Abstract
Treatment of etiolated Vicia sativa seedlings by the plant hormone methyl jasmonate (MetJA) led to an increase of cytochrome P450 content. Seedlings that were treated for 48 h in a 1 mm solution of MetJA stimulated ω-hydroxylation of 12:0 (lauric acid) 14-fold compared with the control (153 versus 11 pmol min−1 mg−1 protein, respectively). Induction was dose dependent. The increase of activity (2.7-fold) was already detectable after 3 h of treatment. Activity increased as a function of time and reached a steady level after 24 h. Northern-blot analysis revealed that the transcripts coding for CYP94A1, a fatty acid ω-hydroxylase, had already accumulated after 1 h of exposure to MetJA and was maximal between 3 and 6 h. Under the same conditions, a study of the enzymatic hydrolysis of 9,10-epoxystearic acid showed that both microsomal and soluble epoxide hydrolase activities were not affected by MetJA treatment.
Hydroxylases that belong to the CYP4 (Cyt P450) family and are capable of hydroxylating the terminal methyl of fatty acids (ω position) have been extensively studied in mammals (Simpson, 1997). A remarkable property is their inducibility by compounds that are known to stimulate peroxisomal proliferation (Simpson, 1997). More than 2 decades have passed since the first fatty acid ω-hydroxylation in a plant was described (Soliday and Kolattukudy, 1977). Previous investigations from our laboratory have extensively characterized P450-dependent ω-hydroxylases oxidizing C10 to C18 fatty acids in pea and Vicia sativa (Benveniste et al., 1982; Salaün et al., 1986; Pinot et al., 1992, 1993). In V. sativa microsomes, oleic acid is subjected to a cascade of reactions that involves at least three distinct enzymes: a peroxygenase, an epoxide hydrolase, and a Cyt P450-dependent ω-hydroxylase (Pinot et al., 1992, 1997). The latter enzymatic system, inducible by the peroxisome proliferator clofibrate, is able to ω-hydroxylate oleic acid and its oxygenated derivatives, 9,10-epoxystearate and 9,10-dihydroxystearate. The interplay of the three enzymes accounts for the formation of the major C18 cutin monomers (Kolattukudy, 1980). Cutin is a component of the cuticle that protects plants against different stresses (i.e. pathogens, chemicals, and drought). It consists of a biopolymer in which monomers are cross-linked via ester bonds between carboxyl and ω-hydroxyl groups. Thus, enzymes capable of ω-hydroxylating fatty acids have a key role in cutin synthesis: by introducing the terminal hydroxyl function, they allow the elongation reaction of the biopolymer to occur. In addition to being a constituent of the cuticle, ω-hydroxy fatty acids may be involved in plant defense in another way, because it has been shown that they act as endogenous signal molecules for the induction of resistance in pathogen-challenged plants (Schweizer et al., 1996a, 1996b).
Inhibition studies performed in our laboratory suggested the presence of at least two enzymes capable of fatty acid ω-hydroxylation in V. sativa microsomes (Pinot et al., 1993). This was confirmed by the recent cloning of distinct ω-hydroxylases from V. sativa (Tijet et al., 1998; R. Le Bouquin and I. Benveniste, unpublished data). One of these enzymes, CYP94A1, when expressed in yeast (Tijet et al., 1998), catalyzed the ω-hydroxylation of 18:1, 18:2, and 18:3 (oleic, linoleic, and linolenic acids, respectively) and of the model substrate 12:0 (lauric acid).
Jasmonates, which derive from 18:3, are important regulatory molecules in plant defense (for review, see Sembner and Parthier 1993; Creelman and Mullet, 1997; Mueller, 1997). The proteinase inhibitor PI-2 was the first well-characterized defense-related protein induced by jasmonate (Farmer and Ryan, 1990). Gundlach et al. (1992) studied the induction of Phe ammonia-lyase, the first enzyme of the phenylpropanoid pathway, which leads to components of the cell wall and to phytoalexins. Chalcone synthase, which produces precursors of flavonoids, and Pro-rich proteins, which participate in cell wall strengthening, are also induced by MetJA (Creelman et al., 1992). In barley jasmonates stimulate the accumulation of JIP5 (jasmonate-inducible proteins), which have antifungal activity (Andresen et al., 1992) and JIP60, a ribosome-inactivating protein (Chaudhry et al., 1994). Lipoxygenases take part in the oxylipin pathway, the source of volatile aldehydes, alcohol, and jasmonates that participate in plant defense (Avdiushko et al., 1995). Different studies have demonstrated the induction of lipoxygenases by jasmonates (Avdiushko et al., 1995; Heitz et al., 1997).
We have studied the effect of MetJA on Cyt P450 content and on ω-hydroxylation of the model substrate 12:0 in microsomes of V. sativa seedlings. We investigated the effect of dose and time of treatment. The expression of CYP94A1 was studied by northern-blot analysis. We also investigated the effect of MetJA treatment on soluble and microsomal epoxide hydrolase activities. The possible involvement of CYP94A1 in plant defense is discussed.
MATERIALS AND METHODS
Chemicals
[1-14C]12:0 (45Ci/mol) was purchased from DuPont-New England Nuclear. Racemic [1-14C]9,10-epoxystearic acid was purchased from CEA (Gif-sur-Yvette, France). TLC plates (Silica Gel G60 F254; 0.25 mm) were purchased from Merck (Darmstadt, Germany). MetJA was from Aldrich-Sigma.
Plant Material
Vicia sativa seedlings were germinated at 26°C on wet paper with an illumination cycle that consisted of 16 h of light and 8 h of dark (3200 lux). Seedlings were then transferred to distilled water or solutions containing different concentrations of MetJA for various periods (concentrations and periods will be specified for each experiment). To measure the effect of MetJA on Cyt P450 content, seedlings were germinated and induced in water or MetJA in the dark.
Preparation of Plant Subcellular Fractions
For each sample 30 g of seedlings was harvested and homogenized with an Ultra-Turrax (15,000 rpm, twice for 30 s; Janke and Kunkel, Staufen i. Br., Germany) in a final volume of 100 mL of 100 mm sodium-phosphate buffer (pH 7.4) containing 250 mm Suc, 40 mm sodium ascorbate, 10 mm β-mercaptoethanol, and 1 mm PMSF. The homogenate was filtered through 50-μm nylon filtration cloth and centrifuged for 20 min at 10,000g. The resulting supernatant was centrifuged for 1 h at 100,000g. The soluble fraction was divided into aliquots and stored at −30°C. To eliminate contamination from the soluble fraction, the microsomal pellet was homogenized with a potter in 100 mm pyrophosphate buffer (pH 7.5) containing 10 mm β-mercaptoethanol. After a second centrifugation at 100,000g, the microsomal pellet was resuspended in 7 mL of 100 mm sodium-phosphate buffer (pH 7.4), 30% (v/v) glycerol, and 1.5 mm β-mercaptoethanol, divided into aliquots, and stored at −30°C. Protein concentrations of the microsomal and soluble fractions were estimated with a microassay from Bio-Rad using BSA as a standard. Cyt P450 content was measured according to the method of Omura and Sato (1964).
Enzyme Activities
12:0 ω-Hydroxylase activity was determined by following the rate of hydroxylated product formation. The standard assay (0.2 mL) contained 20 mm sodium-phosphate buffer (pH 7.4), microsomal proteins (approximately 100 μg), 1 mm NADPH, plus a regenerating system (consisting of final concentration of 6.7 mm Glc-6-P and 0.4 unit of Glc-6-P dehydrogenase), and radiolabeled substrate (100 μm). The reaction was initiated by the addition of the NADPH and was stopped after 15 min at 27°C by the addition of 0.1 mL of acetonitrile (0.2% acetic acid). The reaction products were resolved by TLC as described below. Epoxide hydrolase activities were measured using 9,10-epoxystearic acid, as described previously (Pinot et al., 1997). The standard assay contained approximately 300 or 40 μg of protein from the microsomal or the soluble fraction, respectively, in a final volume of 0.2 mL of 20 mm sodium-phosphate buffer (pH 7.4). The reaction was initiated by the addition of 2 μL of a 10 mm epoxystearic acid solution in ethanol using a Hamilton repeating syringe (Reno, NV) and stopped by the addition of 0.1 mL of acetonitrile (0.2% acetic acid) after 15 min at 27°C. The residual substrate and hydrolysis product were directly spotted on TLC plates (see below). All assays were shown to be linear for both protein content and time.
Chromatographic Methods
Incubation media were directly spotted on TLC plates, which were developed with a mixture of diethyl ether:light petroleum (boiling point, 40°C–60°C):formic acid (50:50:1, v/v). The plates were scanned with a thin-layer scanner (LB 2723, Berthold Analytical [EG&G Wallac, Gaithersburg, MD]). The area corresponding to the metabolites was scraped into counting vials.
Northern-Blot Analysis
Total RNAs were isolated from 15 g of seedlings using the procedure of detergent and phenol-chloroform extraction. For northern-blot analysis, total RNAs (30 μg/lane) were denaturated, subjected to electrophoresis on a 1.2% agarose gel containing formaldehyde, and transferred onto a membrane (Hybond N+, Amersham). The blot was hybridized with 32P-labeled cDNA corresponding to the coding region of CYP94A1 at 65°C for 16 h in 5× SSC. After hybridization, the blot was washed twice with 2× SSC, 0.1% SDS at room temperature for 15 min, and twice with 0.2× SSC, 0.1% SDS at 55°C for 30 min. An 18S ribosomal DNA from radish was used as an internal control. Densitometric quantification of mRNA was performed from scanned autoradiography (Arcus II scanner, Agfa Division, Bayer, Ridgefield Park, NJ) using the NIH-Image program, version 1.59 (National Institutes of Health, Bethesda, MD). The spot-intensity measurement of mRNA was adjusted as a function of the intensity from the internal control (18S ribosomal DNA from radish).
RESULTS
Effect of MetJA on Cyt P450 Content
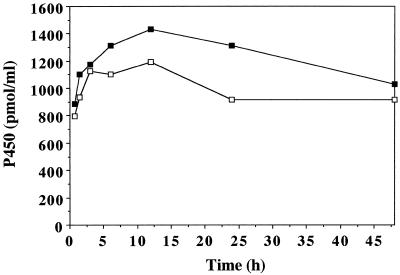
To investigate the effect of MetJA on Cyt P450 content, 5-d-old V. sativa seedlings were induced in water or in water containing 1 mm MetJA. To avoid synthesis of chlorophyll, which interferes with spectrophotometric measurement of Cyt P450 content, germination and induction were performed in obscurity. The results are presented in Figure 1. Cyt P450 content increased during the first 12 h of induction in microsomes of both control and MetJA-treated seedlings. The level of Cyt P450 was consistently higher in microsomes of treated plants. It is possible that we underestimated the level of jasmonate-induced P450 proteins; indeed, gene regulation sometimes requires illumination.
Figure 1.
Cyt P450 content in microsomes of V. sativa seedlings. Five-day-old etiolated seedlings were induced in the dark for different periods in water (□) or in water containing 1 mm MetJA (▪).
Induction of 12:0 ω-Hydroxylation
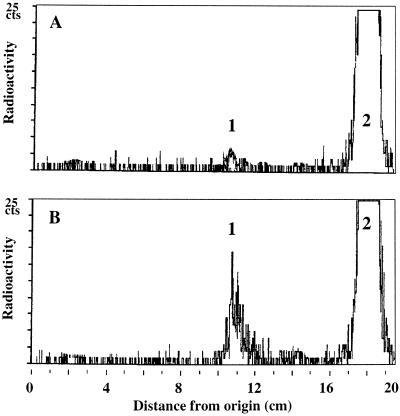
We used the model substrate 12:0 to compare the hydroxylation activity in microsomal fractions from control and treated seedlings. The thin-layer radiochromatograms presented in Figure 2 show that in both cases only the ω-hydroxy-C12:0 was produced. Furthermore, induction of seedlings for 48 h in a 1 mm solution of MetJA enhanced ω-hydroxylation of 12:0 14-fold compared with the control (153 versus 11 pmol min−1 mg−1 protein, respectively).
Figure 2.
Radiochromatographic resolution of 12:0 metabolites generated by microsomal incubations from V. sativa seedlings. 12:0 (100 μm) was incubated with the microsomal fraction from 5-d-old seedlings induced for 48 h in water (A) or in water containing 1 mm MetJA (B). Radiolabeled compounds were resolved into hydroxy-12:0 (peak 1) and 12:0 (peak 2).
Time Course and Dose Effects
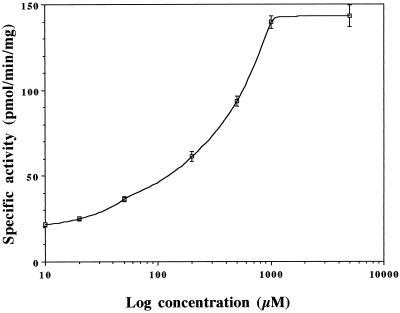
We measured ω-hydroxylation of 12:0 in microsomes of seedlings treated with different concentrations of MetJA. The results are presented in Figure 3. Even at the lowest concentration tested (10 μm) the activity was stimulated by 25% compared with the control. A dose-dependent effect was observed up to 1 mm MetJA. A direct representation shows that this response is linear. The limit of dose dependency could be attributable to aqueous solubility. It could also be attributable to a toxic effect of MetJA when administered at 5 mm. When treated at this dose, the seedlings looked unhealthy compared with seedlings treated at lower doses.
Figure 3.
Dose effect of MetJA on 12:0 ω-hydroxylation in microsomes of V. sativa seedlings. 12:0 ω-Hydroxylation was measured in microsomes of seedlings (5 d old) induced for 24 h in a solution containing increasing concentrations of MetJA. Activity in microsomes of control seedlings (induced for 24 h in water) was 16.3 ± 1.3 pmol min−1 mg−1 protein. Data are means ± sd of three measurements performed in duplicate.
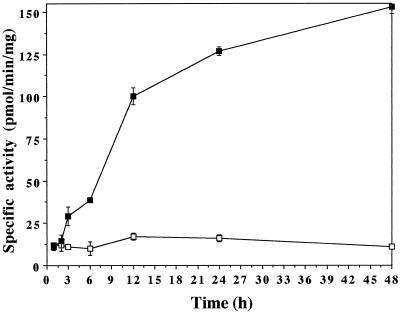
Figure 4 shows 12:0 ω-hydroxylation in microsomes from seedlings induced for different periods in water (control) or in water containing 1 mm MetJA. The activity in control microsomes remained constant during the experiment. To the contrary, induction of seedlings in the presence of 1 mm MetJA led to a drastic enhancement of activity. The effect of the inducer was already measurable after 3 h of treatment: activity was 2.7-fold higher in microsomes of treated plants. Time-course studies with longer exposure times revealed that activity decreased by 65% between 48 and 72 h (not shown).
Figure 4.
Effect of time of treatment with MetJA on 12:0 ω-hydroxylation in microsomes of V. sativa seedlings. 12:0 ω-Hydroxylation was measured in microsomes of seedlings (5 d old) induced for different periods in water (□) or in water containing 1 mm MetJA (▪). Data are means ± sd of three measurements performed in duplicate.
We measured the rate of enzymatic hydrolysis of 9,10-epoxystearic acid in microsomes and soluble fractions of seedlings treated with different MetJA concentrations or with 1 mm MetJA for different periods. Neither the microsomal epoxide hydrolase nor the soluble epoxide hydrolase was affected by these treatments.
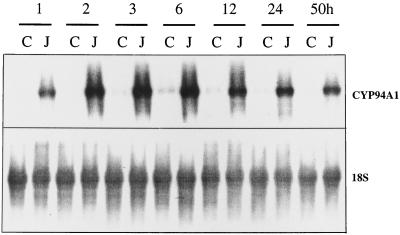
Time Course of CYP94A1 Expression in Control and MetJA-Treated Seedlings
We recently cloned CYP94A1, a Cyt P450-dependent ω-hydroxylase from V. sativa that hydroxylates C12 to C18 fatty acids (Tijet et al., 1998). Here we studied the accumulation of CYP94A1 transcripts during induction of V. sativa seedlings in water or in water containing 1 mm MetJA. The results are presented in Figure 5. Hybridization with a 32P-labeled cDNA probe corresponding to the whole coding sequence of CYP94A1 showed accumulation of hydroxylase-specific mRNA after 1 h of exposure to MetJA. The transcript level increased 14-fold and reached a maximum at 3 h.
Figure 5.
Time-course analysis of CYP94A1 expression in control and MetJA-treated V. sativa seedlings. Seedlings (5 d old) were induced for different periods in water (C) or in water containing 1 mm MetJA (J). Total RNA was extracted from 15 g of seedlings and 30 μg was subjected to RNA-blot analysis. An 18S ribosomal DNA from radish was used as an internal standard.
DISCUSSION
Because we and others (Schweizer et al., 1996a, 1996b) suspect that hydroxy fatty acids are involved in stress signaling, we have examined the effect of the plant hormone MetJA on fatty acid ω-hydroxylation. When using a compound such as MetJA, which is both hydrophobic and volatile, there is no simple relation between the applied dose and the concentration achieved in situ. We applied MetJA using the same protocol described previously for hydrophobic compounds such as clofibrate and DEHP (Salaün et al., 1986; Pinot et al., 1992). A dose-response study (see Results) showed that the response was clearly detectable from 50 μm and remained detectable up to 1 mm MetJA in the treatment solution. It is not possible to directly compare the doses used here with those used in studies in which MetJA was used in the vapor phase or as droplets on leaves. The majority of our experiments were performed with 1 mm MetJA, the concentration that gave the highest induction.
The increase of total spectrophotometrically detectable Cyt P450 that we measured after MetJA treatment illustrates the role of MetJA as an inducer of plant defense. It is recognized that Cyt P450s are involved in the biosynthetic pathway of major phytoalexins (Durst and Benveniste, 1993). Recently, Suzuki et al. (1996) and Ohta et al. (1997) described the first report of MetJA-induced transcription of a Cyt P450 gene (CYP93A1) in soybean. The authors suggest that this Cyt P450 is involved in the plant response to fungal attack.
After 3 h of treatment with MetJA, ω-hydroxylation of 12:0 was already 2.7-fold higher in microsomes of treated versus control seedlings. This rapid response is consistent with an involvement of ω-hydroxylases in the mechanism of plant defense. When examining the role of ω-hydroxy fatty acids as endogenous signal molecules, Schweizer et al. (1996a) have shown that they are perceived by potato cells within 1 to 2 h. In other studies related to plant defense, jasmonates accumulated within 2 h after a stress in cultured cells or in leaves of soybean (Creelman et al., 1992; Creelman and Mullet, 1997). These authors also showed that accumulation of mRNA coding for wound-responsive genes occurred within 4 h after MetJA treatment.
Northern-blot analysis performed with the cDNA probe corresponding to CYP94A1 showed that MetJA treatment led to a rapid accumulation of CYP94A1 transcripts. Jasmonates are thought to act intracellularly as gene inducers. The dose-response effect described in the present study is in favor of the existence of a receptor that remains to be characterized. Mammalian ω-hydroxylases are enhanced by compounds such as clofibrate or DEHP, which induce proliferation of peroxisomes (for review, see Simpson, 1997). This enhancement occurs via PPARs. Isseman and Green (1990) demonstrated that the first PPAR cloned is activated by clofibrate and other peroxisome proliferators, which probably mimic endogenous compounds. Recently, different groups have shown that PPAR can be activated by prostaglandins and other fatty acid derivatives, which could be endogenous ligands of PPAR (Devchand et al., 1996; Wolf, 1996; Forest et al., 1997; Forman et al., 1997; Krey et al., 1997). Clofibrate and DEHP produce similar effects in plants (Salaün et al., 1986; Palma et al., 1991; Pinot et al., 1992), which suggests that the mechanisms of regulation by these compounds may be conserved. There are evident structural analogies between prostaglandins and jasmonates, which are involved in responses to stress. Both are cyclic derivatives of fatty acids (20:4 and 18:3, respectively), and they share a similar five-carbon-ringed structure.
It is tempting to speculate that, like the stimulation of mammalian ω-hydroxylases by prostaglandins, MetJA could induce ω-hydroxylase from V. sativa via activation of a transcriptional regulatory protein analog to PPAR. Recently, Rouster et al. (1997) identified a MetJA-responsive element in the promoter of a lipoxygenase. It is interesting that this element contains the motif TGAC as inverted repeats, which is also found in the promoter region of two mammalian ω-hydroxylases, CYP4A1 and CYP4A6 (Johnson et al., 1996). We are in the process of cloning the complete gene coding for CYP94A1. The knowledge of the sequence will allow a comparison with genes inducible by peroxisome proliferators in mammals and genes coding for proteins implicated in plant defense (Yang et al., 1997).
Recently, we demonstrated the existence of distinct epoxide hydrolases in V. sativa capable of hydrolyzing 9,10-epoxystearic acid (Pinot et al., 1997). Here we show that MetJA treatment does not affect microsomal or soluble epoxide hydrolase activities. This is in contrast to the data from Stapleton et al. (1994), who reported induction by MetJA of the soluble epoxide hydrolase from potato at the transcriptional level. This discrepancy might be explained by the existence of different isozymes of epoxide hydrolases. It could also be attributable to the use of different plant materials. It is noteworthy that epoxides of fatty acid are more effective stress signals than the corresponding diols (Schweizer et al., 1996a). Furthermore, protection of barley against Erysiphe gramini f. sp. hordei after application of 9,10-dihydroxystearic acid was not greater than the protection observed after application of the original epoxide (Schweizer et al., 1996b). Finally, secondary hydroxyls resulting from hydrolysis of an epoxide are not essential for cutin synthesis, which results from the esterification involving mainly primary hydroxyls (Kolattukudy, 1980). Consequently, in the context of plant resistance, epoxide hydrolases might not have key roles.
In conclusion, using V. sativa and 12:0 as models, we show here for the first time, to our knowledge, that MetJA treatment of seedlings stimulates microsomal ω-hydroxylation of fatty acids. This stimulation might be a major event in the general mechanism of plant defense. It leads to the production of ω-hydroxy fatty acids, which (a) are incorporated in cutin, a constituent of the first barrier between the plant and the outer environment, and (b) act as endogenous signals in plants. The comparison of the composition and formation of cutin in control and MetJA-treated plants will help us to assess the involvement of ω-hydroxylases in cutin synthesis. Furthermore, at present we are growing tobacco lines (sense and antisense) with coding sequences of ω-hydroxylases. It will be interesting to measure the cutin formation of these transgenic plants and to determine if cutin modification alters resistance against stress (i.e. pathogen and drought). The comparison of mammalian and plant ω-hydroxylase regulation, together with the similar origins, structures, and functions of prostaglandins and MetJA, suggests that the induction studied here could involve the activation of a receptor analog to PPAR. Cloning of the complete gene coding for CYP94A1 might confirm the existence of cis-elements that could bind such a receptor.
ACKNOWLEDGMENTS
We thank Drs. J.-P. Noël and O. Loreau (Commissariat á l'Energie Atomique, Gif-sur-Yvette, France) for the generous gift of [1-14C]9,10-epoxystearic acid.
Abbreviations:
- DEHP
diethylhexyl-phtalate
- MetJA
methyl jasmonate
- PPAR
peroxisome proliferator-activated receptor
- X:Y
a fatty acyl group containing X carbon atoms and Y cis double bonds
Footnotes
This work was partly supported by grants from the Ministère de la Recherche et de la Technologie (Génétique et Environnement, no. ACC-SV3) and from the Centre National de la Recherche Scientifique (Program Environment, no. GDR 1105).
LITERATURE CITED
- Andresen I, Becker W, Schlüter K, Burges J, Parthier B, Apel K. The identification of leaf thionin as one of the main jasmonate-induced proteins of barley. Plant Mol Biol. 1992;19:193–204. doi: 10.1007/BF00027341. [DOI] [PubMed] [Google Scholar]
- Avdiushko S, Croft KPC, Brown GC, Jackson DM, Hamilton-Kemp TR, Hildebrand D. Effect of volatile methyl jasmonate on the oxylipin pathway in tobacco, cucumber, and Arabidopsis. Plant Physiol. 1995;109:1227–1230. doi: 10.1104/pp.109.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I, Salaün JP, Simon A, Reichhart D, Durst F. Cytochrome P450 dependent ω-hydroxylation of lauric acid by microsomes from pea seedlings. Plant Physiol. 1982;70:122–126. doi: 10.1104/pp.70.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry B, Müller-Uri F, Cameron-Mills V, Gough S, Simpson D, Skriver K, Mundy J. The barley 60 kDa jasmonate-induced protein (JIP60) is a novel ribosome inactivating protein. Plant J. 1994;6:815–824. doi: 10.1046/j.1365-313x.1994.6060815.x. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JA. Jasmonic acid/ methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzales FJ, Wahli W. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- Durst F, Benveniste I. Cytochrome P450 in plants. In: Schenkman JB, Greim H, editors. Cytochrome P450: Handbook of Pharmacology, Vol 105. Berlin: Springer-Verlag; 1993. pp. 293–310. [Google Scholar]
- Farmer EE, Ryan CA. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase in plant leaves. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest C, Franckhauser S, Glorian M, Antras-Ferry J, Robin D, Robin P. Regulation of gene transcription by fatty acids, fibrates and prostaglandins: the phosphoenolpyruvate carboxykinase gene as a model. Prostaglandins Leukotrienes Essent Fatty Acids. 1997;57:47–56. doi: 10.1016/s0952-3278(97)90492-0. [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Evans R. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach H, Muller MJ, Kutchan T, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci USA. 1992;89:2389–2393. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz T, Bergey DR, Ryan CA. A gene encoding a chloroplast-targeted lipoxygenase in tomato leaves is transiently induced by wounding, systemin, and methyl jasmonate. Plant Physiol. 1997;114:1085–1093. doi: 10.1104/pp.114.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isseman I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Johnson EF, Palmer CNA, Griffin KJ, Hsu MH. Role of the peroxisome proliferator-activated receptor in cytochrome P450 4A gene regulation. FASEB J. 1996;10:1241–1248. doi: 10.1096/fasebj.10.11.8836037. [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE. Biopolyester membranes of plants: cutin and suberin. Science. 1980;208:990–1000. doi: 10.1126/science.208.4447.990. [DOI] [PubMed] [Google Scholar]
- Krey G, Braissant O, L'Horset F, Kalkhoven E, Perroud M, Parker MG, Wahli W. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- Mueller MJ. Enzymes involved in jasmonic acid biosynthesis. Physiol Plant. 1997;100:653–663. [Google Scholar]
- Ohta H, Suzuki G, Awai K, Masuda T, Kato T, Shibata D, Takamiya K. Distinct pathways for jasmonate- and elicitor-induced expressions of a cytochrome P450 gene in soybean suspension-cultured cells. Physiol Plant. 1997;100:647–652. [Google Scholar]
- Omura T, Sato R. The carbon monoxide binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–2378. [PubMed] [Google Scholar]
- Palma JM, Garrido M, Rodriguez-Garcia MI, del Rio LA. Peroxisome proliferation and oxidative stress mediated by activated oxygen species in plant peroxisomes. Arch Biochem Biophys. 1991;287:68–74. doi: 10.1016/0003-9861(91)90389-z. [DOI] [PubMed] [Google Scholar]
- Pinot F, Bosch H, Alayrac C, Mioskowski C, Vendais A, Durst F, Salaün JP. ω-Hydroxylation of oleic acid in Vicia sativa microsomes. Inhibition by substrate analogs and inactivation by terminal acetylenes. Plant Physiol. 1993;102:1313–1318. doi: 10.1104/pp.102.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinot F, Bosch H, Salaün JP, Durst F, Mioskowski C, Hammock BD. Epoxide hydrolase activities in the microsomes and the soluble fraction from Vicia sativa seedlings. Plant Physiol Biochem. 1997;35:103–110. [Google Scholar]
- Pinot F, Salaün JP, Bosch H, Lesot A, Mioskowski C, Durst F. ω-Hydroxylation of Z9-octadecenoic, Z9,10-epoxystearic and 9,10-dihydroxystearic acids by microsomal cytochrome P450 systems from Vicia sativa. Biochem Biophys Res Commun. 1992;184:183–193. doi: 10.1016/0006-291x(92)91176-q. [DOI] [PubMed] [Google Scholar]
- Rouster J, Leah R, Mundy J, Cameron-Mills V. Identification of a methyl jasmonate-responsive region in the promoter of a lipoxygenase 1 gene expressed in barley grain. Plant J. 1997;11:513–523. doi: 10.1046/j.1365-313x.1997.11030513.x. [DOI] [PubMed] [Google Scholar]
- Salaün JP, Simon A, Durst F. Specific induction of lauric acid ω-hydroxylase by clofibrate, diethylhexyl-phtalate and 2,4-dichlorophenoxyacetic acid in higher plants. Lipids. 1986;21:776–779. [Google Scholar]
- Schweizer P, Felix G, Buchala A, Müller C, Métraux JP. Perception of free cutin monomers by plant cells. Plant J. 1996a;10:331–341. [Google Scholar]
- Schweizer P, Jeanguenat A, Whitacre D, Métraux JP, Mösinger E. Induction of resistance in barley against Erysiphe gramini f. sp. hordei by free cutin monomers. Physiol Mol Plant Pathol. 1996b;49:103–120. [Google Scholar]
- Sembdner G, Parthier B. The biochemistry and the physiological and molecular actions of jasmonates. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:569–589. [Google Scholar]
- Simpson AECM. The cytochrome P450 A (CYP4) family. Gen Pharmacol. 1997;28:351–359. doi: 10.1016/s0306-3623(96)00246-7. [DOI] [PubMed] [Google Scholar]
- Soliday CL, Kolattukudy PE. Biosynthesis of cutin. ω-Hydroxylation of fatty acids by a microsomal preparation from germinating Vicia faba. Plant Physiol. 1977;59:1116–1121. doi: 10.1104/pp.59.6.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton A, Beetham JK, Pinot F, Garbarino JE, Rockhold DR, Friedman M, Hammock BD, Belknap WR. Cloning and expression of soluble epoxide hydrolase from potato. Plant J. 1994;6:251–258. doi: 10.1046/j.1365-313x.1994.6020251.x. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Ohta H, Kato T, Igarashi T, Sakaki F, Shibata D, Takano A, Masuda T, Shioi Y, Takamiya K. Induction of a novel cytochrome P450 (CYP93 family) by methyl jasmonate in soybean suspension-cultured cells. FEBS Lett. 1996;383:83–86. doi: 10.1016/0014-5793(96)00229-3. [DOI] [PubMed] [Google Scholar]
- Tijet N, Helvig C, Pinot F, Le Bouquin R, Lesot A, Durst F, Salaün JP, Benveniste I. Functional expression in yeast and characterization of a clofibrate-inducible plant cytochrome P450 (CYP94A1) involved in cutin monomer synthesis. Biochem J. 1998;332:583–589. doi: 10.1042/bj3320583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G. Adipocyte differentiation is regulated by a prostaglandin liganded to the nuclear peroxisome proliferator-activated receptor. Nutr Rev. 1996;54:290–292. doi: 10.1111/j.1753-4887.1996.tb03951.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shah J, Klessig DF. Signal perception and transduction in plant defense responses. Genes Dev. 1997;11:1621–1639. doi: 10.1101/gad.11.13.1621. [DOI] [PubMed] [Google Scholar]