Abstract
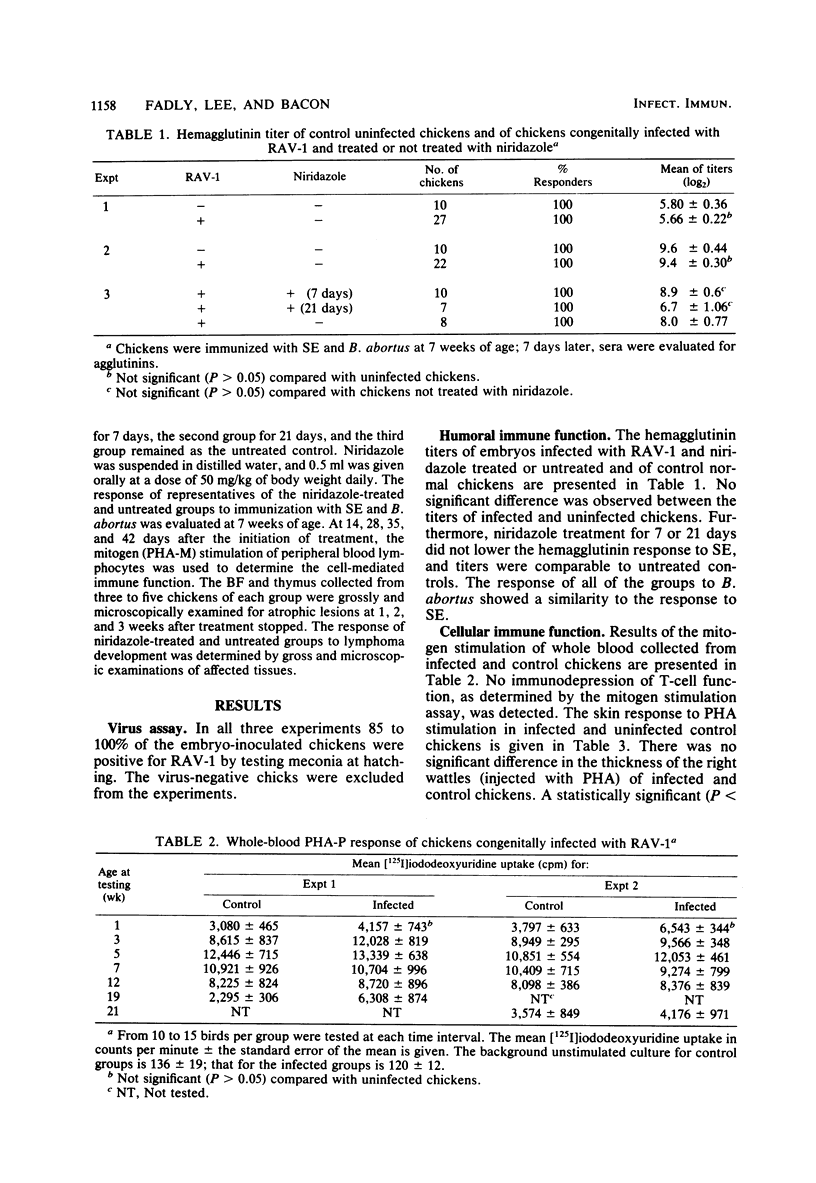
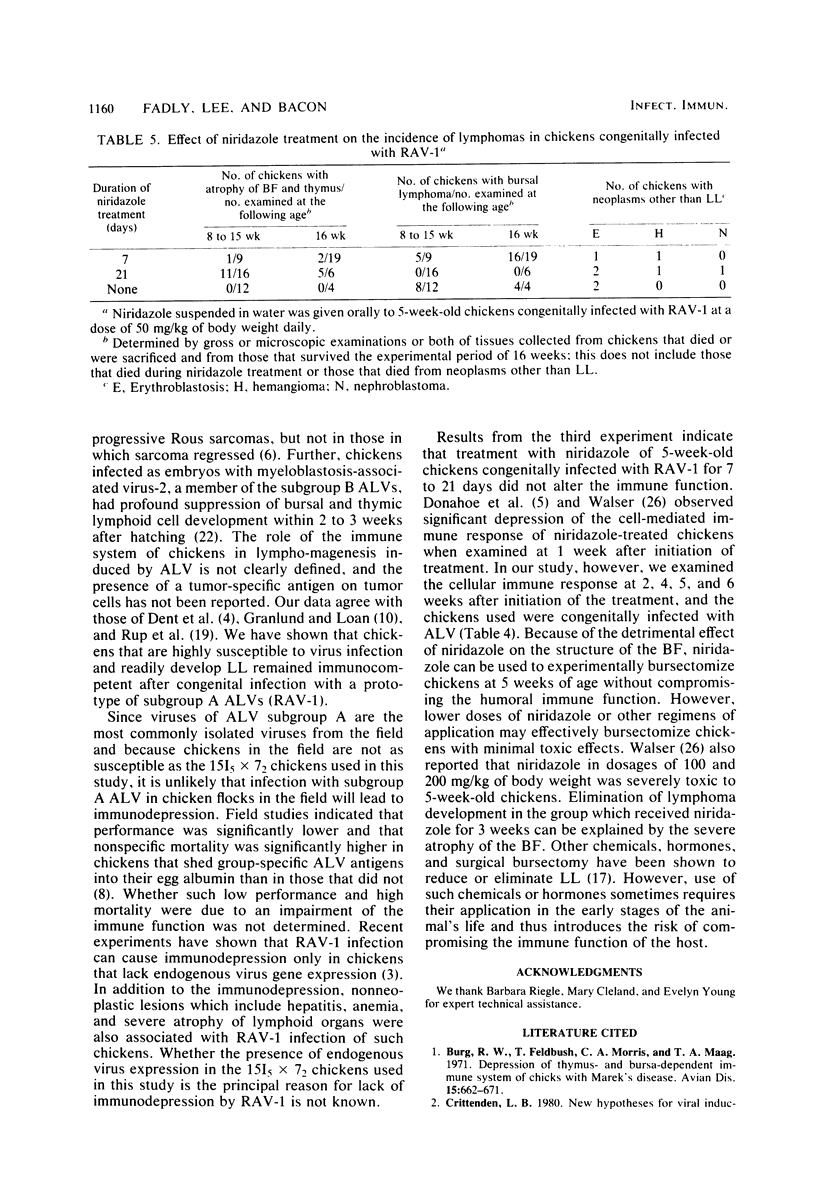
A study was designed to determine the effects of congenital infection with the Rous-associated virus-1 (RAV-1) on the immune function chickens during the early and late tumorigenic stages of infection. In another experiment, the effects of niridazole on the immune competence and the tumor incidence in chickens congenitally infected with RAV-1 were studied. Lymphocyte stimulation by phytohemagglutinin, the phytohemagglutinin skin test, the response to immunization with sheep erythrocytes and Brucella abortus, and histological evaluation of lymphoid organs were used to determine the immune competence in normal and infected chickens. Results indicated that both B- and T-cell immune functions during the early and late stages of RAV-1 infection were comparable to those of normal uninfected chickens. Administration of niridazole to congenitally infected chickens at 5 weeks of age for 7 or 21 days had no effect on the T-cell-mediated immunity; however, administration of the drug for 21 days eliminated lymphoma development. Unlike infection with other oncogenic viruses such as those causing Marek's disease and reticuloendotheliosis, infection with RAV-1 caused no detectable immunodepression during the early and late stages of infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg R. W., Feldbush T., Morris C. A., Maag T. A. Depression of thymus-and bursa-dependent immune systems chicks with Marek's disease. Avian Dis. 1971 Oct-Dec;15(4):662–671. [PubMed] [Google Scholar]
- Dent P. B., Cooper M. D., Payne L. N., Solomon J. J., Burmester B. R., Good R. A. Pathogenesis of avian lymphoid leukosis. II. Immunologic reactivity during lymphomagenesis. J Natl Cancer Inst. 1968 Aug;41(2):391–401. [PubMed] [Google Scholar]
- Donahoe J. P., Giambrone J., Fletcher O. J., Kleven S. H. In vivo function tests of the effect of tilorone and niridazole on cell-mediated immunity in chickens. Am J Vet Res. 1977 Dec;38(12):2013–2017. [PubMed] [Google Scholar]
- Fadly A. M., Purchase H. G., Gilmour D. G. Tumor latency in avian lymphoid leukosis. J Natl Cancer Inst. 1981 Mar;66(3):549–552. [PubMed] [Google Scholar]
- Gavora J. S., Spencer J. L., Gowe R. S., Harris D. L. Lymphoid leukosis virus infection: effects on production and mortality and consequences in selection for high egg production. Poult Sci. 1980 Oct;59(10):2165–2178. doi: 10.3382/ps.0592165. [DOI] [PubMed] [Google Scholar]
- Goto N., Kodama H., Okada K., Fujimoto Y. Suppression of phytohemagglutinin skin response in thymectomized chickens. Poult Sci. 1978 Jan;57(1):246–250. doi: 10.3382/ps.0570246. [DOI] [PubMed] [Google Scholar]
- Granlund D. J., Loan R. W. Effect of lymphoid leukosis virus infection on the cell-mediated immune capacity of the chicken. J Natl Cancer Inst. 1974 Apr;52(4):1373–1374. doi: 10.1093/jnci/52.4.1373. [DOI] [PubMed] [Google Scholar]
- Lee L. F. Chicken lymphocyte stimulation by mitogens: a microassay with whole-blood cultures. Avian Dis. 1978 Apr-Jun;22(2):296–307. [PubMed] [Google Scholar]
- Mahmoud A. A., Warren K. S. Anti-inflammatory effects of tartar emetic and niridazole: suppression of schistosome egg granuloma. J Immunol. 1974 Jan;112(1):222–228. [PubMed] [Google Scholar]
- Meyers P., Ritts G. D., Johnson D. R. Phytohemagglutinin-induced leukocyte blastogenesis in normal and avian leukosis virus-infected chickens. Cell Immunol. 1976 Nov;27(1):140–146. doi: 10.1016/0008-8749(76)90163-5. [DOI] [PubMed] [Google Scholar]
- Okazaki W., Purchase H. G., Burmester B. R. Phenotypic mixing test to detect and assay avian leukosis viruses. Avian Dis. 1975 Apr-Jun;19(2):311–317. [PubMed] [Google Scholar]
- Peterson R. D., Purchase H. G., Burmester B. R., Cooper M. D., Good R. A. Relationships among visceral lymphomatosis, bursa of Fabricius, and bursa-dependent lymphoid tissue of the chicken. J Natl Cancer Inst. 1966 Apr;36(4):585–598. doi: 10.1093/jnci/36.4.585. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Chubb R. C., Biggs P. M. Effect of lymphoid leukosis and Marek's disease on the immunological responsiveness of the chicken. J Natl Cancer Inst. 1968 Mar;40(3):583–592. [PubMed] [Google Scholar]
- Rup B. J., Hoelzer J. D., Bose H. R., Jr Helper viruses associated with avian acute leukemia viruses inhibit the cellular immune response. Virology. 1982 Jan 15;116(1):61–71. doi: 10.1016/0042-6822(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Scofield V. L., Bose H. R., Jr Depression of mitogen response in spleen cells from reticuloendotheliosis virus-infected chickens and their suppressive effect on normal lymphocyte response. J Immunol. 1978 Apr;120(4):1321–1325. [PubMed] [Google Scholar]
- Smith R. E., Ivanyi J. Pathogenesis of virus-induced osteopetrosis in the chicken. J Immunol. 1980 Aug;125(2):523–530. [PubMed] [Google Scholar]
- Smith R. E., Van Eldik L. J. Characterization of the immunosuppression accompanying virus-induced avian osteopetrosis. Infect Immun. 1978 Nov;22(2):452–461. doi: 10.1128/iai.22.2.452-461.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis G. A., McBride R. A., Schierman L. W. Depression of in vitro responsiveness to phytohemagglutinin in spleen cells cultured from chickens with Marek's disease. J Immunol. 1975 Sep;115(3):848–853. [PubMed] [Google Scholar]
- Walser M. M. Evaluation of niridazole as a suppressant of cellular immunity in chickens. Am J Vet Res. 1978 Nov;39(11):1858–1860. [PubMed] [Google Scholar]


