Abstract
Recent work has shown that the primate reticulospinal tract can influence spinal interneurons and motoneurons involved in control of the hand. However, demonstrating connectivity does not reveal whether reticular outputs are modulated during the control of different types of hand movement. Here, we investigated how single unit discharge in the pontomedullary reticular formation (PMRF) modulated during performance of a slow finger movement task in macaque monkeys. Two animals performed an index finger flexion–extension task to track a target presented on a computer screen; single units were recorded both from ipsilateral PMRF (115 cells) and contralateral primary motor cortex (M1, 210 cells). Cells in both areas modulated their activity with the task (M1: 87%, PMRF: 86%). Some cells (18/115 in PMRF; 96/210 in M1) received sensory input from the hand, showing a short-latency modulation in their discharge following a rapid passive extension movement of the index finger. Effects in ipsilateral electromyogram to trains of stimuli were recorded at 45 sites in the PMRF. These responses involved muscles controlling the digits in 13/45 sites (including intrinsic hand muscles, 5/45 sites). We conclude that PMRF may contribute to the control of fine finger movements, in addition to its established role in control of more proximal limb and trunk movements. This finding may be especially important in understanding functional recovery after brain lesions such as stroke.
Key points
The reticulospinal tract is an important pathway communicating instructions for movement from the brain to the spinal cord
Although the reticulospinal tract is usually associated with gross movements such as postural adjustments and walking, recent work has shown that it also connects to spinal centres involved in hand function
In awake monkeys, we recorded from the origin of the reticulospinal tract (the reticular formation) during performance of a fine finger movement task. Cells modulated their firing during finger movements
Stimulation of sites within the reticular formation sometimes activated hand muscles, and some cells within the reticular formation responded to the sensory input following externally imposed movements of the digits
This work supports a role of the reticulospinal tract in hand function in healthy individuals. Additionally, this tract may be able to mediate some recovery of hand function when other pathways are damaged, such as after stroke
Introduction
The reticulospinal tract is a major descending pathway by which the brain controls spinal motor output. Much previous work has focused on the reticulospinal contribution to gross movements such as locomotion (Grillner, 1997; Mori et al. 2001) or postural adjustments (Schepens et al. 2008), and to the control of neck movements in orienting (Grantyn, 1989; Isa & Sasaki, 2002). More recently, we reported evidence suggesting a reticulospinal contribution to control of the primate hand (Baker, 2011). The reticulospinal tract makes mono- and di-synaptic connections to motoneurons innervating forearm muscles in monkey, including those projecting to intrinsic hand muscles (Riddle et al. 2009). In addition, spinal cord interneurons involved in the control of hand movements frequently receive convergent input from reticulospinal and corticospinal tracts (Riddle & Baker, 2010).
Most previous work on the neural control of the hand has investigated corticospinal systems, which appear critical for fine dexterous control of independent finger movements – acting both via direct (corticomotoneuronal; Lemon, 2008) and indirect (propriospinal) pathways (Sasaki et al. 2004). Our previous work on the reticulospinal tract has shown only connectivity, indicating a potential influence on hand muscles. It remains unclear how these connections are actually used, and what the role of the reticulospinal tract might be during different classes of hand movements. For example, one possibility would be that the reticular formation plays no part in fine movements, but rather contributes exclusively to less dexterous uses of the hand such as during climbing or locomotion. An alternative would be that reticular inputs to spinal circuits are also active during finer finger movements, with the final movement produced by a combined output from both brainstem and cortical systems. Such dual control by reticulospinal and corticospinal cells has previously been suggested for locomotion in the cat (Drew et al. 2004; Edgley et al. 2004).
In this study, we addressed the issue of whether the primate pontomedullary reticular formation (PMRF) contributes to fine finger movements. We recorded from neurons within this region in awake behaving monkeys performing a precise index finger tracking task and related their activity to the movement. Many PMRF cells strongly modulated their discharge in a task-dependent manner; in addition, some received peripheral input from the hand and forearm, which could allow for a reticular contribution to short latency corrections following a perturbation. Our results indicate that fine independent movements of the digits are likely to be under collaborative control by both brainstem and cortical systems.
Methods
All animal procedures were carried out under appropriate UK Home Office licenses in accordance with the Animals (Scientific Procedures) Act 1986, and were approved by the Local Research Ethics Committee of Newcastle University. Data presented in this paper are from recordings made from two adult rhesus macaque monkeys (M. mulatta, age 4–4.5 years, weight 4.4 to 8 kg) trained on a behavioural task.
Slow finger movement task
In order to examine PMRF contributions to fine hand movements, Monkeys D and R were trained to track a visually presented target by performing index finger movements (Williams et al. 2009, 2010). The index finger was splinted in a narrow plastic tube which constrained flexion/extension movements to the metacarpophalangeal joint. The hand, and digits 1 and 3–5, rested within a padded pocket which constrained movements in all directions. The tube was attached to the shaft of a torque motor and optical encoder, mounted approximately coaxially with the metacarpophalangeal joint. The target appeared at a stationary position (HOLD 1, 1 s), moved with a constant velocity of 12 deg s−1 (movement period, MOVE) for 1 s, and then remained stationary for a further 1 s (HOLD 2). Movements were either in the extension or flexion direction, chosen randomly; the HOLD 1 and HOLD 2 displacements required flexion by 12 or 24 deg from the neutral position. The lever was attached to a motor which simulated a spring load (torque for initial lever movement, 26.4 mN m; spring constant, 1.8 mN m deg−1). Force on the levers was always in a direction to oppose flexion. Deviations from the target of more than 1.4 deg resulted in an error signal and the trial was terminated with no reward. At the end of a correct trial, the lever was returned by the motor to the start position and the monkey was given a food/liquid reward. The arm was held gently supported in a sleeve to prevent proximal movements, while the contralateral arm was not restrained.
Surgical preparation
Following behavioural training, monkeys were implanted with a stainless steel headpiece to allow atraumatic head fixation (Lemon, 1984). Recording chambers were sited with centres at stereotaxic coordinates P10 R4.5 (monkey D) and P16.5 R6.4 (monkey R) to allow access to the PMRF. Chambers were also placed over the primary motor cortex (A12 L18) to allow single unit recordings from this area.
All procedures were performed using aseptic technique under general anaesthesia comprising 3–5% inhaled sevoflurane in 100% O2, supplemented with a continuous intravenous infusion of alfentanil (25 μg kg−1 h−1). Post-operative care included broad spectrum antibiotic cover (coamoxyclav 140/35 (Synulox): clavulanic acid 1.75 mg kg−1, amoxycillin 7 mg kg−1, Pfizer; cefalexin (Ceporex) 10 mg kg−1, Schering-Plough Animal Health; amoxycillin (Clamoxyl LA) 15 mg kg−1, Pfizer) and analgesics (buprenorphine (Vetergesic) 10 mg kg−1), Reckitt and Colman Products; carprofen (Rimadyl) 5 mg kg−1, Pfizer).
Muscle recordings
Electromyographic (EMG) recordings were available from subcutaneous patch electrodes implanted over the following muscles: flexor digitorum superficialis (FDS), flexor digitorum profundus (FDP), flexor carpi radialis (FCR), flexor carpi ulnaris (FCU), extensor carpi ulnaris (ECU), extensor digitorum communis (EDC), extensor carpi radialis (ECR), abductor pollicis longus (AbPL), first dorsal interosseus (1DI), abductor pollicis brevis (AbPB, Monkey D only). EMG signals were sampled at 5 kHz (gain 0.5–2 K, 30 Hz to 2 kHz bandpass). All of these muscles showed a task-dependent modulation in their activity during the slow finger movement task (see Fig. 1), as expected given the highly complex biomechanical arrangement of the forearm and hand (Schieber, 1995).
Figure 1. Slow finger movement task.
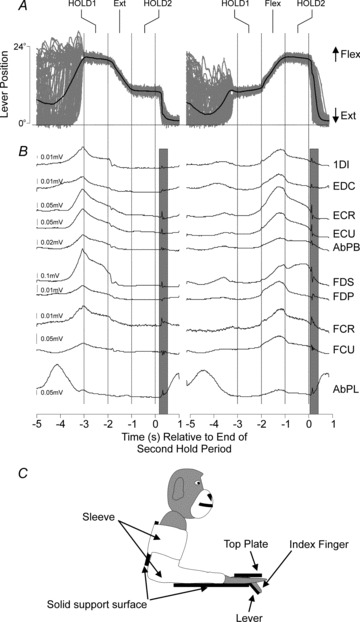
A, lever position signals (grey) during extension (left) and flexion (right) trials. B, mean EMG activity of 10 muscles during the task. Note the muscle stretch responses to the rapid return of the finger back to the neutral position (vertical grey bars). C, sketch of animal's posture during the experiments. The head was rigidly fixed, the arm held in a sleeve, and the hand inserted into a padded well which constrained movement of all digits except the index finger.
Neuronal recordings
In daily sessions, multiple single neuron extracellular recordings were made from cells of the PMRF using an Eckhorn microdrive loaded with glass insulated platinum tetrodes (Thomas Recording, Giessen, Germany). To minimize electrode deviation, sharpened guide tubes (Soteropoulos & Baker, 2006) were driven through the cortical dura up to the tentorium prior to searching for cells. Recordings were made from up to five electrodes simultaneously. Recordings in the PMRF targeted cells with large and stable extracellular spikes; identification as reticulospinal cells was not possible, although it is likely that many of these large neurons did project axons to the spinal cord.
During recordings, spike waveforms (gain 2–10 K; 300 Hz to 10 kHz bandpass) were sampled continuously at 25 kHz from all four contacts of each tetrode and stored to hard disc together with behavioural task markers. Offline, spike occurrence times were discriminated from the raw waveforms using custom-written cluster cutting routines (Getspike, S. N. Baker; SpikeLab, G. Bhumbra). Only clean single units with stable waveforms and an absence of inter-spike intervals less than 1 ms were used for subsequent analysis.
For locating the PMRF the inferior colliculus and the abducens nucleus were useful neurophysiological landmarks as they are relatively easy to identify. Low intensity (<30 μA) stimulation in the abducens nucleus results in ipsilateral eye temporal deviation while cell activity in the inferior colliculus is often responsive to auditory stimuli. Following a small number of initial mapping sessions to locate these areas, electrode penetrations were subsequently angled through the chamber to target a region around 1 mm lateral and 1 mm deeper to the abducens nucleus, via the inferior colliculus when possible. This is the caudal pontine reticular field, which forms one of the predominant locations of primate reticulospinal cells (Sakai et al. 2009).
Where possible, cells within PMRF were tested to determine if they responded to peripheral inputs. With the animal sitting quietly, the experimenter manually stimulated the ipsilateral upper limb, while listening to the cell's discharge on a loudspeaker. By stimulating in a successively more focal manner, the location and nature of the receptive field (cutaneous vs. deep receptors) was determined.
For comparison, recordings were also made in the hand representation of the primary motor cortex (M1) prior to PMRF recordings, while the monkeys performed the same task. The hand representation was located by observing the muscle responses to low current intracerebral microstimulation (ICMS, <30 μA). Recordings in M1 were made with up to 11 electrodes simultaneously (median 9, range 3 to 11). Corticospinal neurones (pyramidal tract neurons, PTNs) were identified through antidromic activation and a collision test through chronically implanted electrodes in the ipsilateral pyramidal tract (Baker et al. 1999).
Responses to microstimulation at recording sites
Output effects from recording sites in both PMRF and M1 were determined by observing the movements evoked by trains of ICMS (train of 13–18 stimuli at 300 Hz, 5–60 μA). Such weak currents are likely to activate neural elements directly only in the immediate vicinity of the electrode tip (Stoney et al. 1968), although in the cortex activity may spread further via transsynaptic activation of neurons following direct stimulation of axons (Baker et al. 1998). It is not known how reticular outputs are activated by such stimulation, although responses appear broadly consistent whether assessed using stimulus trains (as here) or single shocks, where transsynaptic spread is likely to be reduced (Herbert et al. 2010). In the PMRF, although output is most likely to be mediated via the reticulospinal tract, it is possible that there could also be activation of some corticospinal axons, some of which send corticoreticular collateral branches to the PMRF (Keizer & Kuypers, 1989). We therefore tentatively treat responses to ICMS in PMRF as the output of local circuits, of which the recorded neurons at the stimulation site are likely to form a part, while acknowledging that in some cases other factors may also play a role.
In some sessions EMG responses to ICMS were recorded. To detect the presence of a significant effect following the stimulation, the average response of the rectified EMG was computed. The baseline, and the standard deviation (SD) of this baseline, were estimated during the 20 ms-long period before the stimulus train. In some muscles the EMG recording showed appreciable stimulus artefact, making quantification during the stimulus train difficult. Accordingly, we used a standardized response region of duration 20 ms (100 sample points) starting just after the stimulus train. The number of points that exceeded the baseline plus two SD was then counted. Based on a binomial distribution, if 10/100 points crossed this level, this would correspond to an overall significance value of P = 0.03; this was therefore accepted as a significant effect.
Data analysis
A peri-event time histogram (PETH) was compiled (10 ms bin width), averaging neuronal activity relative to the end of the second hold period. As in this task the monkey had to track the visual target, once a trial was initiated, the relative times of the different hold and movement epochs from trial to trial were fixed relative to the chosen alignment event. Only cells that had at least five trials per PETH were used in this paper. The baseline activity was measured as the mean rate during a 500 ms epoch starting 500 ms after the end of the second hold period. The peak modulation was measured from the PETH as the difference between the maximal and minimal rate, over the period from 4 s before to 2 s after the end of the second hold phase. A shuffling method determined whether the modulation was significantly different from zero. Interspike intervals for each trial were shuffled randomly 100 times; for each shuffle, the PETH was recalculated, and the peak modulation measured. If the modulation of the unshuffled PETH was >95% of the modulations after shuffling, the cell was assumed to be significantly modulated by that event (P < 0.05).
Population averages are given in the text as means ± SEM, and error bars on plots show SEM, unless otherwise stated.
Histology
At the end of experiments, monkeys were deeply anaesthetised (60 mg kg−1 pentobarbitone intraperitoneal, or anaesthetic regime described above) and perfused through the heart with phosphate-buffered saline followed by 4% paraformaldehyde. Brains were removed and, after immersion in graded sucrose solutions (final strength 30%) for cryoprotection, sliced at 50 μm on a freezing microtome. Sections were mounted and stained with cresyl violet prior to microscopic examination. Due to the fine nature of the electrodes used, and the large number of total penetrations made, it was not possible to reconstruct the location of individual tracks.
Results
Task related cell firing
Figure 1 shows the lever position and task related modulation of EMGs recorded during the slow finger movement task. Figure 1A shows overlain single trial (grey) and averaged (black) lever position traces for extension (left) and flexion (right) trials. The monkeys tracked the target within low error bounds, as indicated by the similarity of the overlain lever position signals during the hold and movement epochs of the trials. Below the lever position signals are the averaged EMG signals (Fig. 1B). For the extension trials, the greatest muscle activity was at the start of the first hold. This is because the force exerted by the manipulandum motor on the lever was always directed upwards to oppose flexion. In extension trials, the monkey exerted its greatest force at the start of the trial to move the lever to the maximally flexed position, there then followed a controlled reduction of muscle activity to allow the lever to move upwards (extension) towards the starting position. In the case of flexion trials where the monkey had to exert a controlled force increase to move the lever downwards, a peak in EMG activity occurred around the movement phase of the trial. The grey shading on the figure marks a short latency response in the EMG following the fast return of the lever to the neutral position by the torque motor (analysed in more detail later). For the duration of the trial the contralateral arm was usually held still, as this demanding tracking task required all of the monkey's attention to perform correctly.
We recorded a total of 210 cells from M1 (including 70 PTNs) contralateral to the working hand (during 67 recording sessions, 31 in monkey D and 36 in monkey R), and 115 cells from PMRF ipsilateral to the working hand (42 recording sessions, 17 in monkey D, 25 in monkey R). Figure 2 illustrates activity from six example cells (PMRF, Fig. 2A–C M1, Fig. 2D–F). For reference, Fig. 2G shows the average lever displacement traces from a single session in the same time frame as the PETHs. Individual cells showed very different responses in both motor structures, with some units showing rate increases during the movement epochs and others firing maximally during the hold periods. The diagrams next to each PETH indicate the location of the cells’ receptive fields (if any, grey arrows), and the responses to stimulation through the recording electrode at each site (black arrows).
Figure 2. Example PETHs and raster plots during flexion and extension trials from M1 and PMRF.

Left column is for extension trials, and right for flexion trials. A, B and C, three example cells from PMRF during the task. D, E and F, same but for M1 cells – cells D and E were identified PTNs. G, average lever trace during extension and flexion trials. The arm schematic diagram to the right indicates the location of receptive fields (grey triangle) and stimulation effects (black triangles). The threshold for the most distal effect for the stimulation is indicated. The grey vertical dotted line indicates the time of the end of the second hold period. The arrows on PETH plots mark cell responses to the rapid return of the lever to the neutral position.
Measures from the population of all recorded M1 and PMRF cells are illustrated in Fig. 3. The left column (Fig. 3A–D) shows PMRF units while the right column (Fig. 3E–H) is for M1 units. The majority of neurons in both areas showed a significant modulation in rate with the task in either flexion or extension (M1: 87%, RF: 86%, shuffle test, P < 0.05). Timing of peak and trough rate responses are shown in Fig. 3A and E for extension trials and Fig. 3B and F for flexion trials. For both M1 and PMRF cells the time of peak rate modulations tended to cluster around the time of transition between the different task phases, i.e. HOLD 1 to MOVE, MOVE to HOLD 2 and HOLD 2 to end of trial; this effect was especially clear for the maximal rates in M1 extension trials, and PMRF flexion trials.
Figure 3. Population activity during slow finger movements.
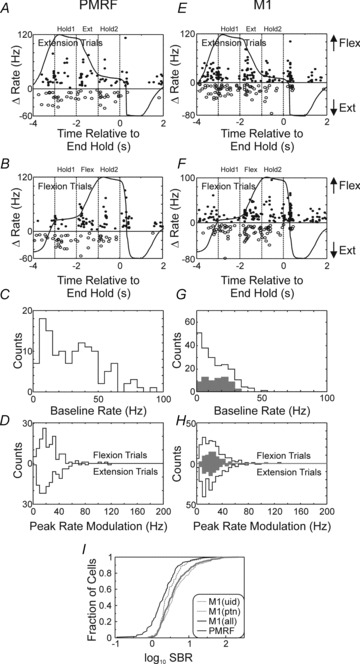
Left column is for PMRF cells and right column is for M1 cells. A, temporal distribution of maximal (filled circles) and minimal (open circles) firing rates during extension trials for PMRF cells. Overlain is the mean lever position trace for reference, and arrows at right hand side of plot show the direction of movement. B, same as A but for flexion trials. C, distribution of baseline activity during non-movement epoch. D, distribution of peak rate modulation during 3 s HOLD–MOVE–HOLD epoch for flexion (top) and extension (bottom) trials. E, F, G and H same as A, B, C and D but for M1 cells. The grey bars in G and H show the data for antidromically identified PTN cells. I, cumulative plot for the signal to baseline ratio (SBR) of M1 (grey) vs. PMRF (black) responses. For M1, the thick grey line corresponds to all M1 cells recorded, the thinner line to unidentified cells, while the thinner hatched line to PTNs.
The mean baseline rates were significantly different between M1 and PMRF cells (Fig. 3C and G; PMRF: 28.9 ± 1.8 Hz, all M1: 17.1 ± 1.1 Hz; M1 PTNs: 16.6 ± 1.1 P < 0.01, unpaired t test). The peak modulations in rate are shown in Fig. 3D and H. For neither structure did the peak modulation of the population differ significantly between extension and flexion trials (P > 0.1, paired t test). However, this should not be taken as evidence that cells coded flexion and extension trials with similar rate profiles. We measured the firing rate over the 1 s-long MOVE phase for each cell during single trials; these rates were compared between flexion and extension trials using a paired t test. For all M1 cells, rate was significantly greater during extension in 105/210 cells, and during flexion in 78/210 cells. For M1 PTNs, the rate was greater in extension trials for 32/70 cells and for flexion in 29/70 cells. In PMRF, 55/115 cells fired significantly more during extension, compared to 42/115 cells during flexion.
PMRF cells showed a significantly greater rate modulation than M1 (PMRF: 35.2 ± 1.9 Hz; all M1 cells: 29.2 ± 1.3 Hz; M1 PTNs:28.4 ± 1.6; comparison of PMRF vs. all M1 P < 0.01, unpaired t test, peak modulation taken as largest of that found for flexion/extension trials). However, when this peak modulation was expressed as a fraction of the baseline rate (signal to baseline ratio, SBR, Fig. 3I), M1 cells had significantly greater values compared to PMRF cells (mean of 4.8, 2.6 and 2.0 respectively for M1 all cells, M1 PTNs and PMRF cells, P < 0.01, unpaired t test). The median SBR values were 1.6 vs. 1.2 for all M1 and PMRF respectively. When comparing cell responses (baseline rate and peak modulation) between the two monkeys there was no difference for cell responses from M1 (unpaired t test P > 0.1). For the PMRF data there was no difference in peak modulation rates (unpaired t test, P > 0.2), but the baseline rate did differ significantly between the two monkeys (32.2 vs. 24.0 Hz, monkey D and R, respectively, unpaired t test, P < 0.03).
Cell responses to rapid lever return
Upon completion of a correct trial (flexion or extension) the lever was rapidly (velocity >200 deg s−1) returned to its starting position by a sudden increase in the force exerted by the motor. This is illustrated in the mean lever trace of Fig. 4A. In Fig. 4B the time around this rapid passive movement is expanded, and the velocity profile of the lever position is also illustrated (Fig. 4C). This will have stretched digit flexor muscles, as well as providing a powerful stimulus to cutaneous and joint receptors. A short latency response increased the active force exerted by the index finger against the lever before it reached the starting position, and produced the transient flexion of the finger and positive lever velocity. The response was highly reproducible across trials, as shown by the overlain traces. In most muscles recorded, a clear response was visible in averages of rectified EMG (Fig. 4D), superimposed on the pre-perturbation baseline activity.
Figure 4. Muscle responses to index finger stretch.

A, averaged lever displacement for flexion trials, plotted relative to end time of second hold. Circle indicates a sharp temporary reversal in the lever's return path to neutral position. B, overlain traces of lever displacement of single trials, showing the robust reversal of the direction of movement of the lever. C, velocity computed from the lever displacement traces shown in B. D, mean rectified EMG responses from several muscles with activity aligned to stretch onset (shown by vertical dotted line). There is a response in all muscles, in some cases polyphasic (e.g. EDC, FDS, FDP) corresponding to classical short and long latency stretch reflex responses. Grey triangles indicate estimated minimal onset latency for a monosynaptic stretch reflex, while grey and white bars indicate early and late responses respectively. The numbers above the triangles and under the grey bars indicate these onset latencies in milliseconds. E, PSTH (1 ms bin width) and raster of PMRF cell recorded during the same session, showing a clear response to the stretch. Plots B–E are on the same time scale.
To aid interpretation of the responses seen in Fig. 4D, we estimated the minimal latency expected for a monosynaptic stretch reflex in each muscle as follows. The peripheral motor conduction time was determined from the EMG response latency in active muscles following stimulation through the PT electrode in the medulla, subtracting 0.7 ms for corticospinal conduction from the medulla to the cervical enlargement, and 1 ms for the corticomotoneuronal synapse (Riddle et al. 2009). Although Group Ia afferents conduct faster than motor efferent fibres, this difference in primates is small (Eccles et al. 1968). Accordingly, we estimated the monosynaptic stretch reflex loop time as twice the peripheral motor conduction time, plus 1 ms to account for the monosynaptic delay. These minimal delay estimates have been indicated for each muscle on Fig. 4D by arrowheads.
In all muscles illustrated there was a short latency response, with an onset between 14.9 and 22.6 ms (marked by grey bars). As this was a rapid finger extension stimulus, we would expect monosynaptic stretch reflexes in the flexor muscles, and indeed the early responses in all flexors except FCR did appear shortly after the estimated minimal monosynaptic latencies. By contrast, the short latency responses in extensor muscles were slightly delayed, suggesting a more complex central pathway, possibly involving cutaneous reflexes. In addition, several muscles showed a longer latency response, with onset latency 53–76 ms (white bars).
One possible concern is that the short latency responses shown in Fig. 4D reflected movement artefacts in the recorded EMG. We checked for this by compiling averages of unrectified EMG. Only the 1DI and AbPB recordings showed small slow deflections consistent with movement artefact, presumably reflecting the close proximity of these intrinsic hand muscles to the moving digit. These were the two muscles with the smallest short latency response, providing confidence that movement artefact is unlikely to underlie these effects.
It has been previously reported that M1 cells respond to such perturbations (Lucier et al. 1975; Cheney & Fetz, 1984). In the present dataset, both M1 and PMRF cells often showed clear perturbation-evoked activity, as shown by the raster and peri-stimulus time histogram (PSTH) for a single PMRF cell in Fig. 4E. To quantify the features of this response, a PSTH was first generated aligned to the onset of the lever return phase (dotted line in Fig. 4B-E). The experimenter determined the onset latency of any response, guided by a display of the mean baseline rate and its SD. The rate during a 20 ms-long window following this onset latency was measured for all available single trials, and compared with a 20 ms-long baseline period prior to perturbation onset. Significance was assessed by a paired t test (P < 0.01).
Example cell responses are shown in Fig. 5, from both PMRF (Fig. 5A) and M1 (Fig. 5B). Although most responses were facilitations, suppressions were also seen (Fig. 5B3). In total 18/115 PMRF cells (16%) and 96/210 M1 cells (46%) showed a response to the lever return stimulus. Thirty-two of the 96 stretch responsive cells in M1 were PTNs (10/32 cells showing a facilitatory response). The distribution of the response latencies is shown in Fig. 5C. There was no significant difference between the mean response latency in M1 (22.2 ms) and PMRF (19.7 ms) (P > 0.1, unpaired t test). The response latencies of PTNs were similar to the latencies of other M1 cells (21.8 ms vs. 22.4 ms, unpaired t test, P > 0.7) Comparison of the latency between M1 and PMRF facilitatory responses revealed no significant difference (23 vs. 22.1 ms respectively, unpaired t test, P > 0.1), while there was a significant difference in latency for the inhibitory responses (21.9 vs. 15 ms, P < 0.01)
Figure 5. PMRF and M1 cells responses to lever return.

A, three example PMRF cells showing a response to the stretch. The PSTH and raster are plotted, with the CUSUM (cumulative sum) overlain in grey. Underneath the raster plot is the mean lever velocity for the particular session where the cell was recorded from. All traces are aligned to the stretch onset (vertical dashed line). B, same as A but for M1 cells. C, distribution of onset latency of responses, white bars show suppressions and black facilitations. D, cumulative plot of SBR of task responses for M1 (black) and PMRF cells (grey) showing a response to the index finger stretch (compare with Fig. 3I).
As is the case with all natural stimuli of this type, it is impossible to be certain which class of peripheral receptors (e.g. muscle spindle vs. cutaneous) contributed to these responses. The monkey's arm was held in a sleeve and resting on an arm rest, and the hand was cushioned in a pocket on the manipulandum. It is thus very unlikely that the passive return of the index finger to its neutral position caused any movements proximal to the elbow, and these responses are most likely to result from inputs associated with the passive finger movement. We were also able manually to test the receptive field of some of the PMRF cells at the end of the recording session (this was not carried out for M1 cells). Of 40 cells with a forelimb receptive field, 27 had a receptive field proximal to and including the elbow; the remaining 13 cells responded to manipulation of the hand or wrist. Manual receptive field testing is likely to underestimate the extent of inputs – this is especially the case here as much of the forearm was covered by the sleeve, requiring exploratory testing to be carried out through the fabric.
Figure 5D shows the cumulative distributions of the SBR of the task responses of M1 and PMRF cells (similar to Fig. 3I), but using only cells with significant responses to the lever return stimulus. The mean SBR was 3.1 and 3.2 for PMRF and M1 respectively; these were not significantly different (Kolmogorov–Smirnoff test, P > 0.4). An alternative approach classified PMRF cells as ‘proximal’ if they had receptive fields proximal to the elbow (as assessed by palpation by the experimenter, n = 25), or ‘distal’ if they had receptive fields distal to the elbow assessed by palpation, or significant responses to the lever return (n = 30). Using this classification, the SBR of the task responses was significantly smaller for proximal than for distal cells (1.8 vs. 3.1; Kolmogorov–Smirnoff test, P < 0.03).
Stimulation effects
Trains of ICMS (13–18 shocks, 3.33 ms inter-stimulus interval) were given regularly in each session to identify the neural landmarks used to locate PMRF (see Methods), but also to identify the output organization of the local circuitry. ICMS at relatively low stimulation currents (<60 μA) usually elicited purely ipsilateral movements, but sometimes produced bilateral effects. As we only had EMG recordings from one arm, we were only able to analyse effects quantitatively from that side (ipsilateral to PMRF sites, contralateral to M1 sites).
Figure 6A summarizes the visually observed responses from stimulation carried out at the end of each recording session in M1/PMRF. Responses were classified depending on which body part showed movement; a movement was classified as ‘finger’ if there was movement in any of the digits, ‘wrist’ if there was any movement of the wrist, similarly for ‘elbow’ and ‘shoulder’. Movements in other body parts were occasionally seen, but the design of our primate chair partially obscured the animal's body, and meant that weak movements of the shoulder, back, trunk, chest and legs could easily be missed. The most common movements elicited from hand area M1 stimulation involved fingers and wrist, while for PMRF the most common responses were movements of the elbow, shoulder and wrist. In some cases (16%), digit movements were observed following PRMF stimulation.
Figure 6. Stimulation effects in PMRF.

A, distribution of main joint movement in visually observed effects from PMRF (grey) and M1 (black) stimulation. B, distribution of threshold currents for stimulation in PMRF (grey) and M1 (black). C, number of muscles activated during ICMS from a single site. D, frequency of muscle activation from a single site. E, example mean response to ICMS of an intrinsic hand muscle (1DI), with single sweeps shown underneath. Vertical grey bars correspond to times of stimuli.
Figure 6B shows the distribution of the threshold intensity required to produce a visible movement. For M1 71% of thresholds were lower than 20 μA, while for PMRF only 7% were below this value. The median threshold intensity for M1 was 12 μA, and for PMRF 30 μA. The mean threshold at distal/wrist PMRF sites was 30 μA compared to 40 μA for all other sites (P < 0.001, unpaired t test). For M1 sites the mean threshold was 14 μA for distal/wrist effects, compared to 21 μA for all others (P < 0.001, unpaired t test)
The rest of Fig. 6 summarizes data from 45 sites in the PMRF where EMG responses to ICMS stimulation were recorded. There was a response in at least one muscle for 19/45 (42%) sites. Only facilitations were observed; the lack of suppressions probably reflects the lack of background EMG, as the animals had ceased working on the behavioural task by this stage of the experiment. Figure 6C plots the distribution of number of muscles activated from a single stimulation site. Activation of more than four muscles was rare (10% of sessions with any effect, 4% of overall ICMS sessions). Activation of more than one muscle was more common than activation of single muscles only (68 vs. 32% of sessions with at least one response).
Figure 6D shows how common effects were in different muscle groups. The extensor group was most frequently activated (47%). However, we also encountered several sites which elicited responses in a forearm muscle controlling the thumb (AbPL n = 6) and in an intrinsic hand muscle (1DI, n = 5). These effects were never isolated, but were always seen in conjunction with the activation of a more proximal forearm muscle. Overall, 13/45 sites produced effects in forearm or hand muscles controlling the digits. An example response from 1DI is shown in Fig. 6E. The top trace is the average while the single sweeps are shown below; the sweep-to-sweep fluctuations give confidence that this is a genuine EMG response, and not a result of a long-lasting stimulus artefact. The current intensities used for the distal effects were all <30 μA, making current spread beyond PMRF unlikely. For all recorded stimulation effects the maximal current used was 60 μA (n = 1) and the modal current used was 30 μA (51/53 effects required currents ≤30 μA).
Histology
Figure 7 shows the histological reconstruction of visible gliosis tracks as well as the estimated location of the electrode tips used to record the data presented here. The fine nature of our electrodes meant that almost all penetrations left no visible scars; these illustrations can therefore only indicate the general location of our recording sites, rather than reconstruct individual tracks. Nevertheless, most of the visible scars head towards the area corresponding to the reticular formation, on the side ipsilateral to the hand performing the slow finger movement task.
Figure 7. Histological reconstruction of PMRF gliosis scars and estimated stereotaxic location of recordings.

Black marks correspond to visible gliosis scars in different histological sections. Yellow areas correspond to approximate location of reticular formation. The number next to each section corresponds to an approximate anterioposterior location (relative to interaural line, P = posterior). Symbols (see key) show different stimulus responses, or cell activity, noted during recordings. Abbreviations: 6n, abducens nucleus; 7n, facial motor nucleus; g7, internal genu of facial nerve; SCP, superior cerebellar peduncle; MCP, medial cerebellar peduncle; IC, inferior colliculus; IO, inferior olive; MLF, medial longitudinal fasciculus; LL, lateral lemniscus; PCRt, parvicellular reticular nucleus; PNC, caudal pontine reticular nucleus; Gi, gigantocellular nucleus.
For each penetration we were able to estimate the likely stereotaxic target of each microelectrode tip – these are also shown on the illustrations. The dorsoventral locations were adjusted relative to the landmarks noted during recordings (inferior colliculus, abducens nucleus and facial nerve). The sections shown in Fig. 7 are at the approximate mean AP location of the penetrations for the two monkeys where the reference landmarks were clearly visible. Based on the gliosis scars and estimated stereotaxic locations of our penetrations, penetrations in monkey R (mean estimated anterior-posterior location 0.06 mm posterior to interaural line (IAL), SD 1.8 mm) were slightly more anterior than monkey D (0.48 mm posterior, SD 1.8 mm); both were mostly in the region of the pontine group of reticular nuclei (pontine reticular nucleus, parvicellular reticular nucleus, nucleus gigantocellularis).
Discussion
The reticular formation is traditionally associated with locomotion and postural control (Mori et al. 2001; Schepens et al. 2008), although some recent studies (Davidson & Buford, 2006; Riddle et al. 2009; Riddle & Baker, 2010) suggest involvement in controlling more distal musculature in primates. Here, we show similar modulation in PMRF and M1 cell activity during a task where the goal is fine control of the index finger. Stimuli within PMRF can activate muscles acting on the digits, and PMRF cells can respond to peripheral input following finger movement. Overall this supports a role for the PMRF in the control of hand movements.
PMRF Role in voluntary finger movements
As with any independent finger movement, a complex synergy of muscle activities was required to produce the apparently simple motor goal of our task (Schieber, 1995). There may have been modulation of proximal (e.g. shoulder) muscles, even though there was no overt movement beyond the wrist: the whole arm usually acts as a functionally interconnected unit (see e.g. Alexander & Harrison, 2003). It is therefore possible that the observed task modulation of PMRF cells occurred in relation to proximal muscles which were co-activated with the hand and forearm muscles responsible for the index finger movements. We did not record EMG from muscles acting around the shoulder and trunk, and cannot therefore completely rule out this explanation. However, the monkeys’ posture during the experiment made this unlikely to be a major factor. Animals were seated comfortably, with the head fixed. The arm was held in a sleeve with a solid back rest, and the forearm rested on a rigid support platform. The hand, and digits 1 and 3–5, rested within a padded pocket which constrained movements in all directions. Only the index finger was free to move, and these movements were limited to the measured small flexion/extension excursions. Under such circumstances, the task would not have affected the animal's centre of gravity, and it is unlikely that task performance would have been associated with postural adjustments.
The available anatomical evidence suggests that the PMRF projects mainly to the medial portion of the intermediate zone and ventral horn of the cervical spinal cord (Kuypers et al. 1962; Matsuyama et al. 1997), pointing to an involvement primarily with the control of proximal and axial muscles (Kuypers et al. 1962). In the primate there have been several studies showing that cells in the PMRF can be recruited for discrete, voluntary arm movements (Werner et al. 1997; Buford & Davidson, 2004, 2005). The reticular formation's involvement in forelimb reaching has also been shown in the cat (Alstermark et al. 1987a; Gibson et al. 1998; Schepens & Drew, 2004, 2006; Schepens et al. 2008). Against this background, the finding that PMRF neurons modulate their activity during finger movements is surprising. However, some previous work in cat also suggests a role in digit movements. There are sparse terminations of reticulospinal axons to the lateral portions of the intermediate zone, which may be more related to distal musculature (Sasaki, 1997). Following lesions of both corticospinal and rubrospinal systems, grasp in cat appears to recover partly via reticulospinal pathways (Pettersson et al. 2007).
Control of fine distal movements has been considered the domain of M1, and a large body of evidence highlights the importance of M1 for fine movement control in higher primates. The activity of M1 cells is strongly modulated by manipulative tasks such as precision grip (Bennett & Lemon, 1996; Morrow & Miller, 2003), while grosser grasping movements such as power-grip do not engage M1 cells to the same extent (Muir & Lemon, 1983). Both permanent and transient lesions involving the corticospinal tract or M1 have their most severe consequences on distal finger movements (Lawrence & Kuypers, 1968a; Hepp-Reymond & Wiesendanger, 1972; Nudo & Milliken, 1996; Schieber & Poliakov, 1998; Lemon et al. 2012), and these movements show worst recovery in the case of permanent lesions. In this earlier work, lesions were made at the brainstem or cortical level, and could not thus differentiate the impact of direct corticomotoneuronal connections versus indirect effects from the corticospinal tract via spinal cord interneurons. Subsequent work has demonstrated that indirect corticospinal connections to motoneurons via C3/C4 propriospinal interneurons can mediate fine grasp in monkey, as well as the direct corticomotoneuronal connections (Sasaki et al. 2004).
Both M1 and PMRF cells modulated their activity during the slow finger movement task. The reticular formation receives substantial information regarding movements from M1 via corticospinal collaterals and from a direct cortico-reticular pathway (Kuypers, 1958; Keizer & Kuypers, 1989; Kably & Drew, 1998a,b), as well as from the cerebellum (Bantli & Bloedel, 1975). These inputs probably partly shape the activity of PMRF cells and its relationship to distal movements. Although we did not antidromically identify as reticulospinal cells the neurons from which we recorded, the area targeted by our penetrations is one of the main origins of the reticulospinal tract (Sakai et al. 2009). The low stimulation currents typically needed to elicit forearm movements also suggest that our electrode tips were amongst reticulospinal cells, and hence that the activity which we observed was likely to be transmitted down the spinal cord. It is then important to determine the specific contribution of the PMRF to the control of fine finger movements.
This role will be in part constrained by the anatomical connectivity of the PMRF with spinal motor controllers. In cats, reticulospinal axons can have broad terminations across many spinal segments. The same neuron can project to both cervical and lumbar enlargements bilaterally (Peterson et al. 1975; Peterson, 1979; Matsuyama et al. 1997; Matsuyama et al. 1999), suggesting a highly divergent control signal targeting a wide range of muscles, although a population of reticulospinal axons which targets only the cervical cord does exist (Sasaki, 1997). In marked contrast, although corticospinal axons also project to multiple motor nuclei, their termination patterns are much more focal and lateralized (Shinoda et al. 1979, 1981; Buys et al. 1986; Lacroix et al. 2004).
The effects of stimulation in M1 and PMRF support a qualitative difference in the extent of the divergence of the two pathways. ICMS in M1 produces focal responses, often confined to single joint movements (Kwan et al. 1978), whereas effects from PMRF are usually more widespread (Davidson & Buford 2006). Although we did not record from muscles proximal to the elbow, during our penetrations we often found locations eliciting visible movements simultaneously in the back, forelimb and hindlimb. In addition, although we found effects from PMRF stimulation in distal muscles, these were never in isolation from activation of more proximal forelimb muscles. We used stimulus trains to identify PMRF outputs, which may have produced less selective effects than would have been seen using either single pulse stimulation, or spike triggered averaging (Herbert et al. 2010). Nevertheless, it seems likely that the widespread termination patterns seen in feline reticulospinal axons are similar in primates. The PMRF is thus unlikely to be capable of sending an isolated signal to target distal motor networks alone. Rather, activity in the PMRF related to distal movements will probably be sent to spinal networks controlling a broad range of muscles – including those in the limb contralateral to the prime mover. This would in turn allow distal and proximal movements to be coordinated, and for the movements of one limb to be placed in a bilateral context. It should be remembered that while isolated distal movements (such as the slow finger movement task used here) are experimentally attractive, almost all natural movements require proximo-distal coordination (Jeannerod, 1988), and many require the use of both hands. A basic template of whole arm – or even whole body – coordinated action generated by the reticulospinal tract may be further sculpted by corticospinal activity to produce the detailed behavioural goal.
Role of the PMRF in long latency reflexes
Muscle stretch elicits reflex contraction, as demonstrated in the familiar tendon tap reflex. The earliest components of the stretch reflex result from monosynaptic feedback from muscle spindle Group Ia afferents to motoneurons. There has been considerable past controversy over the pathway underlying longer latency components. For hand muscles, several lines of evidence now support a trans-cortical contribution via fast corticospinal pathways (Cheney & Fetz, 1984; Matthews et al. 1990; Day et al. 1991), although these studies have not excluded a possible role for other pathways. In the present work, we often saw long latency responses to lever displacement in hand and forearm muscles (see Fig. 4D). Reticular neurons responded to this stimulus at latencies shorter than the long-latency reflex onset in EMG (Fig. 4D and E, Fig. 5A and C). Since some reticulospinal cells provide input to motoneurons controlling the digits (Riddle et al. 2009, and Fig. 6A), this represents evidence – for the first time in a primate – that at least some of the long latency reflex in hand and forearm muscles could be carried not just by a trans-cortical, but also by a trans-reticular pathway.
Due to the nature of our stimulus, we cannot determine which peripheral receptors were most important in mediating reticular responses. Although Group Ia muscle spindle afferents seem a likely possibility, there could also be a contribution from cutaneous or joint receptors (Corden et al. 2000). It is also not possible to be sure of the central pathway mediating these responses in the PMRF. It is possible that M1 activity (transmitted over corticoreticular connections) contributed to PMRF responses as well as more direct pathways in the brainstem, such as input from the cuneate nucleus (Leiras et al. 2010).
Implications for recovery from lesions
The role of the PMRF may become especially important following lesions to other motor structures. Pure corticospinal lesions in monkeys produce initial severe impairment of hand function (Lawrence & Kuypers, 1968a; Lemon et al. 2012). Although fine independent control of the fingers does not recover completely, there is considerable restoration of hand function in the weeks following the lesion, with animals able to grip their cage bars sufficiently to support their entire body weight (Lawrence & Kuypers, 1968a). The functional recovery of fine hand control in monkeys is often ascribed to the rubrospinal tract (Lawrence & Kuypers, 1968b; Belhaj-Saif & Cheney, 2000), but when combined corticospinal and rubrospinal lesions at the level of C2 are made in monkey, power grip recovers within 2 weeks (Alstermark et al. 2011). The reticulospinal tract is the only major surviving pathway in this case, suggesting that alone it is capable of mediating grosser aspects of hand function. In humans the rubrospinal tract may be either vestigial or absent (Nathan & Smith, 1982; Onodera & Hicks, 2010). In that case, patients recovering from brain lesions such as stroke might rely especially on reticulospinal connections (Dewald et al. 1995; Nathan et al. 1996), as has been previously suggested for cats and monkeys (Alstermark et al. 1981, 1987b; Jiang & Drew, 1996; Jankowska & Edgley, 2006; Zaaimi et al. 2012). The failure to achieve full recovery of fractionated finger movement after corticospinal lesions probably reflects a limitation in how well brainstem pathways can achieve independent control of small synergistic muscle groups. In addition, sub-cortical strokes will interrupt not only corticospinal projections, but also cortico-bulbar and cortico-pontine projections to the PMRF, reducing the ability of the cortex to control reticulospinal outputs. Further understanding of these important pathways could allow interventions targeted to induce plasticity in the brainstem and spinal connections, ameliorating functional outcomes for patients recovering from brain lesions.
Acknowledgments
Funded by the Wellcome Trust and MRC (UK). The authors would also like to thank Terri Jackson for her assistance with animal training.
Glossary
- 1DI
first dorsal interosseus
- AbPL
abductor pollicis longus
- AbPB
abductor pollicis brevis
- CUSUM
cumulative sum
- ECR
extensor carpi radialis
- ECU
extensor carpi ulnaris
- EDC
extensor digitorum communis
- EMG
electromyogram
- FCR
flexor carpi radialis
- FCU
flexor carpi ulnaris
- FDP
flexor digitorum profundus
- FDS
flexor digitorum superficialis
- ICMS
intracortical microstimulation
- M1
primary motor cortex
- PETH
peri-event time histogram
- PMRF
pontomedullary reticular formation
- PTN
pyramidal tract neuron
- SBR
signal to baseline ratio
Author contributions
S.N.B. devised experiments and obtained funding. D.S.S., E.R.W. and S.N.B. carried out experiments. D.S.S. and E.R.W. carried out analysis. D.S.S. drafted the manuscript. All authors have approved the final version of the paper.
References
- Alexander CM, Harrison PJ. Reflex connections from forearm and hand afferents to shoulder girdle muscles in humans. Exp Brain Res. 2003;148:277–282. doi: 10.1007/s00221-002-1256-9. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Kummel H, Tantisira B. Monosynaptic raphespinal and reticulospinal projection to forelimb motoneurones in cats. Neurosci Lett. 1987a;74:286–290. doi: 10.1016/0304-3940(87)90311-9. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A, Norrsell U, Sybirska E. Integration in descending motor pathways controlling the forelimb in the cat. 9. Differential behavioural defects after spinal cord lesions interrupting defined pathways from higher centres to motoneurones. Exp Brain Res. 1981;42:299–318. doi: 10.1007/BF00237496. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A, Pettersson LG, Tantisera B, Walkowska M. Motor recovery after serial spinal cord lesions of defined descending pathways in cats. Neurosci Res. 1987b;5:68–73. doi: 10.1016/0168-0102(87)90024-1. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Pettersson LG, Nishimura Y, Yoshino-Saito K, Tsuboi F, Takahashi M, Isa T. Motor command for precision grip in the macaque monkey can be mediated by spinal interneurons. J Neurophysiol. 2011;106:122–126. doi: 10.1152/jn.00089.2011. [DOI] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. An investigation of the intrinsic circuitry of the motor cortex of the monkey using intra-cortical microstimulation. Exp Brain Res. 1998;123:397–411. doi: 10.1007/s002210050585. [DOI] [PubMed] [Google Scholar]
- Baker SN, Philbin N, Spinks R, Pinches EM, Wolpert DM, MacManus DG, Pauluis Q, Lemon RN. Multiple single unit recording in the cortex of monkeys using independently moveable microelectrodes. J Neurosci Methods. 1999;94:5–17. doi: 10.1016/s0165-0270(99)00121-1. [DOI] [PubMed] [Google Scholar]
- Bantli H, Bloedel JR. Monosynaptic activation of a direct reticulo-spinal pathway by the dentate nucleus. Pflugers Arch. 1975;357:237–242. doi: 10.1007/BF00585978. [DOI] [PubMed] [Google Scholar]
- Belhaj-saif A, Cheney PD. Plasticity in the distribution of the red nucleus output to forearm muscles after unilateral lesions of the pyramidal tract. J Neurophysiol. 2000;83:3147–3153. doi: 10.1152/jn.2000.83.5.3147. [DOI] [PubMed] [Google Scholar]
- Bennett KM, Lemon RN. Corticomotoneuronal contribution to the fractionation of muscle activity during precision grip in the monkey. J Neurophysiol. 1996;75:1826–1842. doi: 10.1152/jn.1996.75.5.1826. [DOI] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res. 2004;159:284–300. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2005. Long-term correlations between reticular formation neural activity and upper limb muscle EMG during a bilateral reaching task. Programme No. 181.2. [Google Scholar]
- Buys EJ, Lemon RN, Mantel GW, Muir RB. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol. 1986;381:529–549. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984;349:249–272. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden DM, Lippold OC, Buchanan K, Norrington C. Long-latency component of the stretch reflex in human muscle is not mediated by intramuscular stretch receptors. J Neurophysiol. 2000;84:184–188. doi: 10.1152/jn.2000.84.1.184. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res. 2006;173:25–39. doi: 10.1007/s00221-006-0374-1. [DOI] [PubMed] [Google Scholar]
- Day BL, Riescher H, Struppler A, Rothwell JC, Marsden CD. Changes in the response to magnetic and electrical stimulation of the motor cortex following muscle stretch in man. J Physiol. 1991;433:41–57. doi: 10.1113/jphysiol.1991.sp018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res. 2004;143:251–261. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- Eccles RM, Phillips CG, Chien-Ping W. Motor innervation, motor unit organization and afferent innervation of m. extensor digitorum communis of the baboon's forearm. J Physiol. 1968;198:179–192. doi: 10.1113/jphysiol.1968.sp008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci. 2004;24:7804–7813. doi: 10.1523/JNEUROSCI.1941-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson AR, Horn KM, Pong M, Van Kan PLE. Construction of a reach-to-grasp. In: Bock GR, Goode JA, editors. Sensory Guidance of Movement: Novartis Foundation Symposium. Vol. 218. Chichester, UK: John Wiley & Sons; 1998. [DOI] [PubMed] [Google Scholar]
- Grantyn A. How visual inputs to the ponto-bulbar reticular formation are used in the synthesis of premotor signals during orienting. Prog Brain Res. 1989;80:159–170. doi: 10.1016/s0079-6123(08)62209-8. discussion 127–158. [DOI] [PubMed] [Google Scholar]
- Grillner S. Ion channels and locomotion. Science. 1997;278:1087–1088. doi: 10.1126/science.278.5340.1087. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond M, Wiesendanger M. Unilateral pyramidotomy in monkeys: Effects on force and speed of a conditioned precision grip. Brain Res. 1972;36:117–131. doi: 10.1016/0006-8993(72)90770-6. [DOI] [PubMed] [Google Scholar]
- Herbert WJ, Davidson AG, Buford JA. Measuring the motor output of the pontomedullary reticular formation in the monkey: do stimulus-triggered averaging and stimulus trains produce comparable results in the upper limbs? Exp Brain Res. 2010;203:271–283. doi: 10.1007/s00221-010-2231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Sasaki S. Brainstem control of head movements during orienting; organization of the premotor circuits. Prog Neurobiol. 2002;66:205–241. doi: 10.1016/s0301-0082(02)00006-0. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. How can corticospinal tract neurons contribute to ipsilateral movements? A question with implications for recovery of motor functions. Neuroscientist. 2006;12:67–79. doi: 10.1177/1073858405283392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. The Neural and Behavioural Organisation of Goal-Directed Movements. Oxford: Oxford University Press; 1988. [Google Scholar]
- Jiang W, Drew T. Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. J Neurophysiol. 1996;76:849–866. doi: 10.1152/jn.1996.76.2.849. [DOI] [PubMed] [Google Scholar]
- Kably B, Drew T. Corticoreticular pathways in the cat. I. Projection patterns and collaterization. J Neurophysiol. 1998a;80:389–405. doi: 10.1152/jn.1998.80.1.389. [DOI] [PubMed] [Google Scholar]
- Kably B, Drew T. Corticoreticular pathways in the cat. II. Discharge activity of neurons in area 4 during voluntary gait modifications. J Neurophysiol. 1998b;80:406–424. doi: 10.1152/jn.1998.80.1.406. [DOI] [PubMed] [Google Scholar]
- Keizer K, Kuypers HGJM. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis. Exp Brain Res. 1989;74:311–318. doi: 10.1007/BF00248864. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. An anatomical analysis of cortico-bulbar connexions to the pons and lower brain stem in the cat. J Anat. 1958;92:198–218. [PMC free article] [PubMed] [Google Scholar]
- Kuypers HG, Fleming WR, Farinholt JW. Subcorticospinal projections in the rhesus monkey. J Comp Neurol. 1962;118:107–137. doi: 10.1002/cne.901180109. [DOI] [PubMed] [Google Scholar]
- Kwan HC, MacKay WA, Murphy JT, Wong YC. Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol. 1978;41:1120–1131. doi: 10.1152/jn.1978.41.5.1120. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, Tuszynski MH. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol. 2004;473:147–161. doi: 10.1002/cne.20051. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers H. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968a;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers H. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathway. Brain. 1968b;91:15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Leiras R, Velo P, Martin-Cora F, Canedo A. Processing afferent proprioceptive information at the main cuneate nucleus of anaesthetized cats. J Neurosci. 2010;30:15383–15399. doi: 10.1523/JNEUROSCI.2193-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon RN. Methods for recording in conscious animals. IBRO Handbook Series: Methods in Neuroscience. London, UK: Wiley; 1984. [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Landau W, Tutssel D, Lawrence DG. Lawrence and Kuypers (1968ab) revisited: copies of the original filmed material from their classic papers in Brain. Brain. 2012 doi: 10.1093/brain/aws037. (in press) [DOI] [PubMed] [Google Scholar]
- Lucier GE, Ruegg DC, Wiesendanger M. Responses of neurones in the motor cortex and in area 3a to controlled stretches of forelimb muscles in cebus monkeys. J Physiol. 1975;251:833–853. doi: 10.1113/jphysiol.1975.sp011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol. 1999;410:413–430. [PubMed] [Google Scholar]
- Matsuyama K, Takakusaki K, Nakajima K, Mori S. Multi-segmental innervation of single pontine reticulospinal axons in the cervico-thoracic region of the cat: anterograde PHA-L tracing study. J Comp Neurol. 1997;377:234–250. [PubMed] [Google Scholar]
- Matthews PBC, Farmer SF, Ingram DA. On the localization of the stretch reflex of intrinsic hand muscles in a patient with mirror movements. J Physiol. 1990;428:561–577. doi: 10.1113/jphysiol.1990.sp018228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Matsuyama K, Mori F, Nakajima K. Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv Neurol. 2001;87:25–40. [PubMed] [Google Scholar]
- Morrow MM, Miller LE. Prediction of muscle activity by populations of sequentially recorded primary motor cortex neurons. J Neurophysiol. 2003;89:2279–2288. doi: 10.1152/jn.00632.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir RB, Lemon RN. Corticospinal neurons with a special role in precision grip. Brain Res. 1983;261:312–316. doi: 10.1016/0006-8993(83)90635-2. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith M, Deacon P. Vestibulospinal reticulospinal and descending propriospinal nerve fibres in man. Brain. 1996;119:1809–1833. doi: 10.1093/brain/119.6.1809. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. The rubrospinal and central tegmental tracts in man. Brain. 1982;105:223–269. doi: 10.1093/brain/105.2.223. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Onodera S, Hicks TP. Carbocyanine dye usage in demarcating boundaries of the aged human red nucleus. PloS One. 2010;5:e14430. doi: 10.1371/journal.pone.0014430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BW. Reticulospinal projections to spinal motor nuclei. Annu Rev Physiol. 1979;41:127–140. doi: 10.1146/annurev.ph.41.030179.001015. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and braching of reticulospinal neurons. Exp Brain Res. 1975;23:333–351. doi: 10.1007/BF00238019. [DOI] [PubMed] [Google Scholar]
- Pettersson LG, Alstermark B, Blagovechtchenski E, Isa T, Sasaski S. Skilled digit movements in feline and primate–recovery after selective spinal cord lesions. Acta Physiol (Oxf) 2007;189:141–154. doi: 10.1111/j.1748-1716.2006.01650.x. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Convergence of pyramidal and medial brain stem descending pathways onto macaque cervical spinal interneurons. J Neurophysiol. 2010;103:2821–2832. doi: 10.1152/jn.00491.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009;29:4993–4999. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai ST, Davidson AG, Buford JA. Reticulospinal neurons in the pontomedullary reticular formation of the monkey (Macaca fascicularis. Neuroscience. 2009;163:1158–1170. doi: 10.1016/j.neuroscience.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S. Axonal branching and termination of cervical reticulospinal neurons in the cat brachial segments. Neurosci Lett. 1997;228:83–86. doi: 10.1016/s0304-3940(97)00362-5. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Isa T, Pettersson LG, Alstermark B, Naito K, Yoshimura K, Seki K, Ohki Y. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol. 2006;96:2229–2252. doi: 10.1152/jn.00342.2006. [DOI] [PubMed] [Google Scholar]
- Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol. 2008;100:2235–2253. doi: 10.1152/jn.01381.2007. [DOI] [PubMed] [Google Scholar]
- Schieber MH. Muscular production of individuated finger movements: The roles of extrinsic finger muscles. J Neurosci. 1995;15:284–297. doi: 10.1523/JNEUROSCI.15-01-00284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J Neurosci. 1998;18:9038–9054. doi: 10.1523/JNEUROSCI.18-21-09038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Yokota J, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett. 1981;23:7–12. doi: 10.1016/0304-3940(81)90182-8. [DOI] [PubMed] [Google Scholar]
- Shinoda Y, Zarzecki P, Asanuma H. Spinal branching of pyramidal tract neurons in the monkey. Exp Brain Res. 1979;34:59–72. doi: 10.1007/BF00238341. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Baker SN. Cortico-cerebellar coherence during a precision grip task in the monkey. J Neurophysiol. 2006;95:1194–1206. doi: 10.1152/jn.00935.2005. [DOI] [PubMed] [Google Scholar]
- Stoney SD, Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol. 1968;31:659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1997;115:191–205. doi: 10.1007/pl00005690. [DOI] [PubMed] [Google Scholar]
- Williams ER, Soteropoulos DS, Baker SN. Coherence between motor cortical activity and peripheral discontinuities during slow finger movements. J Neurophysiol. 2009;102:1296–1309. doi: 10.1152/jn.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ER, Soteropoulos DS, Baker SN. Spinal interneuron circuits reduce approximately 10-Hz movement discontinuities by phase cancellation. Proc Natl Acad Sci U S A. 2010;107:11098–11103. doi: 10.1073/pnas.0913373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity following recovery from corticospinal tract lesions in macaque monkeys. Brain. 2012 doi: 10.1093/brain/aws115. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


