Abstract
Whether injuries to the alar ligaments could be responsible for complaints of patients having whiplash injury in the upper cervical spine is still controversially discussed. It is known that these ligaments protect the upper cervical spine against excessive lateral bending and axial rotation movements. The objective of the present in vitro study was therefore to examine whether the alar ligaments or any other structures of the cervical spine are damaged in side collisions. In a specially designed acceleration apparatus, six human osteoligamentous cervical spine specimens were subjected to incremental 90° side collisions from the right (1 g, 2 g, 3 g, etc.) until structural failure occurred. A damped pivot table accounted for the passive movements of the trunk during collision, and a dummy head (4.5 kg) ensured almost physiological loading of the specimens. For quantification of functional injuries, the three-dimensional flexibility of the specimens was tested in a spine tester before and after each acceleration. In all six specimens, structural failure always occurred in the lower cervical spine and always affected the facet joint capsules and the intervertebral discs. In four specimens, this damage occurred during the 2 g collision, while in the other two it occurred during the 3 g and 4 g collision, respectively. The flexibility mainly increased in the lower cervical spine (especially in lateral bending to both sides) and, to a minor extent, in axial rotation. In vitro low-speed side collisions caused functional and structural injury to discoligamentous structures of the lower cervical spine, but did not damage the alar ligaments. Since the effects of muscle forces were not taken into account, the present in vitro study reflects a worst-case scenario. Injury thresholds should therefore not be transferred to reality.
Keywords: Side collision, Whiplash trauma, Cervical spine, Alar ligament, Biomechanics, In vitro experiment
Introduction
Whiplash trauma of the cervical spine is one of the most common injuries in traffic accidents. Even though vehicle safety systems are continually being improved, the incidence of whiplash is rising considerably. According to insurance statistics, approximately 200,000 whiplash traumas are likely to occur in Germany every year. This number of traumas corresponds to about € 0.75 billion in damages [7, 9]. In the United States, it is estimated that whiplash injury costs US$ 4.5 billion annually [21].
The three major factors which increase the relative risk of suffering a whiplash trauma in traffic accidents are an age between 20 and 30 years, female gender, and a collision from the rear [8, 11, 23, 21]. With regard to the risk of whiplash in this age group, it must be considered that the 20–30 year olds constitute the largest group of licensed drivers. In contrast to the relative risk, the absolute number of whiplash traumata seems to be greatest in head-on collisions [10].
Due to the high risk of injury involved, rear-end collisions have already been investigated in various scientific studies. Biomechanical data about loads and deformations of the cervical spine during rear-end collisions are available from numerous trials with volunteers [2, 3] and some in vitro tests with cadaveric cervical spine specimens [4, 7, 15, 16, 25]. Panjabi’s in vitro experiment in particular [16] indicated that rear-end collisions might cause functional injury to the lower cervical spine, but not to the upper segments. However, from a clinical point of view, about 20% of all patients suffer from symptoms in the upper cervical spine. the origin these complaints is still unknown, since both objective clinical findings and visualizable morphological substrates are lacking.
Ligament injuries of the upper cervical spine are known to occur in children [22]. Whether or not morphological changes within the alar ligaments and the other ligament structures of the upper cervical spine in adults (which might be detected on magnetic resonance images) are causally associated with clinical symptoms is still controversial [23].There are no accident analysis data available for injuries of the alar ligaments. Since insurance companies take into account the accident dynamics in the causality assessment of injuries, biomechanical studies on this topic are required.
The alar ligaments, which span the gap between the axis and the occiput, mostly serve to stabilize the craniocervical junction in lateral bending, axial rotation and flexion [17]. Consequently, stress is exerted on these ligaments mainly during side collisions, where lateral bending and additional axial rotation movements of the head relative to the torso are to be expected.
Unfortunately, data from volunteer trials on side collisions are rare and there is a complete lack of biomechanical studies on this topic. The objective of the present in vitro study was therefore to examine whether the alar ligaments or any other structures of the cervical spine are damaged in side collisions.
Materials and methods
A new, specially designed acceleration apparatus allowed cadaveric cervical spine specimens to be accelerated in any desired direction (Fig. 1). Its basic components were a pneumatic acceleration unit, a sled, and a rail track which was 9 m in length. The maximum sled acceleration could be varied continuously between 1 and 15 g by varying the pressure within the pneumatic acceleration unit, and the duration of the acceleration pulse could be changed by varying the distance over which the acceleration unit pushed the sled.
Fig. 1.

The acceleration apparatus consisted of three basic components: a pneumatic acceleration unit, a sled, and a rail track. On the sled, the cervical spine specimens were fixed on a damped pivot table, which accounted for the passive movements of the trunk during collision. On the occipital bone of the specimens, a dummy head was mounted which was suspended by a cord until acceleration, since buckling of the specimens would have occurred otherwise. At the beginning of the acceleration, this cord was cut in order to create unconstrained loading conditions during acceleration
On the sled, the cervical spine specimens were fixed on a damped pivot table which was allowed to pivot passively around an axis that was aligned perpendicularly to the direction of the acceleration (Fig. 1). This pivoting movement accounted for the passive movements of the trunk during collision and reached a maximum of approximately 0.5° when the sled was accelerated with 1 g, 1° when it was accelerated with 2 g, and 2° when it was accelerated with 3 g.
In order to guarantee adequate inertia, a dummy head was designed with a weight of 4.5 kg [4, 5]. Its centre of gravity was localized in the region of the anterior inferior sella [8]. It could be fixed with flanges on the occiput of cadaveric specimens. The removal of the original head was necessary to fix the cervical spine to the spine tester. Before acceleration, the weight of the dummy head was balanced using a suspension cord to prevent an unphysiological buckling of the osteoligamentous specimens. At the beginning of the acceleration, this suspension cord was cut in order to allow the head to move in a completely unconstrained manner and to ensure a realistic loading of the specimen.
Six human osteoligamentous cervical spine specimens (occiput–T1) from donors with an average age of 79.5 years (range 67–91 years) were freshly dissected and frozen at –20°C for storage. Before testing, they were thawed at 4°C and all soft tissue surrounding the discoligamentous spine was carefully removed. In order to fix the specimens on the acceleration apparatus and in the spine tester, C0 and T1 were embedded in polymethylmethacrylate (PMMA) with a horizontally aligned foramen magnum.
In the acceleration apparatus, the specimens were then subjected to an incremental 90° side collision from the right until a macroscopically visible structural failure occurred. For this purpose, maximum sled acceleration was first adjusted to approximately 1 g and then increased stepwise by 1 g to 2 g, 3 g, 4 g, etc. During collision, the dummy head did not contact any head rest.
After the acceleration pulse, the sled was slowed down with no more than –0.5 g in order to prevent any damage to the specimens due to deceleration. The one-dimensional horizontal displacement of the sled was registered within the sled coordinate system using one single uniaxial linear accelerometer (EGE-73AE1-100D1, Entran Sensors and Electronics, Ludwigshafen, Germany). Another three of these accelerometers were mounted around the head’s centre of gravity along the axes of the standardized head coordinate system. Data channel performance was adapted to SAE J211 and DIN ISO 6487. The sled data channel was characterized by a channel amplitude class (CAC) of 100 g and a channel frequency class (CFC) of 60 Hz, while the head data channels had a CAC of 100 g and a CFC of 1,000 Hz.
The whole sled acceleration pulse was considered to lie within the range recommended in the proposal for the ISO/TC22 N 2071/ISO/TS22/SC10 “Test procedure for the evaluation of the injury risk to the cervical spine in a low-speed rear-end impact”. Acceleration was measured during a 2-s measurement period. After each measurement, the velocity of the sled was calculated by integration of the filtered accelerometer data.
Before acceleration, the pivot table was aligned with a pre-inclination of 10° towards the acceleration unit. This pre-inclination accounted for the tilt angle of the trunk at the onset of the acceleration of the shoulders in real side collisions. During acceleration, additional passive damped pivoting was allowed.
After each acceleration, the three-dimensional flexibility of the specimens was quantified. This method allowed us to even detect microscopic injuries (which were then called functional injuries), and to assess the integrity or failure of deep and therefore not directly visible structures, such as the alar ligaments. For this purpose, the PMMA block on T1 was rigidly fixed to the spine tester (in contrast to the non-rigid fixation to the acceleration sled), whereas the upper block on C0 was connected to a cardan joint integrated into a three-dimensional slide system enabling unconstrained movements in all six degrees of freedom [24].
Pure moments in lateral bending, flexion–extension and axial rotation (±1.5 Nm) were applied continuously by stepping motors integrated into the cardan joint of the spine tester at a constant rate of up to 3.0°/s. During testing, the specimens were allowed to move in the five uncontrolled degrees of freedom in an unconstrained manner. In each loading direction, two cycles were applied for preconditioning of the specimens, and data were collected on the third.
The resulting rotations of the whole specimens (C0–C7) in the three principal motion planes were recorded by rotary potentiometers (Novotechnik, Ostfildern, Germany, resolution <0.1°) integrated in the three axes of the cardan joint. Since the focus was put on the middle and upper cervical spine and on the alar ligaments, the monosegmental motion of C0–C1, C1–C2, C2–C3, C3–C4 and C4–C5 was additionally recorded using an ultrasound motion analysis system (Cmstrao, Zebris, Isny, Germany, resolution <0.1°).
Range of motion (ROM), as the rotation at maximum load, and the neutral zone (NZ), as the difference between the loading and unloading curve at zero load, were determined from the resulting load deformation curves. Both parameters were evaluated separately for the positive and the negative loading direction in each of the three principal motion planes.
Descriptive statistics comprised calculation of median, minimum and maximum values across the six specimens. Statistical significance of the destabilizing effect of acceleration on ROM and NZ was evaluated using the one-sided exact Wilcoxon signed rank test. The level of significance was defined at P<0.05. Since this statistical evaluation was purely explorative, P values were not adjusted for multiple comparisons.
Results
Accelerometer data
The acceleration pulse of the sled was characterized by a steep rise, followed by a plateau and a steep decrease (Fig. 2).The resultant pulse of the head, in contrast, showed a slow increase, a sharp maximum and a slow decrease. A gap was observed between the times of onset of the two pulses, which decreased with increasing acceleration from 31 ms (median value) in the 1 g collision, to 20 ms in the 2 g collision to 11 ms in the 3 g collision. After that gap, the resultant head acceleration rose and always exceeded the absolute sled acceleration approximately 100 ms after the beginning of the pulse of the sled.
Fig. 2.
Sled horizontal acceleration and head resultant acceleration during the 1 g and 2 g collisions. Median curves of six specimens. (asled sled acceleration; ares head head resultant acceleration)
The maximum resultant head acceleration was always almost twofold higher than that of the sled, whereas the mean resultant head acceleration tended to be lower than that of the sled (Table 1). Accordingly, the percentage relation between mean and maximum acceleration (adt/amax) was 77% for the sled but only 48% for the head in the 1 g collision, 74 and 37% in the 2 g collision, and 71 and 29% in the 3 g collision.
Table 1.
Maximum acceleration (amax), mean acceleration (adt), duration of the acceleration pulse (Δt) and velocity change (Δv) of the sled and the head (resultant of x- and y-axes) during the 1, 2 and 3 g side collision. Median (minimum...maximum) of six specimens in the 1 and 2 g collision, and median of two specimens in the 3 g collision
| amax [g] | adt [g] | Δt [ms] | Δv [km/h] | ||
|---|---|---|---|---|---|
| 1 g | Sled | 1.28 (1.10...1.34) | 0.94 (0.87...1.04) | 124 (118...127) | 4.27 (4.18...4.43) |
| Head resultant | 2.23 (2.15...2.77) | 1.13 (0.92...1.21) | 223 ( 211...307) | - | |
| Head/sled | 1.74 | 1.20 | 1.80 | - | |
| 2 g | Sled | 2.36 (2.25...2.52) | 1.76 (1.68...1.85) | 125 (121...128) | 8.14 (7.74...9.59) |
| Head resultant | 4.12 (3.11...5.63) | 1.61 (1.13...2.03) | 227 (191...278) | - | |
| Head/sled | 1.75 | 0.91 | 1.82 | - | |
| 3 g | Sled | 3.82 (3.73...3.90) | 2.71 (2.64...2.78) | 125 (122...128) | 12.46 (12.28...12.64) |
| Head resultant | 7.13 (5.93...8.32) | 2.04 (1.94...2.13) | 225 (220...230) | - | |
| Head/sled | 1.87 | 0.75 | 1.80 | - |
The duration of the acceleration pulses of the sled and the head did not vary significantly between the 1 g, 2 g and 3 g collisions. The sled pulse always lasted for about 125 ms and the head pulse for 225 ms. Integration of the filtered accelerometer data enabled the determination of the velocity change (Δv) of the sled, which was 4.3 km/h in the 1 g collision, 8.1 km/h in the 2 g collision, and 12.5 km/h in the 3 g collision. A second integration showed that the difference between the distance covered by the head and that covered by the sled always reached its maximum value approximately 150 ms after the beginning of the acceleration of the sled.
Structural injury
Structural failure always occurred in the lower cervical spine and always affected the facet joint capsules and the intervertebral discs. A complete rupture of the left facet joint capsule in combination with a partial rupture of the intervertebral disc was observed in five specimens, and an additional rupture of the right capsule was observed in one specimen. Two specimens were injured at C5–C6, two at C6–C7 and two at C7–T1. No lesions of the alar ligaments were detected in any of the six cases. In four specimens damage occurred during the 2 g collision, in one during the 3 g collision and in one during the 4 g collision.
Functional injury
Four specimens failed during the 2 g collision, one during the 3 g collision, and one during the 4 g collision. Complete flexibility data from all six specimens could be recorded before and after acceleration with 1 g, whereas flexibility data after 2 g were available from only two specimens and from only one specimen after 3 g.
In general, the side collisions seemed to affect the stability of the specimens mainly in lateral bending and, to a minor extent, in axial rotation (Tables 1, 2, Figs. 3, 4). Almost no destabilizing effect was observed in flexion and extension (Fig. 5).
Table 2.
ROM in degrees before and after acceleration with 1 g in right and left lateral bending, flexion and extension and left and right axial rotation. P values for comparison of the ROM before and after acceleration (one-sided exact Wilcoxon signed rank test)
| Left lateral bending | Right lateral bending | |||||
|---|---|---|---|---|---|---|
| ROM before acceleration | ROM after acceleration | P value | ROM before acceleration | ROM after acceleration | P value | |
| C0–C1 | −4.1 | −4.0 | 0.656 | 3.5 | 4.4 | 0.031 |
| C1–C2 | −2.3 | −1.5 | 0.500 | 2.4 | 2.5 | 0.594 |
| C2–C3 | −3.9 | −3.4 | 0.844 | 4.0 | 3.6 | 0.781 |
| C3–C4 | −1.6 | −1.7 | 0.062 | 1.5 | 1.7 | 0.219 |
| C4–C5 | –2.7 | −2.9 | 0.344 | 2.0 | 2.4 | 0.156 |
| C0–C7 | −17.5 | −19.3 | 0.031 | 16.6 | 19.6 | 0.016 |
| Extension | Flexion | |||||
| C0–C1 | −9.6 | −7.5 | 0.922 | 13.9 | 11.6 | 0.953 |
| C1–C2 | −4.3 | −4.0 | 0.781 | 6.9 | 7.3 | 0.281 |
| C2–C3 | −3.1 | −2.8 | 0.719 | 1.0 | 1.2 | 0.422 |
| C3–C4 | −4.7 | −4.8 | 0.500 | 1.7 | 0.8 | 0.969 |
| C4–C5 | −4.9 | −5.2 | 0.016 | 2.1 | 1.8 | 0.500 |
| C0–C7 | −32.7 | −32.1 | 0.891 | 23.8 | 24.3 | 0.500 |
| Right axial rotation | Left axial rotation | |||||
| C0–C1 | −4.7 | −5.1 | 0.031 | 4.7 | 4.8 | 0.578 |
| C1–C2 | −27.3 | −26.9 | 0.891 | 28.7 | 31.5 | 0.094 |
| C2–C3 | −2.7 | −2.8 | 0.344 | 2.4 | 2.6 | 0.109 |
| C3–C4 | −3.1 | −3.2 | 0.109 | 3.3 | 1.9 | 0.969 |
| C4–C5 | −4.9 | −5.1 | 0.031 | 5.1 | 5.2 | 0.406 |
| C0–C7 | −49.6 | −50.3 | 0.203 | 52.1 | 52.5 | 0.281 |
Fig. 3.
ROM of each of the six specimens before acceleration (open symbols) and after acceleration with 1 g (filled symbols) in the segments C0–C1, C1–C2, C2–C3, C3–C4, C4–C5 and in the whole cervical spine (C0–C7) in right and left lateral bending
Fig. 4.
ROM of each of the six specimens before acceleration (open symbols) and after acceleration with 1 g (filled symbols) in the segments C0–C1, C1–C2, C2–C3, C3–C4, C4–C5 and in the whole cervical spine (C0–C7) in flexion and extension
Fig. 5.
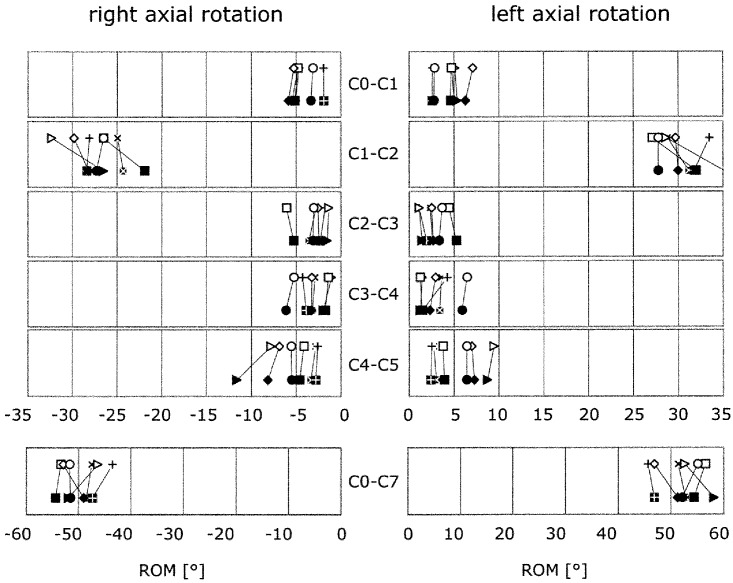
ROM of each of the six specimens before acceleration (open symbols) and after acceleration with 1 g (filled symbols) in the segments C0–C1, C1–C2, C2–C3, C3–C4, C4–C5 and in the whole cervical spine (C0–C7) in left and right axial rotation
In lateral bending to the right, the median ROM of the whole specimens C0–C7 increased from 16.6° before acceleration to 19.6° after acceleration with 1 g (P=0.016), and in lateral bending to the left it increased from −17.5° to −19.3° (P=0.031). This almost symmetrical increase did not seem to be caused by any of the single segments (C0–C1, C1–C2, C2–C3, C3–C4, C4–C5) of the upper and middle cervical spine and, therefore, has to be attributed to the segments (C5–C6 and/or C6–C7) of the lower cervical spine. The overall ROM further increased after the 2 g collision in the specimen which failed at 3 g, and after the 2 g and 3 g collisions in the specimen which failed at 4 g (Fig. 6). The NZ also showed an increase in right lateral bending from 6.8° to 9.0° (P=0.031) and in left lateral bending from −5.3° to −7.3° (P=0.156) in the whole specimens, but no distinct segmental increase in the upper or middle cervical spine.
Fig. 6.
ROM of the one specimen which could not only be tested before acceleration (white) and after acceleration with 1 g (light grey), but also after acceleration with 2 g (dark grey) and 3 g (black). Results for the segments C0–C1, C1–C2, C2–C3, C3–C4, C4–C5 and the whole cervical spine (C0–C7) in right and left lateral bending
In axial rotation to the left, there was a slight increase in C0–C7 from 52.1° to 52.5°, and in axial rotation to the right, there was an increase from −49.6 to −50.3 - however, there is a high probability that this trend was incidental (P=0.281 and P=0.203) (Table 2, Fig. 4). No marked increase in the ROM or NZ was observed in flexion and extension, neither in the whole cervical spine nor in the single segments of the upper and middle cervical spine (Fig. 5).
Discussion
The aim of the present in vitro study was to investigate whether the ligaments of the upper cervical spine or any other structures of the cervical spine are damaged in side collisions. Incomplete injuries to ligaments cannot be identified with imaging techniques. Flexibility tests involving the application of pure moments before and after trauma are able to detect the resulting ligament failures under in vitro conditions [15].
The results of the NZ and ROM showed that the structures of the upper cervical spine (especially the alar ligaments) remained uninjured in all six specimens. The assumed high vulnerability of these ligaments in side collisions could therefore not be confirmed under in vitro conditions.
All macroscopically observed injuries occurred in the lower cervical spine and always affected discoligamentous structures. The kind and localization of the injury were therefore similar to those reported for in vitro rear-end collisions [15] and corresponded well with clinical observations, where whiplash patients more often suffer from symptoms related to the lower than to the middle or upper cervical spine [11].
Head kinematics
In all collisions, a delay was observed between the onset of the acceleration pulse of the sled and that of the head. This delay reflects a translational movement between head and sled and might induce an S-shaped deformation of the cervical spine similar to that reported for rear-end collisions [6, 12].
In rear-end impacts the S-shaped deformation of the spine is assumed to be more critical than the subsequent C-shaped deformation [6]. However, maximum stress and strain within the spine seem to occur much later. In the present study, both the absolute resultant head acceleration (as a measure for the stress) and the relative resultant displacement between head and sled (as a measure for the strain) did not reach their maximum values until 150 ms after the beginning of the acceleration of the sled.
Functional injury
None of the current clinical imaging methods is capable of visualizing specific alterations in acute whiplash patients [1, 18, 19, 20]. The patients’ symptoms could therefore be caused by microscopic injuries, which might best be detected indirectly by quantification of the resulting dysfunction seen as increases in NZ and ROM under in vitro conditions. In the present study, flexibility tests seemed to be the best suited functional tests for identification of microscopic injuries to discoligamentous structures of the cervical spine [14,15]. Therefore, the three-dimensional flexibility of the specimens after acceleration was compared to that before acceleration. A significant increase in flexibility was then interpreted as a functional or microscopic structural injury.
Due to acceleration, the flexibility of the whole cervical spine increased especially in lateral bending and, to a minor extent, in axial rotation, but not in flexion and extension. These results exactly reflect the kind of load that was applied. A 90° side collision mainly causes a deformation of the cervical spine in the frontal plane, which increases the flexibility in lateral bending. Additional deformations in the transverse plane occur since the mass centre of gravity of the head in an upright body position is located anteriorly to the cervical spine [8].This deformation might be responsible for the increase in flexibility in axial rotation.
The increase in flexibility in lateral bending affected both sides to a similar extent, but not, as expected, chiefly that to the right. In its initial phase, the side collision from the right caused the head to move rightwards relative to the sled. However, once this initial phase was passed, a kind of damped oscillation of the head relative to the sled occurred. This resulted in alternating stresses to the left- and right-sided structures of the specimens, and might therefore be responsible for the symmetrical increase in flexibility in this plane. Since the aim of the present study was to create and examine injuries that occur exclusively during the initial phase of the collision, the oscillation of the head had to be suppressed. Maximum deceleration was therefore kept below 0.5 g. A complete suppression, however, was technically not achievable.
Besides the oscillating movement, an S-shaped deformation of the specimens could also have led to the symmetrical increase in flexibility in lateral bending. The upper segments would have been stressed on their right side, while the lower segments would have been stressed on their left side, resulting in an overall increase in flexibility to both sides.
Structural injury
In four specimens, the structural injury occurred in the 2 g collision, in one specimen it occurred in the 3 g collision and in another in the 4 g collision. These thresholds cannot directly be transferred to real accidents for two major reasons: the absence of muscles and the almost rectangular acceleration pulse.
The neck muscles passively and actively stabilize the cervical spine in real accidents. While the passive stabilization acts permanently, the active stabilization depends on a certain reaction time. In low-speed rear-end collisions with volunteers, Magnusson and coworkers found that the average reaction time of deep and superficial neck muscles ranged between 66 ms (levator scapulae) and 119 ms (splenius capitis) relative to the beginning of the sled movement [13]. Even if it is assumed that another 100 ms is needed until efficient muscle tension is developed, single muscle fibres would already have actively stabilized the cervical spine during the phase of maximum stress and strain, which, in the present study, occurred approximately 150 ms after the onset of the acceleration of the sled.
Nevertheless, in in vitro acceleration experiments, Grauer et al. [6] could show that the deformation of osteoligamentous cadaveric cervical spine specimens during simulated rear end collisions was well in accordance with that reported from volunteer tests [12]. The lack of muscles therefore seems to affect motion quantity rather than motion quality. Thus, the kind and site of injury may be transferred to reality, whereas injury thresholds should not. In order to additionally enable a quantitative interpretation of the results, the simulation of neck muscles should be a priority in the future.
Rectangular acceleration pulses are uncommon in real collisions. In most car-to-car collisions, the mean acceleration reaches no more than 25–50% of the maximum value. Since the area below the acceleration-time curve accounts for the energy which is transferred during collision, an increasing percentage could be interpreted as an increasing injury risk to the passenger. The pulses of the present study with mean accelerations between 71 and 77% of the maximum value should therefore be classified as pulses with a high injury risk. They resemble pole rather than car-to-car side-collisions. Nevertheless, in the present study, the resultant acceleration curves of the head were qualitatively similar to those reported in volunteer or dummy tests [10, 12]. Furthermore, the peak acceleration of the head was approximately twofold higher than that of the sled. This observation also corresponds well with reported volunteer and dummy data.
Conclusion
In conclusion, in vitro low-speed 90° side collisions without head contact caused functional and structural injury to discoligamentous structures of the lower cervical spine, but did not damage the alar ligaments. These results should not be transferred to any other type of collision (not even to any other type of side collision) since, for example, an oblique acceleration, an additional rotation or head contact, would exert a significant influence on the loading pattern of the spine. Furthermore, the present in vitro study represents a worst-case scenario due to the absence of muscles. Consequently, the measured critical sled acceleration of 2–4 g cannot directly be transferred to reality. Nevertheless, this study qualitatively shows what kind of functional and structural injury most probably occurs in 90° side collisions, and disproves the assumed vulnerability of the alar ligaments in this kind of impact.
Acknowledgements
The authors would especially like to thank Mr. Voigt of the Department of Anatomy, University of Ulm, for the preparation of the specimens, and gratefully acknowledge the Deutsche Forschungsgemeinschaft DFG (HA 3276/1-1) and the University Hospital of Ulm, Germany (P.648) for their financial support.
References
- 1.BicikNeurology 1998513459710001 [Google Scholar]
- 2.Castro W, Lemcke H, Schilgen M, Lemcke L (1998) [So-called “whiplash trauma”—legal and medical considerations]. Chirurg 69 [Suppl]: 176–184 [PubMed]
- 3.Castro Eur Spine J. 1997;6:366. doi: 10.1007/BF01834062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemens Arch Orthop Unfallchirurg. 1972;73:220. doi: 10.1007/BF01880731. [DOI] [PubMed] [Google Scholar]
- 5.Clemens Arch Orthop Unfallchirurg. 1972;74:116. doi: 10.1007/BF00416163. [DOI] [PubMed] [Google Scholar]
- 6.Grauer Spine. 1997;22:2489. doi: 10.1097/00007632-199711010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Grifka Orthopade. 1998;27:802. doi: 10.1007/s001320050302. [DOI] [PubMed] [Google Scholar]
- 8.Harrison D, Janik T, Jones EW, Cailliet R, Normand M (2001) Comparison of axial and flexural stresses in lordosis and three buckled configurations of the cervical spine. Clin Biomech (Bristol, Avon) 16:276–284 [DOI] [PubMed]
- 9.Hell W, Langwieder K (1998) Epidemiologische Daten zur HWS—Beschleunigungsverletzung. Die Notwendigkeit eines verbesserten Diagnosestandards und bessere Prävention bei Auffahrunfällen. Hefte zu “Der Unfallchirurg” 272:80–82
- 10.Jakobsson Accid Anal Prev. 2000;32:307. doi: 10.1016/S0001-4575(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 11.Johnson J Accid Emerg Med. 1996;13:3. doi: 10.1136/emj.13.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneoka Spine. 1999;24:763. doi: 10.1097/00007632-199904150-00006. [DOI] [PubMed] [Google Scholar]
- 13.Magnusson Eur Spine J. 1999;8:118. doi: 10.1007/s005860050140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panjabi Spine. 1998;23:17. doi: 10.1097/00007632-199801010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Panjabi J Spinal Disord. 1998;11:227. [PubMed] [Google Scholar]
- 16.PanjabiOrthopade 1998278139894235 [Google Scholar]
- 17.Panjabi J Spinal Disord. 1991;4:270. [PubMed] [Google Scholar]
- 18.Pettersson Spine. 1997;22:283. doi: 10.1097/00007632-199702010-00010. [DOI] [PubMed] [Google Scholar]
- 19.Pettersson Acta Orthop Scand. 1994;65:525. doi: 10.3109/17453679409000906. [DOI] [PubMed] [Google Scholar]
- 20.Ronnen Radiology. 1996;201:93. doi: 10.1148/radiology.201.1.8816527. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer Spine. 1995;20:1S. [PubMed] [Google Scholar]
- 22.SunJ Neurosurg 2000932810879755 [Google Scholar]
- 23.Volle Ear Nose Throat J. 2001;80:41. [PubMed] [Google Scholar]
- 24.WilkeEur Spine J 19943917874556 [Google Scholar]
- 25.Yoganandan J Biomech Eng. 1997;119:237. doi: 10.1115/1.2796086. [DOI] [PubMed] [Google Scholar]