Abstract
The influence of additional dorsal structure damage on anterior stabilization of a thoracolumbar fracture is still unknown. Screw-cement enhancement can be used to reinforce the stability of anterior instrumentation. We have developed a new anchorage system for fixation of anterior stabilization devices, adapted through geometric optimization and the additional option of cementation after screw insertion. This study examines the question of whether this enhancement is strong enough to enable a single anterior procedure and still compensate for dorsal instability. Various spinal reconstruction procedures were evaluated biomechanically in an increasing ventrodorsal instability model for thoracolumbar fracture stabilization. A biomechanical in vitro study, simulating stabilized defect situations (corporectomy/vertebrectomy) with strut grafting and overbridging instrumentation, was performed on six human T10–L2 cadaveric specimens. The primary stability parameters, range of motion and neutral zone, were evaluated with or without anterior screw-cement enhancement. This was compared with a single conventional anterior stabilization without a dorsal defect (corporectomy). It was also compared with a single anterior, posterior or combined procedure in the presence of additional dorsal structure damage (vertebrectomy). The use of an additional cementable screw dowel enhanced the primary stability of the anterior instrumentation, compensating for dorsal instability. These results are warranted for the clinical use of minimally open or endoscopic techniques, creating the highest possible primary stability while performing a single anterior enhanced instrumentation with a tissue-preserving approach.
Keywords: Spine, Thoracolumbar fracture treatment, Stabilization, Biomechanics
Introduction
Anterior spinal fixation is commonly used to manage fractures, tumors and deformities of the spinal column and has become an increasingly accepted method. The influence of additional dorsal structure damage on anterior stabilization of a thoracolumbar fracture is still unknown. There have been many biomechanical studies evaluating spinal reconstruction methods in corporectomy models [1, 2, 7, 9, 27, 30]; however, few investigators have examined the biomechanics of spinal reconstruction after additional dorsal structural damage [10, 14]. In the clinical setting, a combination of anterior strut graft and ventrodorsal instrumentation is commonly performed. Because minimally invasive anterior and endoscopic techniques are becoming more widespread in spinal surgery, this topic has again moved into the spotlight [3, 4, 5, 6, 8, 12, 13, 16, 17, 21, 22, 23, 24, 25].
No investigators have evaluated screw-cement enhancement for the reinforcement of the primary stability of anterior stabilization systems in the presence of dorsal structure damage. We have developed a new anchorage system for fixation of anterior stabilization devices, adapted through geometric optimization and the additional option of cementation after screw insertion. This study was designed to investigate the hypothesis that the newly developed additionally cementable hollow screw dowel improves the primary stability of an anterior stabilization system, compensating for additional dorsal structure damage in a biomechanical in vitro model with ventrodorsal instability.
Materials and methods
Polyaxialscrew XL
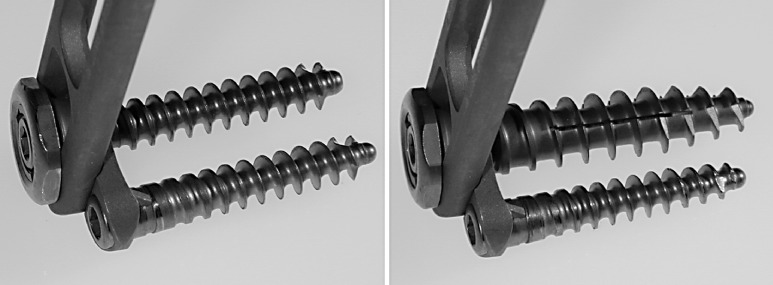
A totally endoscopically implantable anchorage system for implant fixation in the anterior vertebral body was developed (Fig. 1, 2). The self-tapping, threaded hollow screw dowel (Polyaxialscrew XL, Aesculap, Tuttlingen, Germany), made of titanium, is 30 or 40 mm long, with a core diameter of 6 mm, thread diameter of 10 mm, and a screw pitch of 4 mm. Additional cementation after implantation is possible through three slits along the longitudinal axis . Exactly 2 ml of Osteopal bone cement was used (Merck Biomaterial, Darmstadt, Germany), the maximum allowed by the applicator. This small amount sufficed to enhance the screw base; therefore potential problems from runoff or cement heating appear not to be of concern. The viscosity of Osteopal eases absorption in the applicator and injection through the pre-inserted Polyaxialscrew XL. Cement hardening takes about 5 min in situ.
Fig. 1.

Polyaxialscrew XL cemented (caudal) and un-cemented (cranial)
Fig. 2.
MACS TL Twin Screw (left) and MACS TL Polyaxialscrew XL un-cemented (right)
The new Polyaxialscrew XL can optionally be used with the modular anterior construct system (MACS TL, Aesculap, Tuttlingen, Germany). This allows: an endoscopic approach and thoracoscopic instrumentation from T4 to L1; endoscopic diaphragm splitting and thoracoscopic instrumentation to L2; and a minimally invasive retroperitoneal approach to L3/4 for fracture stabilization. As a Twin Screw concept, it consists of a rigid-angle, stable monocortical anchorage, using two convergent screws—a polyaxial posterior screw (length 25–50 mm, diameter 7 mm) and an anterior stabilization screw (length 25–50 mm, diameter 6.5 mm), in each vertebral body. Low-profile plates or rods are available. If an enforced screw hold is needed, the Polyaxialscrew XL is an alternative to the posterior screw of the Twin Screw concept. It is implantable initially or as second chance, in place of the posterior screw, with or without cementation.
Biomechanical testing
The biomechanical in vitro tests were performed with six human T10–L2 specimens. A radiographic examination was done to ensure the absence of structural spinal disorders or previous spinal surgery. Median age was 82 years. The specimens were wrapped in triple-sealed plastic bags and kept frozen at −28°C prior to preparation and testing. Median trabecular bone mineral density (BMD) of the anterior vertebral body was 133 mg/cm3, as measured by peripheral quantitative computed tomography (CT) at each level in a horizontal plane (XCT-9600A pQCT, Stratec, Birkenfeld, Germany). The CT was calibrated using a hydroxylapatite phantom. Before testing, the fresh-frozen specimens were thawed at room temperature. In preparation, surrounding soft tissue and muscles were carefully dissected to preserve bone, discs and spinal ligaments. During testing the specimens were kept moist with saline (0.9% sodium chloride) irrigation solution. Tests were performed at normal room temperature (20°C) and in an average atmospheric humidity of 60%, as continually recorded by a hygrometer to minimize the effects of soft-tissue degradation. T10 and L2 were potted in polymethylmethacrylate (Technovit 3040, Heraeus Kulzer, Wehrheim/Ts, Germany) for fixation in the spine tester [28] (Fig. 3). To achieve better anchorage of the vertebra in this material, short screws were partially driven into the embedded bony structures. L2 was fixed rigidly in the testing device. T10 was fixed to a cardan device containing integrated stepper motors that could introduce pure moments separately around three axes. The remaining five out of six degrees of freedom were free, enabling the specimen to move unconstrained. Tests in flexion/extension, lateral bending and rotation were first performed on the intact specimens.
Fig. 3.

Thoracolumbar human specimen T10–L2 with corporectomy of T12 fixed in the three-dimensional spinal loading simulator and stabilized with the MACS TL System and a strut graft-simulating wooden block
Stabilization was performed after creating the corporectomy and strut grafting between the vertebral bodies, proximally and distally. For strut grafting of the corporectomy defect (corporectomy, resection of the anterior ligaments and adjacent discs were performed), an adapted wooden block representing the strut graft was made. As increasing dorsal instability additional to the corporectomy, a laminectomy and bilateral facetectomy simulating the maximal circumferential structure damage of a vertebrectomy were performed.
Ventral stabilization was done with the MACS TL system (Twin screw or Polyaxialscrew XL) (Aesculap, Tuttlingen, Germany) and/or dorsal stabilization using the US System (Stratec, Oberdorf, Switzerland). Each device was implanted according to the insertion instructions provided by the manufacturer. The MACS TL™ Twin Screws were 40 mm long for the posterior screw and 30 mm for the anterior screw. Plate length ranged 80–100 mm. Length of the Polyaxialscrew XL was 40 mm. All ventral screws were inserted unicortically. After screw insertion, cementation was performed using the above-mentioned technique. To simulate the in vivo, interbody grafting technique, maximal manual compression was applied on the strut graft with the overbridging implants, when tightening the plate/rod junctions. Proper placement of the various instrumentations and cementation was verified radiographically after each step.
Stabilization between the vertebral bodies, proximal and distal to the load cell, was performed using the following common combinations of hardware components, all tested on the same six specimens according to standardization criteria for in vitro testing of spinal implants [29]:
Intact spine
Corporectomy: MACS TL with Twin Screw Concept
Vertebrectomy: MACS TL with Polyaxialscrew XL cemented
Vertebrectomy: MACS TL with Polyaxialscrew XL cemented + US System dorsal
Vertebrectomy: US System dorsal
Pure moments of 3.75 Nm without axial preload in flexion/extension (±My), right/left lateral bending (±Mx), and left/right axial rotation (±Mz) were first applied at a constant rate of 1.7°/s on the intact specimens [19, 20, 29]. Two precycles were applied to precondition the construct so as to minimize the viscoelastic effect, and data of the third cycle were recorded. Resulting three dimensional rotations were measured between all adjacent segments with a non-contacting ultrasound motion measurement system (Cmstrao 1.0, Zebris, Isny, Germany). From the load-deformation curves, T11–L1 range of motion (ROM) and neutral zone (NZ) were determined for the three principal motion planes [19, 20].
Range Of Motion (ROM) was defined as the angular deformation at maximum load. The neutral zone (NZ), a measure for laxity, was defined as the difference at zero load between the angular positions corresponding to the loading and unloading phases of the test cycle. This corresponds to the range in which only very small moments are needed to flex, rotate and bend the specimen.
Data are reported as means and standard deviations of the observed ROM and NZ. Nonparametric tests were used because sample sizes were small and our data were not distributed normally. We performed the Friedman test to determine whether there were significant differences between the tested conditions. Afterwards a Wilcoxon signed rank test was used. Although we tested many conditions and several parameters, we did not adjust the calculated p value for multiple parameters. This would have resulted in a great loss of information. Since a Bonferroni–Holm correction for multiple comparisons would have raised all p values above 0.05, no p- values are presented and no level of significance has been determined.The p values listed in this paper are considered as indicating tendencies and underlining descriptive statistics, but not revealing statistically significant differences.
Results
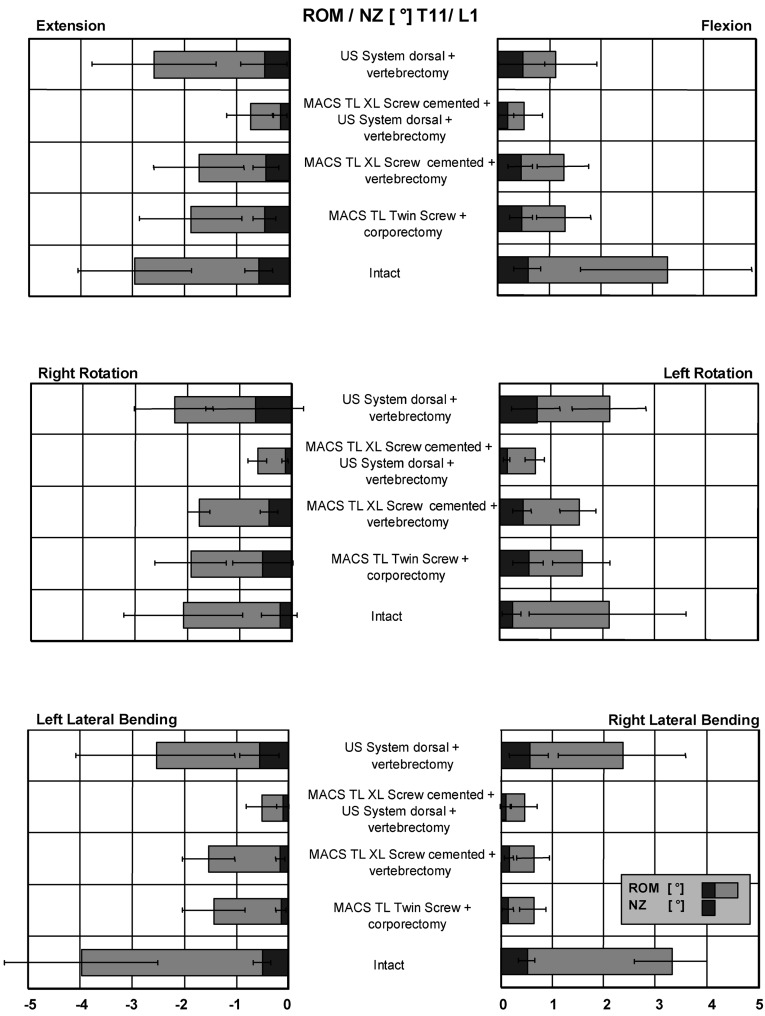
In flexion with the MACS TL Twin Screw system, ROM was reduced from 3.3° (intact) to 1.3° and NZ from 0.6° to 0.5° (Fig. 4). Dorsal destabilization was performed by laminectomy and bilateral facetectomy as described above. Following dorsal destabilization and subsequent stabilization with the cemented MACS TL Polyaxialscrew XL System, the ROM was 1.3° and NZ 0.4°. Measured ROM with dorsoventral instrumentation was 0.5° (NZ 0.2°) and with single dorsal stabilization alone, 1.1°(NZ 0.5°). In extension with the MACS TL Twin Screw system, ROM was reduced from 3.0° (intact) to 1.9° and NZ from 0.6° to 0.5°. Following dorsal destabilization and subsequent stabilization with the cemented MACS TL Polyaxialscrew XL System, the ROM was 1.7° and NZ 0.4°. Measured ROM with dorsoventral instrumentation was 0.7° (NZ 0.2°) and with single dorsal stabilization alone, 2.6° (NZ 0.5°).
Fig. 4.
Mean values and standard deviations for ROM and NZ of T11–L1 for all loading directions with applied maximum moments of 3.75 Nm
In left axial rotation with the MACS TL Twin Screw system, ROM was reduced from 1.5° intact to 1.9° and NZ increased from 0.2° to 0.5°. Following dorsal destabilization and subsequent stabilization with the cemented MACS TL Polyaxialscrew XL System the ROM was 1.7° and NZ 0.4°. Measured ROM with dorsoventral instrumentation was 0.7° (NZ 0.2°) and with single dorsal stabilization alone, 2.3° (NZ 0.7°). In right axial rotation with the MACS TL Twin Screw system, ROM was reduced from 2.1° (intact) to 1.6° and NZ increased from 0.2° to 0.5°. Following dorsal destabilization and subsequent stabilization with the cemented MACS TL Polyaxialscrew XL System, ROM was 1.5° and NZ 0.4°. Measured ROM with dorsoventral instrumentation was 0.7° (NZ 0.2°) and with single dorsal stabilization alone, 2.1° (NZ 0.7°).
In left lateral bending with the MACS TL Twin Screw system, ROM was reduced from 3.3° intact to 0.6°, and NZ decreased from 0.5° to 0.1°. Following dorsal destabilization and subsequent stabilization with the cemented MACS TL Polyaxialscrew XL System, ROM was 0.6° and NZ 0.1°. Measured ROM with dorsoventral instrumentation was 0.4° (NZ 0.1°) and with single dorsal stabilization alone, 2.4° (NZ 0.5°). In right lateral bending with the MACS TL Twin Screw system, ROM was reduced from 4.0° intact to 1.4°, and NZ decreased from 0.5° to 0.1°. Following dorsal destabilization and subsequent stabilization with the cemented MACS TL Polyaxialscrew XL System, ROM was 1.5° and NZ 0.4°. Measured ROM with dorsoventral instrumentation was 0.5° (NZ 0.1°) and with single dorsal stabilization alone, 2.6° (NZ 0.5°).
Statistics
P values concerning differences in ROM and NZ of the instrumented segments for all loading conditions were determined by the Friedman test and the Wilcoxon signed-rank test for all instrumentations (See Table 1).
Table 1.
Significance levels of differences in ROM and NZ of the instrumented segments T11–L1 for all loading conditions, as determined by the Friedman test and the Wilcoxon signed-rank test for all implants (USS US System, Comb. combination)
| Loading condition | Flexion ROM+ | Extension ROM− | NZ | Lateral ROM+ | Bending ROM− | NZ | Axial ROM+ | Rotation ROM− | NZ |
|---|---|---|---|---|---|---|---|---|---|
| 0.001 | 0.002 | 0.01 | 0.001 | 0.001 | 0.001 | 0.003 | 0.01 | 0.02 | |
| Twin Screw vs intact | 0.03 | 0.04 | 0.30 | 0.03 | 0.03 | 0.03 | 0.12 | 0.46 | 0.50 |
| XL Screw vs intact | 0.03 | 0.03 | 0.13 | 0.03 | 0.03 | 0.03 | 0.34 | 0.46 | 0.34 |
| Comb. vs intact | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.24 |
| USS vs intact | 0.03 | 0.05 | 0.40 | 0.12 | 0.11 | 0.60 | 0.75 | 0.91 | 0.60 |
| Twin Screw vs XL Screw | 0.60 | 0.30 | 0.90 | 0.60 | 0.34 | 0.46 | 0.24 | 0.46 | 0.91 |
| Twin Screw vs comb. | 0.03 | 0.04 | 0.03 | 0.12 | 0.03 | 0.25 | 0.03 | 0.03 | 0.03 |
| Twin Screw vs USS | 0.60 | 0.10 | 0.91 | 0.03 | 0.17 | 0.03 | 0.07 | 0.24 | 0.24 |
| XL Screw vs comb. | 0.03 | 0.03 | 0.30 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 |
| XL Screw vs USS | 0.60 | 0.04 | 0.90 | 0.03 | 0.17 | 0.03 | 0.17 | 0.25 | 0.34 |
Discussion
This biomechanical in vitro study investigated the influence of anterior screw-cement enhancement on additional dorsal instability in a human corporectomy/vertebrectomy stabilizing model. This enhancement increased the stability of the ventral instrumented specimen and compensates for dorsal instability. With the cemented Polyaxialscrew XL, the total ROM of the dorsoventral destabilized specimen (vertebrectomy) was the same or better than that of the initial MACS TL Twin Screw instrumentation without dorsal instability in the corporectomy model, stabilized with a strut graft and overbridging implant in the three primary directions. Dorsal stabilization and strut grafting of the circumferential defect (vertebrectomy) alone increased total ROM in all directions over the ROM of the initial MACS TL Twin Screw stabilization without dorsal instability and in flexion/extension and axial rotation over that of the intact specimen. In anticipation, maximal primary stability was achieved with dorsoventral stabilization in all directions for the circumferential instability (vertebrectomy).
There have been many biomechanical studies evaluating spinal reconstruction methods in corporectomy models; however, few investigators have examined the biomechanics of spinal reconstruction techniques including additional dorsal instabilities [10, 11, 14, 18]. From the biomechanical point of view, neither short posterior nor anterior instrumentation alone should be selected after total spondylectomy. Short circumferential reconstruction is better than multilevel posterior instrumentation and requires fewer levels of spinal reconstruction [18]. Combined anterior and posterior reconstruction may not be necessary if anterior reconstruction alone can restore sufficient stability without posterior augmentation. Results of Howard’s studies underscore the importance of meticulous grafting technique to provide a load sharing strut graft [10, 11]. The axial compression force created on the strut graft by the overbridging implant is an important factor on the stability of anterior instrumentation systems[26]. In a case where one unilateral facet was intact, joint stabilization with the Kaneda SR system and anterior strut grafting achieved equivalent stability to circumferential instrumentation [14]. In this study both axial compression force and enhanced screw hold of the Polyaxialscrew XL provided similar primary stability parameters, even in the presence of dorsal structural damage in comparison to the described anterior stabilization without dorsal defects.
There are some noteworthy limitations when applying the acquired data to clinical situations. Ventral fracture reduction the in case of rotational deformity is still an unsolved problem. Often the operative procedure requires an initial dorsal reduction-supporting instrumentation to achieve a physiological alignment. Therefore, single anterior stabilization, even with the previously mentioned cement enhancement, is clinically unsuitable in types of fractures with a rotational malposition of the spine without an initial dorsal reduction supporting instrumentation. Together it demonstrates maximal stability, especially in the elderly and in the osteoporotic specimens we tested. The study was conceived as a worst-case circumferential instability model, to demonstrate the maximal potential biomechanical effects of the screw-cement enhancement, even if the clinical situation often demonstrates smaller additional dorsal injuries, such as fractures of the lamina or ligamentous ruptures with intact articulation of the facet joints. Therefore, the effectiveness of this technique has been proven biomechanically in this study, including all defect situations without malrotation. Stabilizing effects of the extrinsic stabilizers, such as the neuromuscular system, were not considered in this study. On the other hand, a dorsal approach sacrifices the posterior spinal musculature immediately after the operation and through atrophy in the long term.
Comparing studies on biomechanical in vitro testing using cadaveric or animal specimens can be problematic [15]. First, specimen variations are not negligible in age, size and bone mineral density. The second issue is the low reproducibility of the experiments. Therefore, in this study tests have been done according to recommendations for standardization of in vitro stability testing of spinal implants using human specimens [29]. Third, fatigue testing cannot be performed because of soft-tissue degeneration. Fourth, several surgical factors cannot be sufficiently standardized, e.g., screw/plate length, etc. Fifth, a corporectomy model is influenced by an adequate compression force of the overbridging implant and the strut graft, as well as graft placement. Nevertheless, synthetic models mostly simulated the worst-case scenario of corporectomy without strut grafting and did not include the important load-sharing strut graft [15]. The use of pure bending moments in this study provided constant and equal loading conditions in all motion segments, following the currently accepted method for simulating the in vivo environment [19, 20, 29]. Range of motion (ROM) and neutral zone (NZ) of the intact specimens were comparable to values reported in the literature. No preload was used, because it is well known that in the absence of muscles the ligamentous in vitro spine buckles under a very small axial load [10]. While the behavior of the various implant systems under high loads and extended cycles—such as those experienced in vivo—cannot necessarily be extrapolated from these data, their relative performance in vitro provides a basis for estimating their prospects for successful clinical use.
The new Polyaxialscrew XL allows enhanced screw hold through geometric optimization and the possibility of optional, additional cementation after screw insertion. Reinforcement of screws with PMMA (polymethyl methacrylate) has been increasingly used clinically for the insertion of screws in severely osteoporotic bone and for reinsertion after they have been stripped by overtightening. However, using PMMA in these situations may be dangerous, because the application of the cement is performed before insertion of the screw and the amount is uncontrolled. Working without adapted instrumentation systems, handling is delicate. Furthermore, the risk of PMMA passage into the spinal canal and problems with cement heating may occur because of uncontrolled cement volume. In this study, no cement runoff occurred, as verified radiographically. The volume of 2 ml is limited through the cement applicator and no cement loosening is possible. Because of the additional cement application through the pre-inserted hollow Polyaxialscrew XL, the screw-base cement enhancement is predicted, and therefore controlled. In contrast to cement injection before screw insertion, it is safe, even during an endoscopic procedure.
Conclusion
For appropriate clinical circumstances, our new technique may be an option for the enhancement of a single anterior instrumentation and the stabilization of circumferential instabilities without rotational malposition. The results of the previously mentioned biomechanical study are warranted for clinical use, especially in minimally open or endoscopic anterior techniques, creating the highest possible primary stability while performing a tissue-preserving ventral approach only.
References
- 1.Abumi Spine. 1989;14:1249. [PubMed] [Google Scholar]
- 2.An Spine. 1995;20:1979. doi: 10.1097/00007632-199509150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Beisse Unfallchirurg. 1998;101:619. doi: 10.1007/s001130050315. [DOI] [PubMed] [Google Scholar]
- 4.Beisse R, Potulski M, Bühren V (2000) Thoracoscopic-assisted anterior approach to thoracolumbar fractures. In: Mayer HM (ed) Minimally invasive spine surgery. A surgical manual. Springer, Berlin Heidelberg New York
- 5.Buhren Chirurg. 1997;68:1076. doi: 10.1007/s001040050326. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham Spine. 1998;23:1333. doi: 10.1097/00007632-199806150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Dick Spine. 1997;22:744. doi: 10.1097/00007632-199704010-00005. [DOI] [PubMed] [Google Scholar]
- 8.DickmanNeurosurgery 1996382798869055 [Google Scholar]
- 9.Gurr J Bone Joint Surg Am. 1988;70:1182. [PubMed] [Google Scholar]
- 10.Hitchon Spine. 1999;24:213. doi: 10.1097/00007632-199902010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Hitchon PW, Goel VK, Rogge TN, Torner JC, Dooris AP, Drake JS, Yang SJ, Totoribe BS, Totoribe K (2000) In vitro biomechanical analysis of three anterior thoracolumbar implants. J Neurosurg (2)93:252–258 [DOI] [PubMed]
- 12.Huang Surg Endosc. 1997;11:1189. doi: 10.1007/s004649900566. [DOI] [PubMed] [Google Scholar]
- 13.Huang Arch Orthop Trauma Surg. 1998;117:92. doi: 10.1007/s004020050201. [DOI] [PubMed] [Google Scholar]
- 14.Kanayama Spine. 1999;24:445. doi: 10.1097/00007632-199903010-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kotani Spine. 1999;14:1406. doi: 10.1097/00007632-199907150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Mack Ann Thorac Surg. 1995;59:1100. doi: 10.1016/0003-4975(95)00112-x. [DOI] [PubMed] [Google Scholar]
- 17.Mayer HM (2000) Minimally invasive spine surgery. A surgical manual. Springer, Berlin Heidelberg New York
- 18.Oda Spine. 1999;24:1634. doi: 10.1097/00007632-199908150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Panjabi J Spinal Disord. 1992;5:383. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Panjabi J Spinal Disord. 1992;5:390. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Potulski Orthopade. 1999;28:723. doi: 10.1007/s001320050402. [DOI] [PubMed] [Google Scholar]
- 22.Regan Clin Orthop. 1997;335:122. [PubMed] [Google Scholar]
- 23.Regan Spine. 1995;20:831. [PubMed] [Google Scholar]
- 24.Regan J Spinal Disord. 1998;11:183. [PubMed] [Google Scholar]
- 25.Schultheiss M, Hartwig E, Wilke HJ, Neller S, Claes L, Kinzl L (2000) Biomechanical aspects of anterior instrumentation in thoracoscopic spine surgery. In: Mayer HM (ed) Minimally invasive spine surgery. A surgical manual. Springer, Berlin Heidelberg New York
- 26.Schultheiss Clin Biomech. 2003;18:631. doi: 10.1016/S0268-0033(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 27.Shono Spine. 1994;19:1711. doi: 10.1097/00007632-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 28.WilkeEur Spine J 19943917874556 [Google Scholar]
- 29.WilkeEur Spine J 199871489629939 [Google Scholar]
- 30.Zdeblick Spine. 1993;18:513. [PubMed] [Google Scholar]