Abstract
Rapidly activating and rapidly inactivating voltage-gated A-type K+ currents, IA, are key determinants of neuronal excitability and several studies suggest a critical role for the Kv4.2 pore-forming α subunit in the generation of IA channels in hippocampal and cortical pyramidal neurons. The experiments here demonstrate that Kv4.2, Kv4.3 and Kv1.4 all contribute to the generation of IA channels in mature cortical pyramidal (CP) neurons and that Kv4.2-, Kv4.3- and Kv1.4-encoded IA channels play distinct roles in regulating the intrinsic excitability and the firing properties of mature CP neurons. In vivo loss of Kv4.2, for example, alters the input resistances, current thresholds for action potential generation and action potential repolarization of mature CP neurons. Elimination of Kv4.3 also prolongs action potential duration, whereas the input resistances and the current thresholds for action potential generation in Kv4.3−/− and WT CP neurons are indistinguishable. In addition, although increased repetitive firing was observed in both Kv4.2−/− and Kv4.3−/− CP neurons, the increases in Kv4.2−/− CP neurons were observed in response to small, but not large, amplitude depolarizing current injections, whereas firing rates were higher in Kv4.3−/− CP neurons only with large amplitude current injections. In vivo loss of Kv1.4, in contrast, had minimal effects on the intrinsic excitability and the firing properties of mature CP neurons. Comparison of the effects of pharmacological blockade of Kv4-encoded currents in Kv1.4−/− and WT CP neurons, however, revealed that Kv1.4-encoded IA channels do contribute to controlling resting membrane potentials, the regulation of current thresholds for action potential generation and repetitive firing rates in mature CP neurons.
Key points
A-type K+ currents, IA, are key determinants of neuronal excitability. Previous studies suggest critical roles for voltage-gated K+ (Kv) channel pore-forming (α) subunits of the Kv4 subfamily in the generation of neuronal IA channels.
The experiments here examined directly the functional roles of Kv4.2, Kv4.3 and Kv1.4 in the regulation of the intrinsic excitability and the firing properties of mature cortical pyramidal (CP) neurons.
The results demonstrate roles for Kv4.2, Kv4.3 and Kv1.4 in the generation of IA channels and show that Kv4.2-, Kv4.3- and Kv1.4-encoded IA channels play distinct roles in regulating the intrinsic excitability and the firing properties of CP neurons.
These findings demonstrate previously unappreciated molecular and functional diversity of IA in central neurons, insights that will contribute importantly to future studies focused on determining the mechanisms underlying the alterations in neuronal excitability in epilepsy and other neurological disorders.
Introduction
Considerable evidence suggests that A-type K+ currents, IA, in hippocampal and cortical pyramidal neurons are encoded by voltage-gated K+ (Kv) channel pore-forming (α) subunits of the Kv4 subfamily (Kim et al. 2005; Yuan et al. 2005; Chen et al. 2006; Andrasfalvy et al. 2008; Nerbonne et al. 2008; Norris & Nerbonne, 2010). Studies using pharmacological approaches to block IA and dominant negative strategies to reduce Kv4 currents selectively have also demonstrated that Kv4-encoded IA channels play key roles in the regulation of the intrinsic membrane properties of pyramidal neurons (Locke & Nerbonne, 1997; Kim et al. 2005; Yuan et al. 2005). Acute in vitro reductions in IA, for example, dramatically affect the resting membrane potentials, input resistances and current thresholds for action potential generation, as well as the waveforms of action potential and the repetitive firing properties of isolated cortical pyramidal neurons and hippocampal pyramidal neurons in organotypic slice cultures (Locke & Nerbonne, 1997; Kim et al. 2005; Yuan et al. 2005).
Studies performed on hippocampal pyramidal neurons in acute slices prepared from mice harboring a targeted disruption of the Kcnd2 locus (Kv4.2−/−) demonstrated that the elimination of Kv4.2 also affects resting membrane potentials and action potential waveforms (Andrasfalvy et al. 2008). In addition, a mutation in the Kv4.2 gene has been identified in a patient with temporal lobe epilepsy (TLE) (Singh et al. 2006). The functional consequences of the in vivo loss of Kv4.2, however, are modest compared to those observed with acute attenuation or elimination of IA using pharmacological approaches or dominant negative strategies (Locke & Nerbonne, 1997; Shibata et al. 2000; Kim et al. 2005; Yuan et al. 2005). In addition, experiments on cortical pyramidal (CP) neurons isolated from neonatal Kv4.2−/− mice demonstrated that the resting and active membrane properties of these neurons are preserved, in spite of the deletion of Kv4.2 (Nerbonne et al. 2008).
Additional studies on isolated Kv4.2−/− CP neurons, however, revealed that delayed rectifier K+ currents are larger in Kv4.2−/− than in wild-type (WT) cells, suggesting that electrical remodelling compensates for the in vivo loss of Kv4.2 to maintain firing properties (Nerbonne et al. 2008). It is certainly also possible that Kv4.2 is not the only Kv α subunit that contributes to the generation of IA channels in CP neurons. Consistent with this suggestion, immunohistochemical experiments have demonstrated that the Kv4.3 protein is also expressed in clusters in the soma, dendrites and spines of mature CP neurons (Burkhalter et al. 2006). In addition, recent studies revealed that Kv4.3, as well as Kv1.4, contribute to macroscopic IA in isolated neonatal CP neurons (Norris & Nerbonne, 2010). Interestingly, alterations in the expression and localization of Kv4.2, Kv4.3 and Kv1.4 have been reported in animal models of epilepsy (Francis et al. 1997; Kim et al. 2007; Lugo et al. 2008; Monaghan et al. 2008), suggesting that alterations in these three IA channel α subunits might contribute to increased neuronal excitability and epileptogenesis. The functional roles of Kv4.3- and Kv1.4-encoded IA channels in the regulation of intrinsic excitability and the firing properties of mature mammalian pyramidal neurons, however, have not been examined. The experiments here were designed to explore these questions directly. The results presented demonstrate that Kv4.2, Kv4.3 and Kv1.4 all contribute to the generation of IA in mature CP neurons and that IA channels encoded by these three Kv α subunits play distinct roles in regulating the resting and active membrane properties of mature CP neurons.
Methods
Adult (4- to 8-week-old) wild-type (WT) mice and mice harbouring targeted genetic disruption of the Kcna4 (Kv1.4−/−) (London et al. 1998), Kcnd2 (Kv4.2−/−) (Guo et al. 2005) or Kcnd3 (Kv4.3−/−) (Niwa et al. 2008) locus were used in the experiments here. All animals were in the C57BL/6 background; the Kv1.4−/−, Kv4.2−/− and Kv4.3−/− mice, obtained in different backgrounds, were backcrossed onto the C57BL/6 background for >10 generations. Experiments were also conducted using adult C57/BL6 mice lacking both Kv4.2 and Kv4.3 (Kv4.2−/−/Kv4.3−/−), generated by crossing Kv4.2−/− and Kv4.3−/− C57BL/6 animals. All experiments were performed in accordance with the guidelines published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experimental protocols were approved by the Washington University School of Medicine Animal Care and Use Committee. All reagents were from Sigma unless otherwise noted.
Preparation of cortical slices
Slices were prepared from the primary visual cortices of (4- to 8-week-old) mice using standard procedures (Davie et al. 2006). Briefly, the mice were deeply anaesthetized with 1.25% Avertin (2,2,2-tribromoethanol and tert-amyl alcohol in 0.9% NaCl; 0.025 ml (g body weight)−1) and then perfused transcardially with ice-cold cutting solution containing (in mm): sucrose, 240; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; CaCl2, 0.5; and MgCl2 7, saturated with 95% O2–5% CO2. Brains were rapidly removed and placed in ice-cold cutting solution. Coronal slices (350 μm) containing the primary visual cortex were cut on a Leica VT1000 S vibrating blade microtome (Leica Microsystems Inc., Buffalo Grove, IL, USA) and incubated in a holding chamber with oxygenated artificial cerebrospinal fluid (ACSF) containing (in mm): NaCl, 125; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 25; CaCl2, 2; MgCl2, 1; and dextrose, 25 (∼310 mosmol l−1), saturated with 95% O2–5% CO2, at room temperature for at least 30 min before transfer to the recording chamber.
Electrophysiological recordings
Whole-cell current-clamp recordings were obtained at room temperature (22–24°C) from visually identified layer 5 pyramidal neurons using differential interference contrast optics with infrared illumination. Recording electrodes were positioned in layer 5 of primary visual cortex under visual control. The primary visual cortex was identified by its distinctive myeloarchitecture as previously described (Dong et al. 2004). Slices were perfused continually with ACSF, saturated with 95% O2–5% CO2. For the tetraethylammonium (TEA) and the Ba2+ experiments, TEA (3 mm) or BaCl2 (400 μm) was added to the ACSF immediately before recordings. In all experiments, recording pipettes contained (in mm): potassium methylsulfate (KMeSO4) 120, KCl 20, Hepes 10, EGTA 0.2, NaCl 8, Mg-ATP 4, Tris-GTP 0.3 and phosphocreatine 14 (pH 7.25; ∼300 mosmol l−1). Experiments were controlled and data were collected using a Multiclamp 700B patch clamp amplifier interfaced with a Digidata 1332 and the pCLAMP 9 software (Axon Instruments, Union City, CA, USA), to a PC. In all experiments, tip potentials were zeroed before membrane–pipette seals were formed; pipette capacitances and series resistances were compensated using the pCLAMP software. Signals were acquired at 50 kHz and filtered at 10 kHz prior to digitization and storage. Initial resting membrane potentials (Vm) were between −60 and −80 mV. Single action potentials and action potential trains were elicited from resting membrane potentials in response to brief (5 ms) and prolonged (500 ms) depolarizing current injections of variable amplitudes.
Data analysis
Data were compiled and analysed using ClampFit (Molecular Devices), Microsoft Excel, Mini Analysis (v. 6.0, Synaptosoft, Inc., Decatur, GA, USA) and Prism (GraphPad Software Inc., La Jolla, CA, USA). Input resistances (Rin) were determined from the change in membrane potential produced by a 20 pA hyperpolarizing current injection from the resting potential. The current threshold for action potential generation was defined as the minimal current injection, applied (for 5 ms) from the resting membrane potential, required to evoke a single action potential. Action potential widths at half-maximum were determined from measurement of the duration of the action potential when the membrane voltage had returned from the peak halfway back to the resting membrane potential.
Statistics
Results are expressed as means ± SEM. Statistical analyses were performed using Student's (unpaired) t test or two-way analysis of variance (ANOVA) using GraphPad Prism (v. 4.0.
Results
Deletion of Kv4.2 or Kv4.3 differentially alters repetitive firing in cortical pyramidal neurons
As illustrated in Fig. 1A, layer 5 visual cortical pyramidal (CP) neurons in acute slices prepared from adult (4- to 8-week-old) wild-type (WT) mice fire repetitively in response to prolonged (500 ms) depolarizing current injections. Although all CP cells studied displayed some degree of frequency adaptation, the majority (∼90%) of the cells fired action potentials throughout the duration of the current injection. Similar to previous studies on isolated neonatal CP neurons (Yuan et al. 2005; Nerbonne et al. 2008), the repetitive firing rates of mature layer 5 CP neurons increased significantly (P < 0.001) as a function of the amplitude of the injected current (Fig. 1B).
Figure 1. Deletion of Kv4.2 or Kv4.3 increases firing rates in layer 5 CP neurons.

Repetitive firing was evoked in mature layer 5 CP neurons in acute slices prepared from WT, Kv4.2−/− or Kv4.3−/− animals in response to prolonged (500 ms) depolarizing current injections of varying amplitudes. A, representative voltage records are shown; the injected current amplitudes are illustrated under the voltage records. B, mean ± SEM numbers of action potentials evoked during 500 ms current injections are plotted as a function of the amplitudes of the injected currents. The input–output curves (number of spikes vs. injected current amplitude) for Kv4.2−/− (n = 30) and Kv4.3−/− (n = 21) CP neurons were significantly (P < 0.001) different from the curve for WT (n = 20) CP neurons. Bonferroni post hoc analysis further revealed that the responses of Kv4.2−/− and Kv4.3−/− CP neurons to depolarizing current injections of small and large amplitudes were distinct. ‡†Values measured in Kv4.2−/− or Kv4.3−/− were significantly (‡P < 0.01; †P < 0.05) different from those determined in WT cells.
To examine the functional roles of Kv4.2 and Kv4.3 in regulating the repetitive firing properties of mature CP cells, additional experiments were conducted on slices prepared from mice lacking Kv4.2 (Kv4.2−/−) or Kv4.3 (Kv4.3−/−). As in WT cells, prolonged depolarizing current injections elicited repetitive firing in Kv4.2−/− and Kv4.3−/− CP neurons (Fig. 1A). Two-way analysis of variance (ANOVA) revealed, however, that the input–output curves (number of spikes vs. injected current amplitude) of Kv4.2−/− (n = 30) and Kv4.3−/− (n = 21) CP neurons were significantly (P < 0.001) different from WT (n = 20) CP neurons (Fig. 1B). Bonferroni post hoc analysis further revealed that the responses of Kv4.2−/− and Kv4.3−/− CP neurons to depolarizing current injections of small and large amplitudes are distinct (Fig. 1B). Firing rates in response to small (80–120 pA) amplitude current injections, for example, were significantly (P < 0.05) higher in Kv4.2−/−, than in WT, CP neurons, but were indistinguishable in WT and Kv4.3−/− CP neurons (Fig. 1B). In contrast, the responses to large (180–220 pA) amplitude current injections were indistinguishable in WT and Kv4.2−/− CP neurons but were significantly (P < 0.05) higher in Kv4.3−/−, than in WT, CP neurons (Fig. 1B). These combined results demonstrate distinct functional roles for Kv4.2 and Kv4.3 in the generation of IA channels and in the regulation of repetitive firing in mature CP neurons (see Discussion).
To study the effect of the combined loss of Kv4.2 and Kv4.3, recordings were also obtained from CP neurons in slices prepared from mice lacking both Kv4.2 and Kv4.3 (Kv4.2−/−/Kv4.3−/−). As illustrated in Fig. 2, the simultaneous loss of both the Kv4.2 and Kv4.3 α subunits markedly altered the responses to prolonged depolarizing current injections. Repetitive firing, similar to that seen in WT, Kv4.2−/− and Kv4.3−/− CP neurons, was observed in the majority (16 of 26, ∼60%) of Kv4.2−/−/Kv4.3−/− CP neurons (Fig. 2A). Two-way ANOVA revealed that the input–out curves for this subset of Kv4.2−/−/Kv4.3−/− CP neurons, however, was significantly (P < 0.001) different from the curves for either Kv4.2−/− or Kv4.3−/− CP neurons (Fig. 2C). Bonferroni post hoc analysis further revealed that firing rates in response to both small and large amplitude current injections in Kv4.2−/−/Kv4.3−/− CP neurons were significantly (P < 0.01) higher than in WT neurons, suggesting additive effects of the combined loss of Kv4.2 and Kv4.3 (see Discussion). In the remaining 10 of 26 (∼40%) Kv4.2−/−/Kv4.3−/− CP neurons (labelled Kv4.2−/−/Kv4.3−/− doublets in Fig. 2), spike doublets were observed in response to depolarizing current injections (Fig. 2B). In contrast, none of the WT (n = 20), Kv4.2−/− (n = 30) or Kv4.3−/− (n = 21) CP neurons examined (Fig. 1A) displayed spike doublets in response to depolarizing current injections (see Discussion). The resting and active membrane properties of Kv4.2−/−/Kv4.3−/− CP neurons firing doublets are summarized in Table 1. Because of the marked difference in firing properties, however, Kv4.2−/−/Kv4.3−/− neurons firing doublets were not compared directly to WT, Kv4.2−/−, Kv4.3−/− or Kv4.2−/−/Kv4.3−/− neurons and were not included in the quantitative analyses presented here.
Figure 2. Simultaneous loss of Kv4.2 and Kv4.3 reveals two distinct repetitive firing patterns in CP neurons.
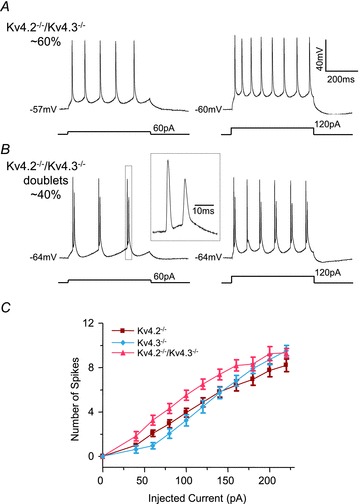
A and B, representative voltage recordings from Kv4.2−/−/Kv4.3−/− CP neurons during prolonged (500 ms) depolarizing current injections of varying amplitudes are illustrated. A, repetitive firing of single action potentials was observed in the majority (∼60%) of Kv4.2−/−/Kv4.3−/− CP neurons. B, in the remaining (∼40%) Kv4.2−/−/Kv4.3−/− CP neurons (Kv4.2−/−/Kv4.3−/− doublets), however, action potential doublets were observed. The inset shows the third spike doublet on an expanded time scale. C, mean ± SEM numbers of action potentials evoked in Kv4.2−/−/Kv4.3−/− CP neurons (A) during 500 ms current injections are plotted as a function of the amplitudes of the injected currents. Mean ± SEM numbers of action potentials in Kv4.2−/− and Kv4.3−/− CP neurons are replotted from Fig. 1C for comparison purposes. The input–output curve (number of spikes vs. injected current amplitude) for Kv4.2−/−/Kv4.3−/− (n = 16) CP neurons were significantly (P < 0.001) different from those of Kv4.2−/− (n = 30) or Kv4.3−/− (n = 21) CP neurons.
Table 1.
Resting and active membrane properties of Kv4.2−/−/Kv4.3−/− CP neurons
| Vm (mV) | Rin (MΩ) | Ithr (pA) | Width (ms) | |
|---|---|---|---|---|
| Kv4.2−/−/Kv4.3−/− | −63 ± 1 | 281 ± 25 | 335 ± 42 | 2.24 ± 0.10 |
| n | 15 | 16 | 15 | 15 |
| Kv4.2−/−/Kv4.3−/− doublets | −59 ± 1 | 213 ± 29 | 180 ± 24 | 1.65 ± 0.06 |
| n | 10 | 10 | 10 | 10 |
All values are means ± SEM; n, number of cells; Vm, resting membrane potential; Rin, input resistance; Ithr, current threshold for action potential generation; Width, action potential duration at 50% repolarization.
Kv4.2 and Kv4.3 play distinct roles in controlling the resting membrane properties of CP neurons
To investigate the functional roles of Kv4.2- and Kv4.3-encoded IA channels in shaping action potential waveforms and in controlling resting membrane potentials, input resistances and current thresholds for action potential generation, single action potentials were elicited by brief (5 ms) depolarizing current injections in WT, Kv4.2−/−, Kv4.3−/− and Kv4.2−/−/Kv4.3−/− layer 5 CP neurons (Fig. 3A). Similar to previous studies on isolated WT neonatal CP neurons (Yuan et al. 2005; Nerbonne et al. 2008), evoked action potentials in mature CP neurons rose rapidly to a maximal potential of ∼+40 mV, then repolarized rapidly (Fig. 3A). In contrast to isolated WT neonatal CP neurons (Yuan et al. 2005; Nerbonne et al. 2008), however, afterdepolarizations (not afterhyperpolarizations) were observed following individual action potentials in mature WT layer 5 CP neurons in slices (Fig. 3A).
Figure 3. Targeted deletion of Kv4.2 or Kv4.3 differentially affects the resting membrane properties of layer 5 CP neurons.
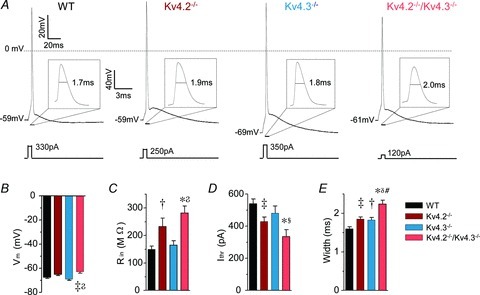
A, representative action potentials, evoked in response to brief (5 ms) current injections in WT, Kv4.2−/−, Kv4.3−/− and Kv4.2−/−/Kv4.3−/− CP neurons are illustrated. The insets show each action potential on an expanded time scale. The amplitudes of the injected currents are given under the voltage records. Resting and active membrane properties were analysed in individual WT (n = 24), Kv4.2−/− (n = 30), Kv4.3−/− (n = 21) and Kv4.2−/−/Kv4.3−/− (n = 15) CP neurons, and mean ± SEM values are presented in B–E. Vm, resting membrane potential; Rin, input resistance; Ithr, current threshold for action potential generation; Width, action potential duration at half-maximum (50%) repolarization. *‡†Values measured in Kv4.2−/−, Kv4.3−/− or Kv4.2−/−/Kv4.3−/− cells were significantly (*P < 0.001; ‡P < 0.01; †P < 0.05) different from those determined in WT cells. In addition, 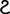
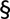
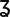 values in Kv4.2−/−/Kv4.3−/− cells were significantly (
values in Kv4.2−/−/Kv4.3−/− cells were significantly (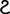 P < 0.001;
P < 0.001; 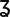 P < 0.01;
P < 0.01; 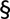 P < 0.05) different from those in Kv4.3−/− cells, and #values in Kv4.2−/−/Kv4.3−/− cells were significantly (#P < 0.001) different from Kv4.2−/− cells.
P < 0.05) different from those in Kv4.3−/− cells, and #values in Kv4.2−/−/Kv4.3−/− cells were significantly (#P < 0.001) different from Kv4.2−/− cells.
Visual inspection revealed that the waveforms of individual action potentials in Kv4.2−/− and Kv4.3−/− CP neurons were qualitatively similar to those in WT neurons, rising rapidly to a maximal potential of ∼+40 mV, then repolarizing rapidly, and with afterdepolarization following the individual action potentials (Fig. 3A). Quantitative analyses further revealed that the mean ± SEM resting membrane potentials (Fig. 3B) as well as the action potential afterdepolarizations (data not shown) of Kv4.2−/− (n = 30) and Kv4.3−/− (n = 21) CP neurons were indistinguishable from WT (n = 24) CP neurons. Further analyses, however, revealed that the in vivo loss of Kv4.2 or Kv4.3 differentially altered the intrinsic membrane properties of mature CP neurons (Fig. 3C and D). The mean ± SEM input resistance was significantly (P < 0.05) higher, for example, and the mean ± SEM current threshold for action potential generation was significantly (P < 0.01) lower, in Kv4.2−/− (n = 30), than in WT (n = 24), CP neurons (Fig. 3C and D). In contrast, input resistances and current thresholds for action potential generation in Kv4.3−/− (n = 21) CP neurons were indistinguishable from those in WT (n = 24) CP neurons (Fig. 3C and D).
The mean ± SEM input resistance determined in Kv4.2−/−/Kv4.3−/− (n = 16) CP neurons was also significantly (P < 0.001) higher than in WT (n = 24) and Kv4.3−/− (n = 21) CP neurons (Fig. 3C). Similarly, the mean ± SEM current threshold for action potential generation was significantly (P < 0.05) lower in Kv4.2−/−/Kv4.3−/− (n = 16) than in WT (n = 24) or Kv4.3−/− (n = 21) CP neurons. The mean ± SEM current thresholds to evoke action potentials were similar, however, in Kv4.2−/− (n = 30) and Kv4.2−/−/Kv4.3−/− (n = 16) CP neurons (Fig. 3D). Taken together, these results suggest that Kv4.2- (but not Kv4.3-) encoded IA channels regulate input resistances and current thresholds for action potential generation in mature CP neurons. Interestingly, the mean ± SEM resting membrane potential in Kv4.2−/−/Kv4.3−/− (n = 16) CP neurons was also significantly (P < 0.01) more depolarized than in WT (n = 24) or Kv4.3−/− (n = 21) CP neurons, but was similar to that measured in Kv4.2−/− (n = 30) CP neurons (Fig. 3B). Importantly, in Kv4.2−/−/Kv4.3−/− CP neurons, input resistances and current thresholds for action potential generation measured from a membrane potential of −70 mV, which corresponds to the average resting membrane potential of WT cells, were indistinguishable from those measured from the resting membrane potential (data not shown). The alterations in the input resistances and the current thresholds for action potential generation determined in Kv4.2−/−/Kv4.3−/− CP neurons, therefore, cannot be attributed to the slightly depolarized resting membrane potentials of these cells.
Analyses of action potential durations (widths at half-maximum) in Kv4.2−/−, Kv4.3−/− and Kv4.2−/−/Kv4.3−/− neurons revealed that loss of either Kv4.2 or Kv4.3 affected action potential repolarization in mature CP neurons (Fig. 3E). The mean ± SEM action potential width at half-maximum was significantly (P < 0.05) longer, for example, in Kv4.2−/− (n = 30) and Kv4.3−/− (n = 21) than in WT (n = 24) CP neurons (Fig. 3E). In addition, action potential widths at half-maximum measured in Kv4.2−/−/Kv4.3−/− (n = 16) CP neurons (when action potentials are elicited from rest or from −70 mV) were significantly (P < 0.01) longer than in either Kv4.2−/− (n = 30) or Kv4.3−/− (n = 21) CP neurons, suggesting that Kv4.2 and Kv4.3 independently contribute to action potential repolarization (see Discussion).
Blockade of TEA-sensitive K+ currents markedly prolongs action potential waveforms in Kv4.2−/− CP neurons
The prolongation of action potentials evident in mature CP neurons with the simultaneous in vivo loss of both Kv4.2 and Kv4.3 (Fig. 3E) is modest compared to the effects reported in previous in vitro studies that used pharmacological approaches to block IA or dominant negative strategies to reduce Kv4-encoded currents (Locke & Nerbonne, 1997; Malin & Nerbonne, 2000; Shibata et al. 2000; Hu & Gereau IV, 2003; Kim et al. 2005; Yuan et al. 2005). Previous studies on neonatal Kv4.2−/− CP neurons, however, demonstrated that TEA-sensitive delayed rectifier K+ currents (IK) are larger in Kv4.2−/− than in WT cells (Nerbonne et al. 2008; Norris & Nerbonne, 2010), suggesting that the upregulation of IK partially compensates for the loss of Kv4.2 to normalize action potential waveforms in Kv4.2−/− neurons. To examine directly the role of TEA-sensitive K+ currents in shaping the waveforms of individual action potentials in mature CP neurons, the intrinsic membrane properties of WT, Kv4.2−/− and Kv4.3−/− layer 5 CP neurons measured in the absence and presence of 3 mm TEA were compared (Fig. 4). At this low concentration, TEA has been previously reported to partially attenuate IK without affecting IA in isolated CP neurons (Nerbonne et al. 2008; Norris & Nerbonne, 2010). Importantly, in the presence of 3 mm TEA, IK densities are indistinguishable in isolated WT, Kv4.2−/− and Kv4.3−/− CP neurons (unpublished observations).
Figure 4. TEA markedly prolongs action potential waveforms in Kv4.2−/− CP neurons.
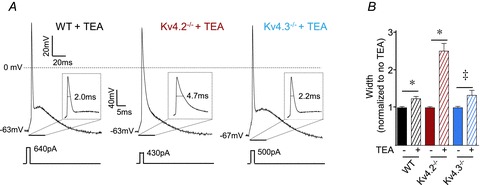
A, representative action potential waveforms evoked by brief (5 ms) current injections in WT, Kv4.2−/− and Kv4.3−/− layer 5 CP neurons in the presence of 3 mm TEA. B, action potential widths at half-maximum (50%) repolarization in individual WT (n = 10), Kv4.2−/− (n = 11) and Kv4.3−/− (n = 8) CP neurons in the presence or absence of 3 mm TEA were normalized to the average action potential widths measured in neurons of the same genotype in the absence of TEA; mean ± SEM action potential widths at half-maximum are presented. TEA significantly (*P < 0.001; ‡P < 0.01) increased action potential durations in WT, Kv4.2−/− and Kv4.3−/− CP neurons. In addition, the magnitude of the effect of TEA on action potential durations was significantly (P < 0.001) larger in Kv4.2−/− than in either WT or Kv4.3−/− CP neurons.
Bath applications of TEA (3 mm) did not measurably affect resting membrane potentials, input resistances or the current thresholds for action potential generation in CP neurons of any of the genotypes examined (data not shown). Analyses of action potential durations, however, revealed that the mean ± SEM action potential width at half-maximum was significantly (P < 0.01) longer in WT (n = 10), Kv4.2−/− (n = 11) and Kv4.3−/− (n = 8) CP neurons in the presence of TEA (3 mm) than in CP neurons of the same genotype in the absence of TEA (Fig. 4B). Importantly, as illustrated in Fig. 4, the TEA-induced increases in action potential durations were significantly (P < 0.001) larger in Kv4.2−/− (∼2.5-fold) than in WT (∼1.2-fold) or Kv4.3−/− (∼1.3-fold) CP neurons. In contrast, TEA-induced increases in action potential durations (Fig. 4B) were indistinguishable in WT and Kv4.3−/− CP neurons (see Discussion).
Interestingly, bath applications of TEA (3 mm) also revealed that Kv4.2, but not Kv4.3, modulates rebound firing following prolonged (500 ms) hyperpolarizing current (−300 pA) injections in mature CP neurons (data not shown). In the presence of TEA (3 mm), for example, firing of one action potential was observed in the majority (8 of 11, ∼70%) of Kv4.2−/− CP neurons examined, while only 1 of 10 (∼10%) WT and 1 of 8 (∼10%) Kv4.3−/− CP neurons displayed rebound firing. In contrast, in the absence of TEA (3 mm), rebound firing was rarely observed in WT (0 of 20), Kv4.2−/− (3 of 30) and Kv4.3−/− (1 of 21) CP neurons.
Upregulation of Kv4-encoded IA functionally compensates for the in vivo loss of Kv1.4
Unexpectedly, recent studies revealed that Kv1.4 also contributes to macroscopic IA in isolated neonatal CP neurons (Norris & Nerbonne, 2010). To examine the possible contributions of Kv1.4 to the intrinsic excitability of mature CP neurons, recordings were obtained from layer 5 CP cells in slices prepared from mice (Kv1.4−/−) lacking Kv1.4. As illustrated in Fig. 5A, prolonged (500 ms) depolarizing current injections elicited repetitive firing in Kv1.4−/− CP neurons that was indistinguishable from that observed in WT CP cells. Further analyses revealed that the in vivo loss of Kv1.4 did not significantly alter firing rates in mature CP neurons (Fig. 5A). As illustrated in Fig. 5B, the waveforms of individual action potentials were also qualitatively similar in mature WT and Kv1.4−/− CP neurons. In addition, mean ± SEM resting membrane potentials, input resistances and current thresholds for action potential generation in WT (n = 24) and Kv1.4−/− (n = 13) CP neurons were indistinguishable (Table 2).
Figure 5. Intrinsic properties of Kv1.4−/− and WT CP neurons are similar.
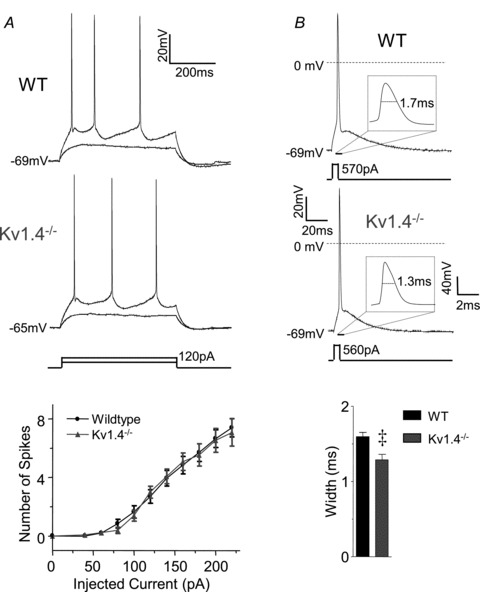
A, representative recordings from WT and Kv1.4−/− CP neurons in response to prolonged (500 ms) depolarizing current injections of varying amplitudes are shown. The mean ± SEM numbers of action potentials evoked during 500 ms current injections in WT (n = 24) and Kv1.4−/− (n = 13) CP neurons are similar at all current injection amplitudes. B, representative single action potential waveforms evoked in response to brief (5 ms) depolarizing current injections in WT and Kv1.4−/− CP neurons. The mean ± SEM action potential width at half-maximum was significantly (‡P < 0.01) shorter in Kv1.4−/− (n = 13) than in WT (n = 24) CP neurons (see text).
Table 2.
Resting and active membrane properties of WT and Kv1.4−/− CP neurons
| Vm (mV) | Rin (MQ) | Ithr (PA) | Width (ms) | |
|---|---|---|---|---|
| WT | −68 ± 1 | 149 ± 12 | 540 ± 30 | 1.60 ± 0.06 |
| n | 24 | 24 | 24 | 24 |
| Kv1.4−/− | −66 ± 1 | 140 ± 20 | 476 ± 26 | 1.29 ± 0.07* |
| n | 13 | 16 | 13 | 13 |
| WT + Ba2+ | −60 ± 2* | 422 ± 84* | 396 ± 35* | 3.56 ± 0.15* |
| n | 10 | 10 | 10 | 10 |
| Kv1.4−/−+ Ba2+ | −52 ± 2#† | 460 ± 77* | 213 ± 62#§ | 3.82 ± 0.35* |
| n | 6 | 6 | 6 | 6 |
| WT + Ba2+ doublets | −56 ± 3 | 169 ± 20 | 390 ± 64 | 3.13 ± 0.14 |
| n | 6 | 6 | 6 | 6 |
| Kv1.4−/−+ Ba2+ doublets | −58 ± 1 | 274 ± 82 | 276 ± 31 | 3.22 ± 0.19 |
| n | 7 | 7 | 7 | 7 |
All values are means ± SEM; n, number of cells; Vm, resting membrane potential; Rin, input resistance; Ithr, current threshold for action potential generation; Width, action potential duration at 50% repolarization. **Values indicated are significantly (*P < 0.01; *P < 0.001) different from WT. In addition, #values indicated are significantly (#P < 0.001) different from Kv1.4−/−. †§Values indicated are significantly (§P < 0.05; †P < 0.001) different from WT + Ba2+.
Although these combined observations suggest that Kv1.4 does not contribute to the resting and active membrane properties of mature CP neurons, action potential durations were significantly (P < 0.01) affected in Kv1.4−/− CP neurons (Fig. 5). Unexpectedly, however, the mean ± SEM action potential width at half-maximum was actually shorter in Kv1.4−/− (n = 13) than in WT (n = 24) CP neurons (Fig. 5B). An alternative interpretation of the lack of effect of loss of Kv1.4 on the intrinsic membrane properties of these cells, therefore, is that upregulation of other K+ currents compensates functionally for the in vivo loss of Kv1.4. Indeed, previous studies suggested that Kv4-encoded IA is upregulated in isolated neonatal Kv1.4−/− CP neurons (Norris & Nerbonne, 2010). To explore this hypothesis directly, the properties of WT and Kv1.4−/− layer 5 CP neurons were examined in the absence and presence of 400 μm Ba2+, which has been previously reported to reduce Kv4-encoded transient outward K+ currents in cardiomyocytes (Li et al. 1998; Li et al. 2000). In pyramidal neurons, Ba2+ (150–400 μm) attenuates Kv4-encoded A-type currents without affecting the Kv1.4-encoded IA (Gasparini et al. 2007; Norris & Nerbonne, 2010). Consistent with the Kv4 specificity of 400 μm Ba2+, the Ba2+-resistant component of IA was shown to be significantly larger in WT than in Kv1.4−/− CP neurons (Norris & Nerbonne, 2010).
Exposure to 400 μm Ba2+ increased the excitability of both WT and Kv1.4−/− CP neurons (Fig. 6, Table 2). Repetitive firing of single action potentials was observed in 10 of 16 (∼60%) WT and 6 of 13 (∼50%) Kv1.4−/− CP neurons in response to prolonged depolarizing current injections in the presence of 400 μm Ba2+ (Fig. 6A). Although firing rates were significantly (P < 0.001) higher in both cell types in the presence of 400 μm Ba2+, the rates were significantly (P < 0.001) higher in Kv1.4−/− than in WT CP neurons (Fig. 6B). In addition, in the presence of 400 μm Ba2+, spontaneous firing was observed in 4 of 6 (∼65%) Kv1.4−/− CP neurons, but not in any of the WT CP neurons examined (Fig. 6B). In the remaining 6 of 16 (∼40%) WT and 7 of 13 (∼50%) Kv1.4−/− CP neurons exposed to 400 μm Ba2+, spike doublets, similar to those observed in the Kv4.2−/−/Kv4.3−/− neurons firing doublets (see Fig. 2B), were observed in response to depolarizing current injections (see Discussion). The resting and active membrane properties of WT and Kv1.4−/− CP neurons firing spike doublets in the presence of Ba2+ are summarized in Table 2. As with the Kv4.2−/−/Kv4.3−/− cells firing doublets described above (Fig. 2B), data from WT and Kv1.4−/− CP neurons firing spike doublets in the presence of Ba2+ were not included in the quantitative analyses presented here.
Figure 6. Ba2+ blockade of Kv4-encoded IA reveals a functional role for Kv1.4 in regulating the excitability of layer 5 CP neurons.
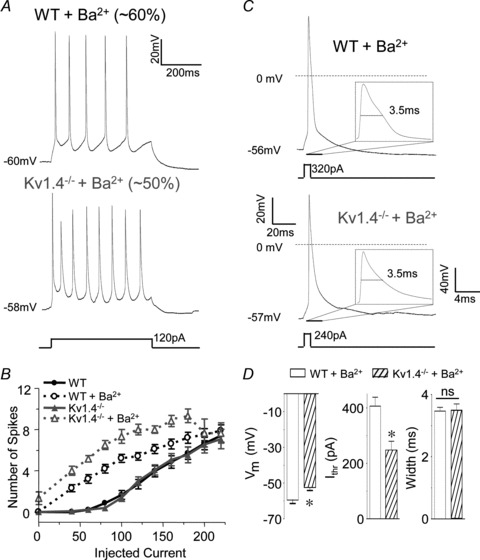
A, representative voltage recordings from WT and Kv1.4−/− CP neurons in the presence of 400 μm Ba2+ in response to prolonged (500 ms) depolarizing current injections are illustrated. Repetitive firing of single action potentials was evoked in the majority (∼60%) of WT and in ∼50% of Kv1.4−/− CP neurons in the presence of Ba2+ (see text). B, the mean ± SEM numbers of action potentials evoked in WT and Kv1.4−/− CP neurons in the presence and absence of Ba2+ are plotted as a function of the amplitudes of the injected currents; mean ± SEM numbers of action potentials in WT and Kv1.4−/− CP neurons in the absence of Ba2+ are replotted from Fig. 5A for comparison. Firing rates in WT (n = 10) and Kv1.4−/− (n = 6) CP neurons in the presence of Ba2+ were significantly (P < 0.001) higher than in WT (n = 24) and Kv1.4−/− (n = 13) CP neurons in the absence of Ba2+. In addition, in the presence of Ba2+, mean ± SEM firing rates were significantly (P < 0.001) higher in Kv1.4−/− than in WT CP neurons. C, representative single action potentials evoked in response to brief (5 ms) depolarizing current injections in WT and Kv1.4−/− CP neurons in the presence of 400 μm Ba2+. D, in the presence of Ba2+, the mean ± SEM current threshold for action potential generation (Ithr) was significantly (P < 0.001) lower in Kv1.4−/− (n = 10) than in WT (n = 6) CP neurons, whereas the mean ± SEM action potential widths at half-maximum were not significantly different.
The effects of 400 μm Ba2+ on the resting and active membrane properties of WT and Kv1.4−/− CP neurons are summarized in Table 2. In both WT and Kv1.4−/− CP neurons, bath application of 400 μm Ba2+ significantly (P < 0.01) depolarized resting membrane potentials, increased input resistances and decreased current thresholds for action potential generation (Table 2). In the presence of 400 μm Ba2+, the input resistances of WT and Kv1.4−/− CP neurons were indistinguishable (Table 2). The mean ± SEM resting membrane potential, however, was significantly (P < 0.001) more depolarized and the mean ± SEM current threshold for action potential generation was significantly (P < 0.001) lower in Kv1.4−/− than in WT CP neurons (Fig. 6D). Further analyses of individual action potential waveforms revealed that action potential widths at half-maximum were prolonged significantly (P < 0.001) in the presence of 400 μm Ba2+ in both WT and Kv1.4−/− CP neurons (Table 2), but that action potential widths at half-maximum in WT and Kv1.4−/− CP neurons in the presence of 400 μm Ba2+ were indistinguishable (Fig. 6D, Table 2). Acute pharmacological blockade of Kv4-encoded IA with 400 μm Ba2+, therefore, differentially affects the membrane properties of WT and Kv1.4−/− CP neurons, unmasking the roles of Kv1.4-encoded IA channels in controlling the resting membrane potentials, the current thresholds for action potential generation and the repetitive firing rates of mature CP neurons. In contrast, Kv1.4-encoded IA channels do not appear to contribute functionally to action potential repolarization in these cells (see Discussion).
Discussion
Kv4.2, Kv4.3 and Kv1.4 generate functional IA channels in mature CP neurons
The results presented here demonstrate that the Kv4.2, Kv4.3 and Kv1.4 α subunits all contribute to the generation of functional IA channels in mature CP neurons. The findings presented here also suggest, however, that IA channels encoded by the individual α subunits function over different voltage ranges and play distinct functional roles in the regulation of the intrinsic excitability of mature CP neurons. To the best of our knowledge, this is the first demonstration that native Kv4.3- and Kv1.4-encoded IA channels regulate the resting and active membrane properties of mature mammalian pyramidal neurons.
Functionally distinct Kv.2- and Kv4.3-encoded IA channels in CP neurons
Immunohistochemical studies demonstrated that Kv4.2 and Kv4.3 are expressed in partially overlapping clusters in the soma, dendrites and spines of adult mouse CP neurons (Burkhalter et al. 2006). In addition, Kv4.2 and Kv4.3 co-immunoprecipitate from adult mouse brain lysates (Marionneau et al. 2009). Together, these observations have been interpreted as suggesting that Kv4.2 and Kv4.3 are likely to assemble to form heteromeric IA channels in CP neurons (Burkhalter et al. 2006; Marionneau et al. 2009). The results presented here, however, demonstrate that the functional consequences of the targeted deletion of the Kv4.2 and Kv4.3 α subunits on the properties of mature CP neurons are distinct. The in vivo loss of Kv4.2, for example, alters the input resistances, the current thresholds for action potential generation and the repetitive firing properties of CP neurons. Loss of Kv4.3 also affects the firing properties of mature CP cells (Fig. 1), but does not affect the input resistances or the current thresholds for action potential generation of these cells (Fig. 3). In addition, the resting properties of Kv4.2−/−/Kv4.3−/− CP neurons are indistinguishable from those of Kv4.2−/− CP neurons, consistent with a distinct role for Kv4.2-encoded IA channels in controlling the input resistances and current thresholds for action potential generation of mature CP neurons.
The results presented here also suggest that the biophysical properties of native Kv4.2- and Kv4.3-encoded IA channels are distinct in mature CP neurons. Previous studies have demonstrated marked differences in the voltage-dependent properties of heterologously expressed Kv4.2- and Kv4.3-encoded IA channels (Serodio et al. 1994, 1996; Guo et al. 2002). The higher input resistances and lower current thresholds for action potential generation observed in Kv4.2−/−, compared with WT, CP neurons suggest that Kv4.2-encoded, but not Kv4.3-encoded, IA channels are open at subthreshold membrane potentials in these cells. Studies on hippocampal CA1 pyramidal neurons have demonstrated the presence of a Kv4.2-encoded IA window current at potentials between −75 and −40 mV (Kim et al. 2005). The loss of Kv4.2-encoded IA channels, however, did not result in a significantly different mean ± SEM resting membrane potential, although there was a trend for more depolarized resting membrane potentials in Kv4.2−/− than in WT CP neurons. Interestingly, however, the mean ± SEM resting membrane potential in CP neurons lacking both Kv4.2 and Kv4.3 was more depolarized than Kv4.2−/−, Kv4.3−/− or WT CP neurons.
Neuronal Kv4-encoded IA channels recover rapidly from inactivation (Jerng et al. 2004; Yuan et al. 2005; Nerbonne et al. 2008). During low frequency repetitive firing, for example, IA recovers from inactivation between spikes, functioning to regulate repetitive firing rates (Connor & Stevens, 1971; Kim et al. 2005; Yuan et al. 2005). Interestingly, the experiments here demonstrate that although repetitive firing is affected by the loss of either Kv4.2 or Kv4.3 in mature CP neurons, the responses of Kv4.2−/− and Kv4.3−/− CP neurons to prolonged depolarizing current injections are distinct (Fig. 1). In vivo loss of Kv4.2, for example, increased firing rates in response to small, but not large, amplitude depolarizing current injections, whereas the loss of Kv4.3 resulted in increased firing only during large amplitude current injections (Fig. 1B). With the combined loss of both Kv4.2 and Kv4.3, firing rates were increased significantly, compared with cells lacking only Kv4.2 or Kv4.3, over the entire range of depolarizing current injections (Fig. 2B). The differential responses of Kv4.2−/− and Kv4.3−/− CP neurons to small and large amplitude depolarizing current injections suggest that the kinetics of recovery from inactivation in native Kv4.2- and Kv4.3-encoded IA channels are distinct in mature CP neurons. The experimental observations here suggest that, although both Kv4.2- and Kv4.3-encoded IA channels recover rapidly from inactivation, recovery is faster in Kv4.3- than in Kv4.2-encoded channels, such that at higher firing frequencies, Kv4.3-encoded channels recover from inactivation between spikes whereas Kv4.2-encoded channels only recover between spikes at lower firing frequencies. Consistent with this hypothesis, previous studies have shown faster recovery from inactivation in heterologously expressed Kv4.3- compared with Kv4.2-encoded channels (Guo et al. 2002). The experiments presented here also revealed that firing rates in response to low amplitude depolarizing current injections are unaffected in Kv4.3−/− CP neurons, suggesting that other channels, like Kv4.2-encoded channels, are the primary determinants of firing rates in response to low amplitude depolarizing current injections. Together, these results are consistent with the suggestion that the biophysical properties of native Kv4.2- and Kv4.3-encoded IA channels are distinct in mature CP neurons, differentially affecting the resting and active membrane properties of these cells (Table 3).
Table 3.
Distinctroles of Kv4.2-, Kv4.3- and Kv1.4-encoded IA channels in the regulation of the resting and active membrane properties of mature CP neurons
| Vm | Rin | Ithr | Action potential durations | Firing frequency (small amplitude current injections) | Firing frequency (large amplitude current injections) | |
|---|---|---|---|---|---|---|
| Kv4.2 | × | × | × | × | × | |
| Kv4.3 | × | × | ||||
| Kv1.4 | × | × | × | × |
Individual action potential waveforms were prolonged in Kv4.2−/−, Kv4.3−/− and Kv4.2−/−/Kv4.3−/− CP neurons (Fig. 3E). The magnitude of the prolongation was similar in Kv4.2−/− and Kv4.3−/− CP neurons, but was significantly larger in Kv4.2−/−/Kv4.3−/− CP neurons, demonstrating that both Kv4.2- and Kv.3-encoded IA channels contribute to action potential repolarization. Interestingly, although blocking TEA-sensitive delayed rectifier K+ currents (IK) significantly increased action potential durations in both Kv4.2−/− and Kv4.3−/− CP neurons, the magnitude of the effect of TEA was substantially larger in Kv4.2−/− than in Kv4.3−/− (or WT) CP neurons (Fig. 4). Taken together, these results demonstrate that the Kv4.2-encoded IA channels are the major determinants of action potential repolarization in CP neurons, but that the previously reported upregulation of IK densities (Nerbonne et al. 2008; Norris & Nerbonne, 2010) functionally compensates for the in vivo loss of Kv4.2. Action potential waveforms in Kv4.3−/− and WT CP neurons in the presence of TEA are similar (Fig. 4), revealing differential electrical remodelling in response to the in vivo loss of Kv4.2 or Kv4.3.
The combined in vivo loss of Kv4.2 and Kv4.3 as well as the acute pharmacological blockade of Kv4-encoded IA with 400 μm Ba2+ in WT and Kv1.4−/− CP neurons unexpectedly revealed novel firing patterns in a subset of layer 5 CP neurons (Fig. 2A). These results clearly suggest differences in the intrinsic properties of layer 5 CP neurons that are only revealed when Kv4-encoded channels are eliminated. Previous studies have demonstrated that the morphology, intrinsic membrane and firing properties of layer 5 CP neurons projecting to different targets are distinct (Hattox & Nelson, 2007). It is therefore possible that the heterogeneity in the firing properties of layer 5 CP neurons observed in the present study represents morphologically distinct neurons that project to different targets. Additional experiments will be necessary to identify the anatomical and ionic mechanisms underlying the observed phenotypic heterogeneity.
Functional roles of Kv1.4-encoded IA channels in mature CP neurons
Combining genetic and pharmacological approaches, the experiments presented here have also revealed multiple functional roles for Kv1.4-encoded IA channels in regulating repetitive firing rates and in determining resting membrane potentials and the current thresholds for action potential generation in mature CP neurons (Fig. 6, Table 3). Previous studies have reported that Kv4-encoded IA densities are upregulated in Kv1.4−/− CP neurons (Norris & Nerbonne, 2010). The experiments here demonstrate that the upregulation of Kv4-encoded current densities functionally affects the excitable membrane properties of Kv1.4−/− CP neurons. Interestingly, the upregulation of Kv4-encoded IA densities actually decreased action potential durations in Kv1.4−/− CP neurons (Fig. 5B), further demonstrating the pivotal role of Kv4.2-encoded IA channels in action potential repolarization in mature CP neurons. Blockade of Kv4-encoded IA with 400 μm Ba2+ resulted in similar action potential durations in WT and Kv1.4−/− neurons (Fig. 6D), revealing that the Kv1.4-encoded component of IA is not a major contributor to action potential repolarization in mature CP neurons (Table 3).
Blockade of Kv4-encoded IA with 400 μm Ba2+ also revealed that the in vivo loss of Kv1.4 depolarizes the resting membrane potential and decreases the current thresholds for action potential generation. These observations suggest that Kv1.4-encoded IA channels operate at subthreshold membrane potentials in mature CP neurons (Table 3), consistent with previous studies demonstrating a Kv1.4-encoded window current at potentials between −70 and −40 mV (Roeper et al. 1997). It is important to note here that inwardly rectifying K+ channels are also blocked by Ba2+ (Schoots et al. 1996; Hughes et al. 2000), suggesting that some of the observed effects of Ba2+ could be due to reduced inwardly rectifying K+ currents. The Ba2+-resistant component of IA, however, is larger in WT than in Kv1.4−/− CP neurons (Norris et al. 2010), suggesting that the differential effects of Ba2+ on the intrinsic membrane properties of WT and Kv1.4−/− neurons are due to the loss of the Kv1.4-encoded component of IA in Kv1.4−/− CP neurons.
The results presented here further demonstrated that spontaneous firing occurs in the majority (∼65%) of Kv1.4−/− CP neurons when Kv4-encoded IA was blocked pharmacologically by the addition of 400 μm Ba2+, indicating that Kv1.4-encoded IA channels, operating at subthreshold membrane potentials, also function to prevent spontaneous firing in mature CP neurons. The observation that the remaining (∼35%) Kv1.4−/− CP neurons did not fire spontaneously in the presence of 400 μm Ba2+ is consistent with the suggestion above that there are subtle, but functionally relevant, differences in the intrinsic properties of mature CP neurons. Additional experiments will be required to determine the ionic mechanisms underlying this heterogeneity.
Acknowledgments
This work was supported by National Institutes of Neurological Disorders and Stroke (RO1-NS041417 and RO1-NS065295) (J.M.N.) of the NIH. Y.C. was supported by the Institutional Training Grant T32-HL007275 and the Individual National Research Service Award F32-NS065581. The authors also thank Mr Rick Wilson for maintaining and genotyping mice used in the studies detailed here.
Glossary
- CP
cortical pyramidal
- IA
A-type K+ current
- Kv
voltage-gated K+
- TLE
temporal lobe epilepsy
- TEA
tetraethylammonium
- Vm
resting membrane potentials
- Rin
input resistance
- IK
delayed rectifier K+ current
Author contributions
The conception and design of this study was by Y.C., A.B. and J.M.N. Experiments were carried out by Y.C. The data were analysed and interpreted by Y.C., A.B. and J.M.N. All authors contributed to writing the manuscript, and approved the final version for publication.
References
- Andrasfalvy BK, Makara JK, Johnston D, Magee JC. Altered synaptic and non-synaptic properties of CA1 pyramidal neurons in Kv4.2 knockout mice. J Physiol. 2008;586:3881–3892. doi: 10.1113/jphysiol.2008.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter A, Gonchar Y, Mellor RL, Nerbonne JM. Differential expression of IA channel subunits Kv4.2 and Kv4.3 in mouse visual cortical neurons and synapses. J Neurosci. 2006;26:12274–12282. doi: 10.1523/JNEUROSCI.2599-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE, Schwarz TL, Sweatt JD, Johnston D. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:12143–12151. doi: 10.1523/JNEUROSCI.2667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971;213:31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie JT, Kole MH, Letzkus JJ, Rancz EA, Spruston N, Stuart GJ, Hausser M. Dendritic patch-clamp recording. Nat Protoc. 2006;1:1235–1247. doi: 10.1038/nprot.2006.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Shao Z, Nerbonne JM, Burkhalter A. Differential depression of inhibitory synaptic responses in feedforward and feedback circuits between different areas of mouse visual cortex. J Comp Neurol. 2004;475:361–373. doi: 10.1002/cne.20164. [DOI] [PubMed] [Google Scholar]
- Francis J, Jugloff DG, Mingo NS, Wallace MC, Jones OT, Burnham WM, Eubanks JH. Kainic acid-induced generalized seizures alter the regional hippocampal expression of the rat Kv4.2 potassium channel gene. Neuroscience Lett. 1997;232:91–94. doi: 10.1016/s0304-3940(97)00593-4. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Losonczy A, Chen X, Johnston D, Magee JC. Associative pairing enhances action potential back-propagation in radial oblique branches of CA1 pyramidal neurons. J Physiol. 2007;580:787–800. doi: 10.1113/jphysiol.2006.121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM. Targeted deletion of Kv4.2 eliminates Ito,f and results in electrical and molecular remodeling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, Nerbonne JM. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res. 2002;90:586–593. doi: 10.1161/01.res.0000012664.05949.e0. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Gereau RW., IV ERK integrates PKA and PKC signaling in superficial dorsal horn neurons. II. Modulation of neuronal excitability. J Neurophysiol. 2003;90:1680–1688. doi: 10.1152/jn.00341.2003. [DOI] [PubMed] [Google Scholar]
- Hughes BA, Kumar G, Yuan Y, Swaminathan A, Yan D, Sharma A, Plumley L, Yang-Feng TL, Swaroop A. Cloning and functional expression of human retinal kir2.4, a pH-sensitive inwardly rectifying K+ channel. Am J Physiol Cell Physiol. 2000;279:C771–784. doi: 10.1152/ajpcell.2000.279.3.C771. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Kim DS, Kim JE, Kwak SE, Won MH, Kang TC. Seizure activity affects neuroglial Kv1 channel immunoreactivities in the gerbil hippocampus. Brain Res. 2007;1151:172–187. doi: 10.1016/j.brainres.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol. 2005;569:41–57. doi: 10.1113/jphysiol.2005.095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GR, Sun H, Nattel S. Characterization of a transient outward K+ current with inward rectification in canine ventricular myocytes. Am J Physiol Cell Physiol. 1998;274:C577–585. doi: 10.1152/ajpcell.1998.274.3.C577. [DOI] [PubMed] [Google Scholar]
- Li GR, Yang B, Sun H, Baumgarten CM. Existence of a transient outward K+ current in guinea pig cardiac myocytes. Am J Physiol Heart Circ Physiol. 2000;279:H130–138. doi: 10.1152/ajpheart.2000.279.1.H130. [DOI] [PubMed] [Google Scholar]
- Locke RE, Nerbonne JM. Role of voltage-gated K+ currents in mediating the regular-spiking phenotype of callosal-projecting rat visual cortical neurons. J Neurophysiol. 1997;78:2321–2335. doi: 10.1152/jn.1997.78.5.2321. [DOI] [PubMed] [Google Scholar]
- London B, Wang DW, Hill JA, Bennett PB. The transient outward current in mice lacking the potassium channel gene Kv1.4. J Physiol. 1998;509:171–182. doi: 10.1111/j.1469-7793.1998.171bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Barnwell LF, Ren Y, Lee WL, Johnston LD, Kim R, Hrachovy RA, Sweatt JD, Anderson AE. Altered phosphorylation and localization of the A-type channel, Kv4.2 in status epilepticus. J Neuroch. 2008;106:1929–1940. doi: 10.1111/j.1471-4159.2008.05508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM. Elimination of the fast transient in superior cervical ganglion neurons with expression of KV4.2W362F: molecular dissection of IA. J Neurosci. 2000;20:5191–5199. doi: 10.1523/JNEUROSCI.20-14-05191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marionneau C, LeDuc RD, Rohrs HW, Link AJ, Townsend RR, Nerbonne JM. Proteomic analyses of native brain KV4.2 channel complexes. Channels (Austin) 2009;3:284–294. doi: 10.4161/chan.3.4.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan MM, Menegola M, Vacher H, Rhodes KJ, Trimmer JS. Altered expression and localization of hippocampal A-type potassium channel subunits in the pilocarpine-induced model of temporal lobe epilepsy. Neuroscience. 2008;156:550–562. doi: 10.1016/j.neuroscience.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerbonne JM, Gerber BR, Norris A, Burkhalter A. Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents. J Physiol. 2008;586:1565–1579. doi: 10.1113/jphysiol.2007.146597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa N, Wang W, Sha Q, Marionneau C, Nerbonne JM. Kv4.3 is not required for the generation of functional Ito,f channels in adult mouse ventricles. J Mol Cell Cardiol. 2008;44:95–104. doi: 10.1016/j.yjmcc.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Foeger NC, Nerbonne JM. Interdependent roles for accessory KChIP2, KChIP3, and KChIP4 subunits in the generation of Kv4-encoded IA channels in cortical pyramidal neurons. J Neurosci. 2010;30:13644–13655. doi: 10.1523/JNEUROSCI.2487-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris AJ, Nerbonne JM. Molecular dissection of IA in cortical pyramidal neurons reveals three distinct components encoded by Kv4.2, Kv4.3, and Kv1.4 α-subunits. J Neurosci. 2010;30:5092–5101. doi: 10.1523/JNEUROSCI.5890-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeper J, Lorra C, Pongs O. Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J Neurosci. 1997;17:3379–3391. doi: 10.1523/JNEUROSCI.17-10-03379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoots O, Yue KT, MacDonald JF, Hampson DR, Nobrega JN, Dixon LM, Van Tol HH. Cloning of a G protein-activated inwardly rectifying potassium channel from human cerebellum. Brain Res. 1996;39:23–30. doi: 10.1016/0169-328x(95)00349-w. [DOI] [PubMed] [Google Scholar]
- Serodio P, Kentros C, Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. J Neurophysiol. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Serodio P, Vega-Saenz de Miera E, Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. J Neurophysiol. 1996;75:2174–2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J Neurosci. 2000;20:4145–4155. doi: 10.1523/JNEUROSCI.20-11-04145.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Ogiwara I, Kaneda M, Tokonami N, Mazaki E, Baba K, Matsuda K, Inoue Y, Yamakawa K. A Kv4.2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiol Dis. 2006;24:245–253. doi: 10.1016/j.nbd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Yuan W, Burkhalter A, Nerbonne JM. Functional role of the fast transient outward K+ current IA in pyramidal neurons in (rat) primary visual cortex. J Neurosci. 2005;25:9185–9194. doi: 10.1523/JNEUROSCI.2858-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


