566. doi: 10.1097/00005131-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Richards J Bone Joint Surg Am. 1995;4:524. doi: 10.2106/00004623-199504000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Schneider Acta Neurochir. 1995;136:16. doi: 10.1007/BF01411430. [DOI] [PubMed] [Google Scholar]
- 16.Schulitz Spine. 1994;19:1172. doi: 10.1097/00007632-199405001-00016. [DOI] [PubMed] [Google Scholar]
- 17.Stambough J Spinal Disord. 1992;5:277. doi: 10.1097/00002517-199209000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Thelander Spine. 1992;17:400. doi: 10.1097/00007632-199204000-00004. [DOI] [PubMed] [Google Scholar]
- 19.William KD (1998) Arthrodesis of spine. In: Canale ST (ed) Campbell’s operative orthopaedics, 9th edn. Mosby, Philadelphia, pp 2791–2810
- 20.Wimmer J Spinal Disord. 1996;9:505. [PubMed] [Google Scholar]